Abstract
KIF (kinesin superfamily) proteins are microtubule-dependent molecular motors that play important roles in intracellular transport and cell division. The extent to which KIFs are involved in various transporting phenomena, as well as their regulation mechanism, are unknown. The identification of 16 new KIFs in this report doubles the existing number of KIFs known in the mouse. Conserved nucleotide sequences in the motor domain were amplified by PCR using cDNAs of mouse nervous tissue, kidney, and small intestine as templates. The new KIFs were studied with respect to their expression patterns in different tissues, chromosomal location, and molecular evolution. Our results suggest that (i) there is no apparent tendency among related subclasses of KIFs of cosegregation in chromosomal mapping, and (ii) according to their tissue distribution patterns, KIFs can be divided into two classes–i.e., ubiquitous and specific tissue-dominant. Further characterization of KIFs may elucidate unknown fundamental phenomena underlying intracellular transport. Finally, we propose a straightforward nomenclature system for the members of the mouse kinesin superfamily.
The dynamic aspect of intracellular organelle transport and cell division involve proper functioning of microtubule-dependent molecular motors such as kinesin and dynein superfamily proteins. Since the biochemical identification (1) and cDNA cloning (2) of kinesin heavy chain, cDNAs of kinesin superfamily genes were genetically identified from various species (3–6). The deduced amino acid sequences of these members revealed the presence of a motor domain of high homology, and a more divergent nonmotor domain. The latter comprises a large portion of the whole molecule, with amino acid sequences that are characteristic for each members. These findings support the observation by quick-freeze, deep-etch electron microscopy techniques that in nerve axons, many different types of crossbridge structures are observed between cellular organelles and microtubules (7, 8). Some of these crossbridges were later identified to be kinesin (9). These and other findings suggest that the machinery for intracellular transport is highly sophisticated, and that a large family of genes encoding the kinesin superfamily is to be expected.
Molecular biological approaches were instrumental in identifying members of the kinesin superfamily. Several amino acid motifs in the motor domain, such as IFAYGQT, DLAGSE, and HIPYR, are highly conserved among species. Fragments flanked by these sequences can therefore be amplified by PCR using degenerate oligonucleotide primers. This strategy identified many kinesin superfamily members in various organisms, such as Drosophila (10, 11), mouse (12, 13), Xenopus (14), fish (15), yeast (3), and rat (16). The reason for the diversity of the kinesin superfamily remains elusive, but it appears that different members are assigned specific cellular functions. In the mouse there are neuron-specific members such as KIF5A (12), nKHC (17), KIF1A (18), and KIFC2 (13) that are apparently not involved in cell division. [Note that Aizawa et al. (12) originally described KIF5. However, further careful studies revealed that KIF5 consists of three members that are referred to as KIF5A, -5B, and -5C in this paper. Henceforth, the name KIF5A is used for the sequence referred to as KIF5 in Aizawa et al.]
For neurons, our data suggest that certain classes of membranous organelles are transported separately down the axon by different motors. For example, KIF1A is associated with organelles that contain synaptotagmin, synaptophysin, and Rab3A, but not SV2, syntaxin 1A, or SNAP-25. Motors such as conventional kinesin or KIF3 were not associated with KIF1A-containing organelle (18). This evidence suggests that different members of the KIF carry different cargos, even though the possibility of functional redundancy still remains. In neurons, at least, motor molecules other than KIF1A may exist that transport vesicles containing SV2, syntaxin 1A, and SNAP-25. Thus with our present knowledge of KIF we may be unable to obtain the overall view of the complex intracellular transport system. These and other considerations strongly motivated us to search for the additional members of the KIF. Initially, based on PCR approach, our group has identified seven KIFs—i.e., KIF1A (18), KIF1B (19), KIF2 (20), KIF3A (21), KIF3B (22), KIF4 (23), and KIF5A (ref. 12; see notes in the previous paragraph) in mouse brain. Three more KIFs were identified by another PCR strategy (13). In the present study, the search for additional KIFs was intensified further by an improved PCR strategy. We identified 16 new members of KIF, virtually doubling the number of known KIFs in the mouse. In this report the partial amino acid sequences of these new KIFs are presented with the classification based on molecular evolutionary analysis and tissue distribution. We also present the chromosomal mapping for some of the known KIFs. Finally, we propose the use of a simplified nomenclature as for the mouse kinesin superfamily.
MATERIALS AND METHODS
Isolation of mRNA.
Mice were killed to obtain the following material: 0-day-old whole brain, 4-week-old hippocampus, 4-week-old olfactory bulb, 4-week-old kidney, and 4-week-old intestine. Each tissue was dissected from separate mice to maintain freshness. The rest of the purification procedures are performed as described (24).
Synthesis of First-Strand cDNA.
Two hundred units of Super Script II (GIBCO/BRL) reverse transcriptase was used to transcribe 50–500 ng of poly(A)+ RNA per 20 μl reaction by an oligo(dT) primer (45°C for 50 min). Other reaction conditions follow the manufacturer’s protocols.
