Abstract
In several cell types, an intriguing correlation exists between the position of the centrosome and the direction of cell movement: the centrosome is located behind the leading edge, suggesting that it serves as a steering device for directional movement. A logical extension of this suggestion is that a change in the direction of cell movement is preceded by a reorientation, or shift, of the centrosome in the intended direction of movement. We have used a fusion protein of green fluorescent protein (GFP) and γ-tubulin to label the centrosome in migrating amoebae of Dictyostelium discoideum, allowing us to determine the relationship of centrosome positioning and the direction of cell movement with high spatial and temporal resolution in living cells. We find that the extension of a new pseudopod in a migrating cell precedes centrosome repositioning. An average of 12 sec elapses between the initiation of pseudopod extension and reorientation of the centrosome. If no reorientation occurs within approximately 30 sec, the pseudopod is retracted. Thus the centrosome does not direct a cell’s migration. However, its repositioning stabilizes a chosen direction of movement, most probably by means of the microtubule system.
Cells migrating on a two-dimensional substratum have a distinct polarity that arises from the fact that protrusive activity is confined to a small portion of the cell cortex, the leading edge or lamellipod, and retraction takes place at the opposite end. In contrast to, e.g., polarized but stationary epithelial cells or neurons, locomoting cells may change their polarity frequently and rapidly, in some cells on a time scale of seconds. How moving cells establish and change their direction of movement is a central question in cell motility (1). Microtubules have long been suspected to be involved in the coordination of cell movement (refs. 2 and 3; for reviews, see refs. 4–6). Moreover, in several cell types an intriguing correlation exists between the position of the microtubule-organizing center (the centrosome) and the direction of cell movement in that the centrosome is positioned between the leading edge of the cell and the nucleus (e.g., refs. 7 and 8). These findings were interpreted to mean that the centrosome determines the direction of cell locomotion. Any changes in the direction of cell movement, then, would be preceded by a reorientation of the centrosome in the intended direction of movement. This attractive hypothesis has subsequently been reinforced by studies in other cell types (5, 9, 10). However, microtubules may not always be essential for cell polarization (11) or movement (12–14), and the centrosome may also occupy a position other than behind the leading edge in certain locomoting cells (15–17). All these studies, however, are based on an analysis of cells fixed for immunofluorescence or electron microscopy to determine centrosome position.
We have chosen amoebae of the slime mold Dictyostelium discoideum as a model system to address the question of the relationship between centrosome position and cell locomotion in living cells. We have generated a fusion protein of the green fluorescent protein (GFP) (18) and γ-tubulin, a protein involved in microtubule nucleation (19–21) that is localized at the centrosome (21–23) in all cell types examined so far, including Dictyostelium (U.E., R.G., and M.S., unpublished data). The fusion protein labels the centrosome brightly and stably, allowing us to follow its position during cell locomotion with unprecedented spatial and temporal resolution. We show that centrosome repositioning never precedes pseudopod extension or a change in the direction of movement, arguing against a role as a coordinator of directional changes of cell movement.
MATERIALS AND METHODS
Construction of the Vector.
The GFP sequence was modified by site-directed mutagenesis to generate S65T-GFP, whose emission is red-shifted (24). An in-frame fusion of the full-length D. discoideum γ-tubulin (accession no. AJ000492) and the mutated GFP was cloned into pB15 (kindly provided by D. Manstein, Max Planck Institute, Heidelberg). The coding sequences of γ-tubulin and GFP are separated by a spacer of 4 amino acids (i.e., SRGS). The resulting vector, pB15γtubGFP, was transformed into AX2 cells by using calcium phosphate (25).
Cell Handling.
Amoebae of D. discoideum (strain AX2) expressing the γ-tubulin-GFP fusion protein were grown axenically on a rotary shaker (150 rpm) at 21°C using standard culture techniques (26). For microscopic observation cells were harvested, resuspended in 17 mM Sörensen’s phosphate buffer, pH 6.0, and allowed to settle on a glass coverslip. For chemotaxis assays, aggregation-competent cells were stimulated by using a glass micropipette filled with 0.1 mM cAMP in Sörensen’s phosphate buffer. First, cells were stimulated to establish morphological polarity. The micropipette was then quickly moved to the tail of the cell to force the cell to change its direction of movement.
Microscopy.
Cells were observed using either a Zeiss Axiophot upright microscope or a Zeiss Axiovert inverted microscope equipped with standard filter sets for fluorescein and rhodamine. Images were captured using a silicon intensified tube (SIT) camera (Hamamatsu, Herrsching, Germany) and recorded onto video tape using a Panasonic AG 6720 video recorder. For image analysis frames were captured from the recorded tapes at 2-sec intervals with a personal computer (Macintosh IIfx) equipped with an analog–digital converter for video images (PixelPipeline; Perceptics, Knoxville, TN) and were analyzed using the NIH Public Domain image software. Immunofluorescence microscopy was carried out using standard procedures (26, 27).