PCRs to Identify New KIFs.
The oligonucleotide primers used in this study are listed in Table 1. The primers were designed according to the conserved amino acid sequences IFAYGQT and VDLAGSE in the motor domain. For all PCRs, AmpliTaq DNA polymerase (Perkin–Elmer) and GeneAmp PCR system 9600 (Perkin–Elmer) were used. Primers IFAYGQT and DLAGSE were used to amplify 0-day-old mouse whole brain first-strand cDNA; the reaction program was as follows: 30 cycles at 94°C for 1 min, at 56°C for 1 min, and at 72°C for 1 min. The reaction mixture contained 0.5 μl of cDNA solution (directly used from cDNA synthesis after heat inactivation), 1 μl of 10 mM dNTP, 5 units of AmpliTaq DNA polymerase, 100 pmol of each primer, 10 mM Tris⋅HCl (pH 8.3) (at 25°C), 50 mM KCl, 1.5 mM MgCl2, and 0.001% (wt/vol) gelatin, in a total volume of 50 μl. Multiple bands appeared when separated by agarose gel electrophoresis. For most of the known kinesin superfamilies, the nucleotide sequences between IFAYGQT and VDLAGSE correspond to ≈500 bp. Thus, bands around 500 bp were cut out and the DNAs were recovered by Gene CleanII (Bio 101). Purified fragments were treated with T4 DNA polymerase and subcloned into EcoRV site of pBlueScript (Stratagene). Sequencing was done by Applied Biosystems auto sequencer models 373 and 377. For the other template cDNAs the remaining primers were used for amplification. PCR schedule was as follows: 96°C for 45 sec, 55°C for 4 min, and 72°C for 3 min with 6-sec extension per cycle for 35–40 cycles (25). Primers IFAY-RI, IFAY-BAM, and IFAY-CLA contain EcoRI, BamHI, and ClaI sites, respectively. These sites were used to subclone the amplified fragments into pBlueScript. Calf intestine alkaline phosphatase (Toyobo, Osaka) treatment of the subcloning vectors was done with less than 1 μg of DNA in 50 μl volume. The buffer used for the reaction was 50 mM Tris⋅HCl (pH 9.0), 1 mM MgCl2, 0.1 mM ZnCl2, and 1 mM Spermidine (Sigma). Three units of enzyme was added to start the reaction (1 h at 37°C). An additional three units of enzyme was added at the middle of the reaction. Competent cells were prepared with DH5α bacteria as described (26).
Table 1.
Degenerate oligonucleotide primers used in this study
| Primer | Sequence |
|---|---|
| IFAYGQT | 5′-AT(T/C/A),TT(T/C),GCI,TA(T/C),GGI,CA(A/G),AC-3′ |
| DLAGSE | 5′-(C/T)TC,I(A/C)(A/T),ICC,IGC,IAG,(A/G)TC-3′ |
| IFAY | 5′-AT(A/T/C),TT(C/T),(A/G)CI,TA(C/T),GGI,CA(A/G),AC-3′ |
| IFAY-RI | 5′-GGG,AAT,TCA,T(A/T/C)T,T(C/T)(A/G),CIT,A(C/T)G,GIC,A(A/G)A,C-3′ |
| IFAY-BAM | 5′-CGG,GAT,CCA,T(A/T/C)T,T(C/T)(A/G),CIT,A(C/T)G,GIC,A(A/G)A,C-3′ |
| IFAY-CLA | 5′-CCA,TCG,ATA,T(A/T/C)T,T(C/T)(A/G),CIT,A(C/T)G,GIC,A(A/G)A,C-3′ |
| LAGSE1 | 5′-CTC,(A/G)CT,ICC,IGC,(C/T)A(A/G),(A/G)TC,IA-3′ |
| LAGSE2 | 5′-CTC,(A/G)CT,ICC,IGC,(A/G)AG,(A/G)TC,IA-3 |
| LAGSE3 | 5′-CTC,IGA,ICC,IGC,(C/T)A(A/G),(A/G)TC,IA-3′ |
| LAGSE4 | 5′-CTC,IGA,ICC,IGC,(A/G)AG,(A/G)TC,IA-3′ |
| LAGSE5 | 5′-TTC,(A/G)CT,ICC,IGC,(C/T)A(A/G),(A/G)TC,IA-3′ |
| LAGSE6 | 5′-TTC,(A/G)CT,ICC,IGC,(A/G)AG,(A/G)TC,IA-3′ |
| LAGSE7 | 5′-TTC,IGA,ICC,IGC,(C/T)A(A/G),(A/G)TC,IA-3′ |
| LAGSE8 | 5′-TTC,IGA,ICC,IGC,(A/G)AG,(A/G)TC,IA-3′ |
Northern Blot Analysis.
Total RNA was purified as described (24) from brain, lung, heart, liver, spleen, kidney, intestine, and testis tissue of 4-week-old mice. Twenty micrograms of total RNA was loaded on each lane of a 1% agarose gel. The concentration of RNAs was measured by light absorbance as described (27). Probes were labeled with T7 Quick prime kit (Pharmacia). Quick Hyb (Stratagene) was used for hybridization according to manufacturer’s protocol. The wash schedule was 2× SSC/0.1% SDS at 65°C for 10 min (three times). For some probes additional wash in 0.1× SSC/0.1% SDS for 10 min was needed to eliminate the cross-hybridization signal.
Sequence Alignment and Molecular Evolutional Analysis.
A phylogenetic tree of the motor domain sequences of the KIF was generated using the clustalw program (28) based on the neighbor joining (NJ) method (29). The confidence limits on the tree were calculated by performing bootstrap resampling 1,000 times. The region between IFAYGQT and LAGSE was used for the alignment. However, for some of the newly identified KIFs, only a partial sequence of the corresponding domain was obtained due to the cloning artefacts. The accuracy of the tree obtained was confirmed by comparing the trees deduced by the NJ method and the maximum parsimony (MP) method. MP method was performed according to the described procedures (30). Sequences other than the newly identified KIFs were obtained from the GenBank database. The tree was drawn using the software treeview (31).
Cytogenetic Mapping.
A λ EMBL3 library of genomic DNA derived from the mouse embryonic stem cell line J1 was screened with cDNA probes containing the motor domains of KIF1A, -1B, -2, -3A, -3B, -5A, -5B, -5C, and -C2 by standard methods (27). Overlapping clones of about 20 kb long were isolated. Clones were proved to contain the correct genes by partial sequencing. For fluorescence in situ hybridization mapping standard methods were used (32–34) [see DNA Biotech (Ontario, Canada) and Nippon Gene (Sendai, Japan)].
RESULTS
Identification of New KIFs.
Fig. 1 shows the alignment of the amino acid sequences of the PCR products of the new KIFs identified together with the previously identified ones (12, 29). The identification of the new KIFs are summarized in Table 2.
Figure 1.
Amino acid sequence of the KIF motor domains. Sequences between IFAYGQT and DLAGSE, the highly conserved kinesin motor domain motif, were aligned using the program clustalw. Arrows indicate the positions of the primers. Asterisks are identical amino acids. Dots are similar amino acids. For some new KIFs only a partial sequence within this domain is shown. This is due to the cloning artefact. The rest of the region was either lost by the restriction enzyme digestion or appeared as a hybrid molecule with another KIF which may be caused by low stringency PCR. Various cDNA templates were obtained from whole brain, hippocampus, olfatory bulb, kidney, and intestine of 4-week-old mice.
Table 2.
Summary of searching the new KIFs
| Library | No. of clones sequenced | Identification |
|---|---|---|
| 0 day whole brain | 83 | KIF3C, KIF1C, KIF8, KIF11 |
| 4 week hippocampus | 50 | KIF6, KIF7, KIF9, KIF10 |
| 4 week olfactory bulb | 100 | KIF16B |
| 4 week kidney | 200 | KIF12, KIF13A, KIF13B, KIF14, |
| KIF16A, KIF17 | ||
| 4 week intestine | 100 | KIF15 |
Tissue Distribution Patterns of the Newly Identified KIFs.
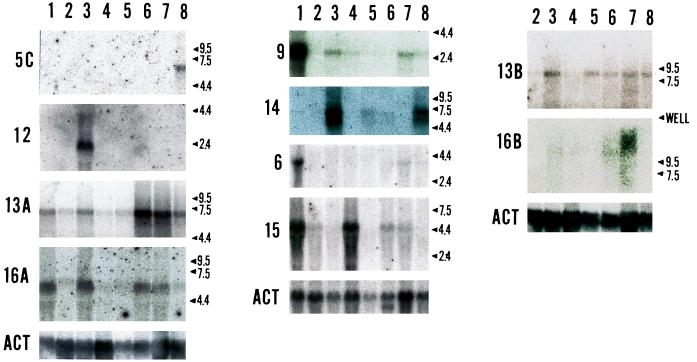
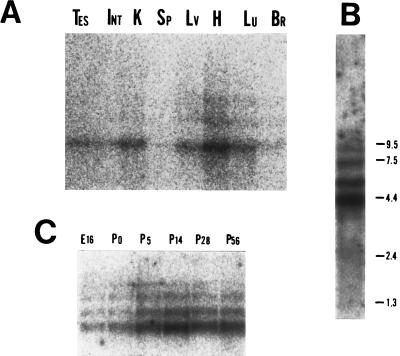
Some of the new KIFs turned out to be homologues of previously identified and characterized kinesin related proteins in other species. Fig. 2 shows the tissue distribution patterns of the transcripts of some of the new KIFs as determined by Northern blot analysis. Twenty micrograms of total RNA was loaded per lane unless otherwise specified. KIF5C turned out to be brain specific. The transcript of KIF1C (Fig. 3A) was ubiquitously distributed and 4 bands were detected, suggesting alternative splicing or multiple polyadenylaytion sites. Two micrograms of poly(A)+ RNA from 4-week-old mouse brain was blotted against KIF1C probe in Fig. 3B. Four bands appear clearly in this Northern blot even after harsh wash condition. In Fig. 3C developmental changes of the KIF1C transcript is shown by applying 2 μg of brain poly(A)+ RNA for each developmental stage. Northern blotting revealed KIF6 and KIF7 (data not shown) to be testis-specific. KIF8 was a mouse homologue of yeast KIP1 (37). KIF9 was testis-dominant with less signals detected from brain, lung, and kidney. KIF10 was a mouse homologue of CENP-E (38). KIF11 was a mouse homologue of Xenopus Eg5 (39). KIF12 was kidney-dominant. KIF13A transcript was overall ubiquitous but dominant in brain, lung, heart, kidney, and testis. The transcript of KIF13B was distributed ubiquitously. KIF14 transcript was detected in brain and kidney. KIF15 transcript was dominantly detected in spleen and testis. KIF16A was overall ubiquitous but dominant in lung, heart, kidney, and testis. KIF16B was lung-dominant.
Figure 2.
Our first approach to elucidate the function of each new KIFs is to examine the tissue distribution. We performed Northern blot analyses for the new KIFs that have almost no functional implication from the updated database. Twenty micrograms of total RNA from various mouse tissues (4 week old) was loaded on each lanes. Probes used are from the PCR amplified and subcloned fragment of the motor domain listed in Fig. 1. Controls were taken by probing the membrane with rat β-actin probe. The arrowhead with the number indicates the molecular weight in kilobases. The numbers in the left of each membrane is the name of the molecule (KIF is abbreviated). ACT is the beta actin control blot. Lanes: 1, testis; 2, intestine; 3, kidney; 4, spleen; 5, liver; 6, heart; 7, lung; 8, brain.
Figure 3.
Northern blot analysis of KIF1C. Twenty micrograms of total RNA from various mouse tissues (4 week old) was loaded on each lanes. Probes used are from the PCR amplified and subcloned fragment of the motor domain. KIF1C appears as four bands in the Northern blot. (A) Tissue distribution of KIF1C transcripts. (B) Two micrograms of mRNA from 4-week-old mouse brain was resolved and blotted likewise. The molecular weights are marked in the right side in kb. (C) Two micrograms of mRNA was resolved to examine the developmental transcription pattern of KIF1C in brain. Br, brain; Lu, lung; H, heart; Lv, liver, Sp, spleen; K, kidney; Int, intestine; Tes, testis.
As seen from the Northern blot results, the source of the cDNA material does not necessarily correlate with transcript distribution pattern. For example, most of the testis-dominant KIFs were identified with nervous tissue as a starting material. Our results suggest that we can roughly classify the KIFs by their transcript distribution patterns into two classes—i.e., ubiquitous and tissue-dominant. KIF1C, -13B, and -16A are the ubiquitously distributed, whereas the others (KIF5C, -6, -9, -12, -13A, -14, -15, and -16B) have the biased tissue distribution.
Although the washing procedure of the Northern blot membranes were done with care, the possibility of cross-hybridization still remains. However, considering the difference of the molecular weight of each transcript, the cross-hybridization may not be between the different families.
Two Brain-Specific Kinesin Heavy Chains Exist in the Mouse.
In the previous search, we identified KIF5 as a brain-specific type of conventional kinesin heavy chain (12). Another kinesin heavy chain identified by Kato (35) was similar to both KIF5 (12) and to ubiquitous kinesin (36). Thus to avoid confusion in nomenclature, we decided to use the name KIF5A for the sequence referred to as KIF5 in Aizawa et al. (12), KIF5B for the ubiquitous kinesin heavy chain, and KIF5C for the molecule identified by Kato (35). Using cDNA fragment of the motor domain kindly provided by K. Kato, we found that this was also specifically expressed in the brain (Fig. 2). Thus there are three different kinesin heavy chains in mouse brain.
Phylogenetic Analysis of Known Kinesin Superfamily Proteins.
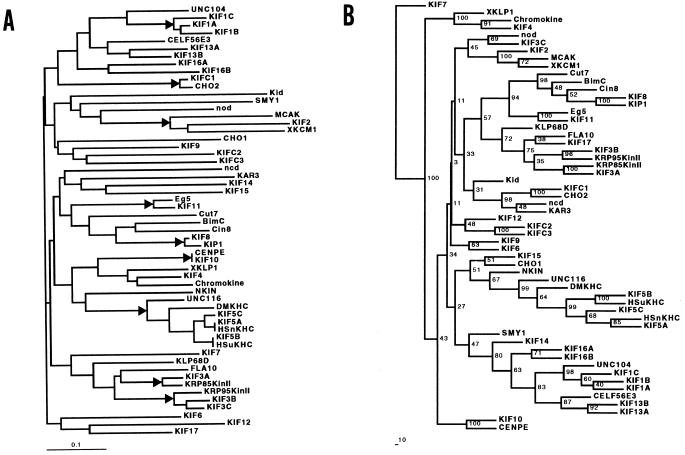
To classify the newly identified KIFs, molecular evolutionary analysis was performed. Phylogenetic trees were obtained by both neighbor joining (Fig. 4A) (29) and maximum parsimony methods (Fig. 4B) (40). Both trees are unrooted. By comparing the two trees, some of the branching patterns of the peripheral branches are nearly identical. For example, the branching patterns of the KIF1, KIF13, and KIF16 families are identical in both methods. In other cases differences are seen. For example, KIF14 locates in a completely different branch. The reliability of each branch point was evaluated by calculating the bootstrap values.
Figure 4.
Phylogenetic tree was constructed using the new sequence data of the KIFs listed in Fig. 1. Other sequences were available from the database and added to generate the tree. The amino acid sequence between IFAYGQT and DLAGSE was used to align the sequences. The tree is unrooted. We confirmed the accuracy of the resultant phylogenetic tree by comparing the trees deduced via the neighbor joining (NJ) method (A) and maximum parsimony (MP) method (B). In A, branching that gives bootstrap values higher than 90 are marked. In B, bootstrap values are listed in the tree. The GenBank accession numbers of the sequence data used are as follows: KIF1A, D29951; KIF1B, D17577; KIF1C, AB001456; KIF2, D12644; KIF3A, D1264; KIF3B, D26077; KIF3C, AB001433; KIF4, D12646; KIF5A, C44259; KIF5B(MMuKHC), L27153; KIF5C(MMnKHC), X61435; KIF6, AB001434; KIF7, AB001435; KIF8, AB001436; KIF9, AB001437; KIF10, AB001426; KIF11, AB001427; KIF12, AB001428; KIF13A, AB001429; KIF13B, AB001430; KIF14, AB001431; KIF15, AB001432; KIF16A, AB001425; KIF16B, AB001423; KIF17, AB001424; KIFC1, D49545; KIFC2, D49544; KIFC3, AB001457; unc104, M58582; CELF56E3, U41536; CHO1, X67155; CHO2, X83576; Kid, D38751; SMY1, M69021; nod, M94188; MCAK, U11790; XKCM1, U36485, U36486; ncd, X57475; KAR3, M31719; Eg5, X54002; Cut7, X57513; BimC, M32075; Cin8, M90522; KIP1, Z11962; CENP-E, Z15005; XKLP1, X82012; Chromokinesin(Chromokine), U18309; NKIN, L47106; unc116, L19120; DmKHC, M24441; HsnKHC, U06698; HsuKHC, X65873; KLP68D, U15974; FLA10, L33697; KRP85(KinII), L16993; KRP95(KinII), U00996.
These results allow us to assess whether a newly identified KIF is a homologue of previously identified motors in other organisms. For example, KIF11 is highly homologous to Eg5 in Xenopus. However, careful interpretation is needed. First, only those that are shown to be evolutionarily close by the two tree-generating methods may be considered to be highly homologous. Second, even if two genes from different species appear evolutionarily close in both trees, there still remains the possibility that the two are not species homologues but rather orthologues. One good example is seen in the branching patterns for KIF13A, KIF13B, and CELF56E3 (a sequence obtained from the Caenorhabditis elegans genome project). KIF13A can be a species homologue of CELF56E3 just as likely as KIF13B. Thus, the information obtained from the phylogeny tree should be interpreted with caution. Full cDNA sequences of each of the molecules are required to finally determine whether two molecules are species homologues or not. In any case, by neglecting the details of molecular evolution we were able to name the newly identified molecules. The numbers were given according to the chronological orders of identification. However, some of the names were later changed when we noticed that the molecule can be considered to consist a subfamily with another molecule. Thus, the numbering itself has no more meaning than each molecules are different.
Chromosomal Mapping of the KIFs and KIFCs.
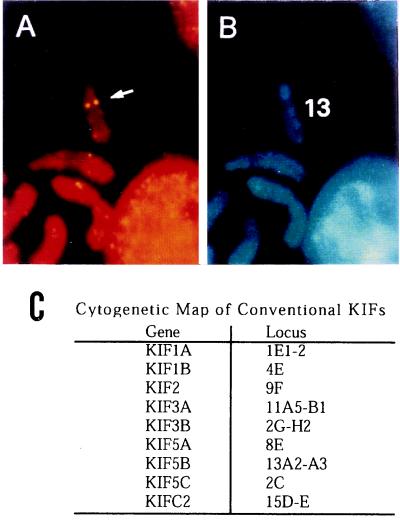
To examine the distribution of the KIF genes on the chromosome, we performed fluorescence in situ hybridization using the genomic sequences as probes. The results are shown in Fig. 5. There seems to be little relation between closely related KIFs and their chromosomal localization. For example KIF1A and 1B, KIF3A and -3B, KIF5A, -5B, and -5C, respectively, are closely related members. However, their chromosomal locations differ and no apparent segregation seems to exist. These data implies that the evolution of the KIF genes may be different from those genes that are clustered in certain regions of the genome, such as globin genes.
Figure 5.
Cytogenetic mapping were performed for some of the known KIFs. (A and B) Examples of the fluorescence in situ hybridization (FISH) mapping (probed for KIF5B). (A) The FISH signals on chromosome (arrow). (B) The same mitotic figure stained with 4′,6-diamidino-2-phenylindole (DAPI) to identify chromosome 13. (C) Rest of the results are summarized.
DISCUSSION
The improved PCR strategy lead to the identification of 16 new KIFs in the mouse, virtually doubling the number of the known mouse KIF molecules. The total number of the known KIFs in mouse has now reached more than 30. Although 3 (KIF8, KIF10, and KIF11) are likely to be the homologues of the previously characterized genes in other species, still 13 are new. In a PCR with a mixture of degenerate oligonucleotide primers, the outcome of the amplified cDNA fragment is strongly biased by the annealing efficiency between the template and the primers. Thus, the probability of discovering the new KIFs does not necessarily correlate with the level of the transcript of that particular KIF in the cDNA template. Our findings does not give any information about the actual size of the KIF family in the mouse genome, and there are reasons to believe there may be even more unknown KIFs.
What Are the Functions of the New KIFs?
When focusing on microtubule-dependent intracellular transport, highly differentiated cells such as neurons and epithelial cells are good model systems to study. However, can we actually distinguish whether the new KIFs are for intracellular transport or for cell division on the basis of the information we have at this moment? Even if the two molecules from different species are shown to be evolutionarily very close, it does not necessarily mean that the two are the species homologues. This is because the phylogenetic analysis performed by us uses only partial amino acid sequences of the motor domain.
From our previous study, KIF1A and KIF1B are highly homologous in the region that covers the entire motor domain, but the cargos they carry are completely different. However, there seems to be some relationship between molecular evolution and function. For example, the branching pattern in the phylogenetic tree deduced by two different methods matches for KIF8, KIP1, CIN8, BimC, Eg5, and Cut7. This is the so called the BimC/Eg5 family (8), and most of the molecules in this class are concerned in cell division. The branching patterns for KIF1A, -1B, -1C, unc104, CELF56E3, KIF13A, -13B, KIF16A, and -16B are also same between the two different analysis method. If the relationship between molecular evolution and function found for the BimC/Eg5 family holds true for this family, the five new KIFs in this KIF1, -13, and -16 branch may be involved in intracellular organelle transport. Because KIF12 and KIF14 are put in different branches in the two trees, we interpret these are unclassified for the moment. KIF12 is dominantly expressed in kidney and KIF14 in brain and kidney. Taking into account the tissue distribution patterns, the evidence is enough to encourage us to further characterize these two molecules too.
What Can Be Said from the Tissue Distribution Northern Blotting Data?
We classified the newly identified KIFs into ubiquitous and tissue-dominant types according to the Northern blotting data. In general, one might suggest that molecules that are ubiquitously expressed in the entire organism may function in some sort of fundamental processes of cells in general. KIF1B, for example, is a ubiquitous molecule and functions as a transporter of mitochondria (19). Mitochondria are ubiquitous organelle, which is consistent with the distribution of KIF1B. However, cell division is also a process observed in all cells except certain highly differentiated cells, such as neurons. In new born animals, when the actual number of cells in the body is increasing, a certain population of almost every tissue should be undergoing cell division. But in relatively grown up animals cell division events should be limited to specific area of the organism, such as testes, lymphatic organs (spleen), and certain epithelial tissues (alimentary duct, dermis, etc.). Our materials for the Northern blot analyses were obtained from 4-week-old mice. By the criteria above, KIF15 may be involved in cell division. KIF6, -7, and -9 are dominantly transcribed in testes compared with other tissues, but it may be premature to conclude that they are involved in cell division. We believe the situation in testes is complicted. In this tissue active spermatogenesis (involving cell division) take place in a vectorial manner from the basement to the apical lumen (directional intracellular transport). However, the sperm cells thus formed also have active flagella which include a lot of KIFs and dyneins. Thus it is difficult to say anything at this moment about the function of KIF6, -7, and -9.
On the other hand, the KIFs that are neither testes- nor spleen-dominant, such as KIF12, -13A, -14, and -16B, are potentially interesting as motors for specific intracellular transports.
What Can We Learn from the New KIFs.
Much of the studies aimed toward intracellular transport are done in neurons and polarized epithelial cell systems. The involvement of KIFs in axonal transport, dendritic transport, and intracellular trafficking in epithelial cells remain elusive. The functional specificity of each KIF is believed to reside in the nonmotor domain of the molecule. This belief is based on the evidence that the amino acid sequences of the nonmotor domain are quite distinct between different members. Initially, the only tools to further devide the nonmotor domains into different subdomains were the prediction of three-dimensional structure by analysis softwares based on the primary amino acid sequence such as the coiled–coil algorithm (41, 42). However, as more and more information on full-length cDNA sequence accumulated, subdomains in the nonmotor domains became clear for some of the new KIFs. One example is the AF6/cno domain (43). The function of this domain is unknown, but the domain exists in the region flanking the motor domain toward the carboxyl terminal in KIF1A and -1B. The question is whether this domain exists in KIF1C. The full cDNA sequence will give an answer. In other KIFs as well subdomains that are shared within the subfamilies of the new KIFs may be revealed.
Nomenclature of the KIF.
The growing number of newly identified KIF proteins may lead to some confusion because different nomenclatures are used in different species. We propose to use the following rule: (i) the term KIF is used for the names of the mouse kinesin superfamily genes; (ii) individual members of the superfamily in mouse are termed KIF followed by a number such as KIF2, KIF4, and so on; (iii) a capital letter is added after the number when there are subfamilies such as, KIF1A, KIF1B, and so on; and (iv) a “C” is added before the number when the molecule has its motor domain in the carboxyl-terminal half, such as KIFC1, KIFC2, and so on. In this way, we think the nomenclature will help people both in and outside the field of molecular motors.
Acknowledgments
We thank Hiroto Okayama for helpful discussion, and Masae Sugaya, Hiromi Sato, and Haruyo Fukuda for their technical and secretarial assistance. This study was supported by a grant for Center of Excellence (COE) from the Ministry of Education, Science and Culture to N.H. T.N. is supported by the Japan Society for the Promotion of Science Research Fellowship for Young Scientists.
Footnotes
Data deposition: The sequences reported in this paper have been deposited in the GenBank database [accession nos. AB001456 (KIF1C), AB001433 (KIF3C), AB001434 (KIF6), AB001435 (KIF7), AB001436 (KIF8), AB001437 (KIF9), AB001426 (KIF10), AB001427 (KIF11), AB001428 (KIF12), AB001429 (KIF13A), AB001430 (KIF13B), AB001431 (KIF14), AB001432 (KIF15), AB001425 (KIF16A), AB001423 (KIF16B), AB001424 (KIF17), AB001457 (KIFC3)].
References
- 1.Vale R D, Reese T S, Sheetz M S. Cell. 1985;42:39–50. doi: 10.1016/s0092-8674(85)80099-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yang J T, Saxton W M, Goldstein L S B. Proc Natl Acad Sci USA. 1998;85:1864–1868. doi: 10.1073/pnas.85.6.1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Meluh P B, Rose M D. Cell. 1990;60:1029–1041. doi: 10.1016/0092-8674(90)90351-e. [DOI] [PubMed] [Google Scholar]
- 4.Enos A P, Morris N R. Cell. 1990;60:1019–1027. doi: 10.1016/0092-8674(90)90350-n. [DOI] [PubMed] [Google Scholar]
- 5.Zhang P, Knowles B A, Goldstein L S B, Hawley R S. Cell. 1990;62:1053–1062. doi: 10.1016/0092-8674(90)90383-p. [DOI] [PubMed] [Google Scholar]
- 6.Hagan I, Yanagida M. Nature (London) 1990;347:563–566. doi: 10.1038/347563a0. [DOI] [PubMed] [Google Scholar]
- 7.Hirokawa N. J Cell Biol. 1982;94:129–42. doi: 10.1083/jcb.94.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hirokawa N. Trends Cell Biol. 1996;6:135–141. doi: 10.1016/0962-8924(96)10003-9. [DOI] [PubMed] [Google Scholar]
- 9.Hirokawa N, Pfister K K, Yorifuji H, Wagner M C, Brady S T, Bloom G S. Cell. 1989;56:867–878. doi: 10.1016/0092-8674(89)90691-0. [DOI] [PubMed] [Google Scholar]
- 10.Endow S A, Hatumi M. Proc Natl Acad Sci USA. 1991;88:4424–4427. doi: 10.1073/pnas.88.10.4424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stewart R J, Pesavento P A, Woerpel D N, Goldstein L S B. Proc Natl Acad Sci USA. 1991;88:8470–8474. doi: 10.1073/pnas.88.19.8470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aizawa H, Sekine Y, Takemura R, Zhang Z, Nangaku M, Hirokawa N. J Cell Biol. 1992;119:1287–1296. doi: 10.1083/jcb.119.5.1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Saito N, Okada Y, Noda Y, Kinoshita Y, Kondo S, Hirokawa N. Neuron. 1997;18:425–438. doi: 10.1016/s0896-6273(00)81243-x. [DOI] [PubMed] [Google Scholar]
- 14.Vernos I, Heasman J, Wylie C. Dev Biol. 1993;157:232–239. doi: 10.1006/dbio.1993.1127. [DOI] [PubMed] [Google Scholar]
- 15.King-Smith C, Bost-Usinger L, Burnside B. Cell Motil Cytoskeleton. 1995;31:66–81. doi: 10.1002/cm.970310108. [DOI] [PubMed] [Google Scholar]
- 16.Sperry A O, Zhao L. Mol Biol Cell. 1996;7:298–305. doi: 10.1091/mbc.7.2.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Niclas J, Navone F, Hom-Booher N, Vale R. Neuron. 1994;12:1059–1072. doi: 10.1016/0896-6273(94)90314-x. [DOI] [PubMed] [Google Scholar]
- 18.Okada Y, Yamazaki H, Sekine-Aizawa Y, Hirokawa N. Cell. 1995;81:769–780. doi: 10.1016/0092-8674(95)90538-3. [DOI] [PubMed] [Google Scholar]
- 19.Nangaku M, Sato-Yoshitake R, Okada Y, Noda Y, Takemura R, Yamazaki H, Hirokawa N. Cell. 1994;79:1209–1220. doi: 10.1016/0092-8674(94)90012-4. [DOI] [PubMed] [Google Scholar]
- 20.Noda Y, Sato-Yoshitake R, Kondo S, Nangaku M, Hirokawa N. J Cell Biol. 1995;129:157–167. doi: 10.1083/jcb.129.1.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kondo S, Sato-Yoshitake R, Noda Y, Aizawa H, Nakata T, Matsuura Y, Hirokawa N. J Cell Biol. 1994;125:1095–1107. doi: 10.1083/jcb.125.5.1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yamazaki, Nakata T, H, Okada Y, Hirokawa N. J Cell Biol. 1995;130:1387–1399. doi: 10.1083/jcb.130.6.1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sekine Y, Okada Y, Noda Y, Kondo S, Aizawa H, Takemura R, Hirokawa N. J Cell Biol. 1994;127:187–201. doi: 10.1083/jcb.127.1.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Okayama H, Kawaichi M, Brownstein M, Lee F, Yokota T, Arai K. Methods Enzymol. 1987;154:3–28. doi: 10.1016/0076-6879(87)54067-8. [DOI] [PubMed] [Google Scholar]
- 25.Buck L, Axel R. Cell. 1991;65:175–187. doi: 10.1016/0092-8674(91)90418-x. [DOI] [PubMed] [Google Scholar]
- 26.Inoue H, Nojima H, Okayama H. Gene. 1990;96:23–28. doi: 10.1016/0378-1119(90)90336-p. [DOI] [PubMed] [Google Scholar]
- 27.Sambrook J, Fritsch E F, Maniatis T. Molecular Cloning: A Laboratory Manual. 2nd Ed. Plainview, NY: Cold Spring Harbor Lab. Press; 1989. [Google Scholar]
- 28.Thompson J D, Higgins D G, Gibson T J. Nucleic Acids Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Saitou N, Nei M. Mol Biol Evol. 1987;4:406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- 30.Tanaka Y, Zhang Z, Hirokawa N. J Cell Sci. 1995;108:1883–1893. doi: 10.1242/jcs.108.5.1883. [DOI] [PubMed] [Google Scholar]
- 31.Page R D M. Comput Appl Biosci. 1996;12:357–358. doi: 10.1093/bioinformatics/12.4.357. [DOI] [PubMed] [Google Scholar]
- 32.Heng H H Q, Squire J, Tsui L-C. Proc Natl Acad Sci USA. 1992;89:9509–9513. doi: 10.1073/pnas.89.20.9509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Feng G S, Shen R, Heng H H Q, Tsui L-C, Kazlauskas A, Pawson T. Oncogene. 1994;9:1745–1750. [PubMed] [Google Scholar]
- 34.Heng H H Q, Tsui L-C. Chromosoma. 1993;102:325–332. doi: 10.1007/BF00661275. [DOI] [PubMed] [Google Scholar]
- 35.Kato K. Eur J Neurosci. 1991;2:704–711. doi: 10.1111/j.1460-9568.1990.tb00460.x. [DOI] [PubMed] [Google Scholar]
- 36.Gudkov A V, Kazarov A R, Thimmapaya R, Axenobich S A, Mazo I A, Roninson I B. Proc Natl Acad Sci USA. 1994;91:3744–3748. doi: 10.1073/pnas.91.9.3744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Roof D M, Meluh P B, Rose M D. J Cell Biol. 1992;118:95–108. doi: 10.1083/jcb.118.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yen T J, Compton D A, Wise D, Zinkowski R P, Brinkley B R, Earnshaw W C, Cleveland D W. EMBO J. 1991;10:1245–1254. doi: 10.1002/j.1460-2075.1991.tb08066.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Le Guellec R, Paris J, Couturier A, Roghi C, Philippe M. Mol Cell Biol. 1991;11:3395–3398. doi: 10.1128/mcb.11.6.3395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Eck R V, Dayhoff M O. Atlas of Protein Sequence and Structure. Silver Spring, MD: Natl. Biomed. Res. Found.; 1966. [Google Scholar]
- 41.Lupas A, Van Dyke M, Stock J. Science. 1991;252:1162–1164. doi: 10.1126/science.252.5009.1162. [DOI] [PubMed] [Google Scholar]
- 42.Lupas A. Trends Biochem Sci. 1996;21:375–382. [PubMed] [Google Scholar]
- 43.Ponting C P. Trends Biochem Sci. 1995;20:265–266. doi: 10.1016/s0968-0004(00)89040-4. [DOI] [PubMed] [Google Scholar]