RESULTS AND DISCUSSION
Full-length red-shifted GFP was fused to the COOH terminus of full-length Dictyostelium γ-tubulin. A complete characterization of γ-tubulin from D. discoideum will be provided elsewhere (U.E., R.G., and M.S., unpublished data). The fusion protein was inserted into the vector pB15 and transformed into AX2 cells. The resulting stable transformants expressed the fusion protein (henceforth termed γtub-GFP) in addition to endogenous γ-tubulin. In cytoplasmic extracts as well as isolated centrosome–nucleus complexes γtub-GFP was present in an approximately 5-fold excess over endogenous γ-tubulin, as determined by immunoblotting (not shown). γtub-GFP localized to the centrosome in both living and fixed cells, as shown by video microscopy (Fig. 1 a and b) and double-label immunofluorescence microscopy with antibodies against tubulin (Fig. 1c) and a 350-kDa centrosomal protein (26) (Fig. 1 d–f). Cells expressing γtub-GFP were indistinguishable from AX2 cells on the basis of a number of criteria. Growth rates were identical, with average cell cycle times of 12.0 hr for AX2 cells and 11.8 hr for γtub-GFP cells. Microtubule number and distribution were unaltered in transfected cells. The average number of microtubules in AX2 cells was 28.5 ± 3.0 (n = 54) in AX2 cells and 29.9 ± 2.4 (n = 51) in γtub-GFP cells, as determined by immunofluorescence microscopy. Thus γtub-GFP is correctely targeted to the centrosome, allowing us to use it as a convenient, specific, and physiological marker for the centrosome that is fully compatible with normal cellular activities. To what extent γtub-GFP is functional in microtubule nucleation remains to be determined. γtub-GFP fluorescence of the centrosome is bright and surprisingly stable. The excitation light from a 50-W halogen lamp is sufficient to allow for continuous illumination for at least 10 min without photobleaching or any visible signs of damage to the cells. Despite its association with the nucleus (26, 28), the Dictyostelium centrosome is surprisingly mobile. In both migrating and stationary cells it continuously shifts its position, though these movements are restricted to the central portion of the cell.
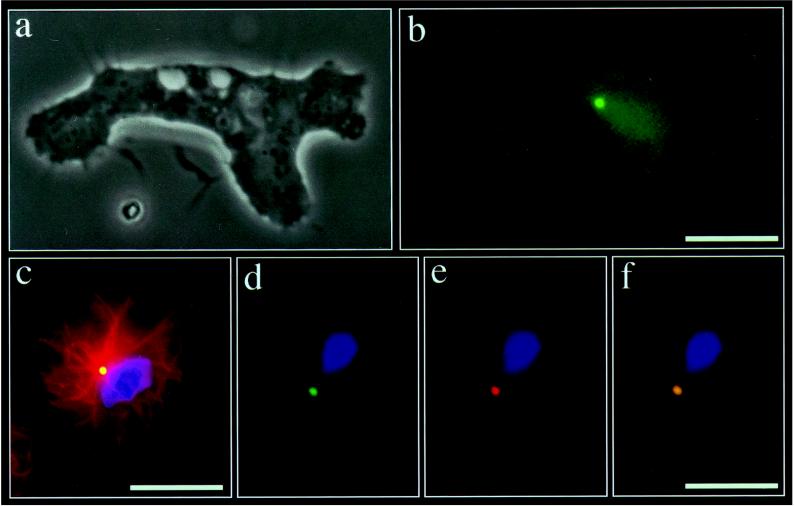
Figure 1.
Expression and localization of γtub-GFP. (a and b) Phase-contrast (a) and γtub-GFP fluorescence (b) microscopy of a moving D. discoideum amoeba. A single brightly fluorescent dot is visible near the cell center. (Bar = 10 μm.) (c) Immunofluorescence microscopy using an antibody against tubulin (27) in conjunction with a rhodamine-labeled secondary antibody demonstrates that the green γtub-GFP spot (which appears yellow due to the superimposition with the red tubulin fluorescence) is located at the center of the microtubule aster. (Bar = 10 μm.) (d–f) Immunofluorescence microscopy of a cell expressing γtub-GFP stained with an antibody against a 320-kDa centrosomal protein (26), demonstrating that γtub-GFP colocalizes with the centrosome. Endogenous γtub-GFP fluorescence (d), fluorescence of the 320-kDa centrosomal protein stained with a rhodamine-labeled secondary antibody (e), and superimposition of d and e (f). (Bar = 10 μm.)
Randomly migrating cells are particularly well suited for an analysis of the importance of centrosome positioning for directional changes because cells undergo a biased random walk (29) in the absence of known external cues or stimuli. If centrosome position plays a role in setting up a new direction of movement (i.e., extension of a pseudopod in a new direction), it is best studied under these conditions. A procedure was developed to monitor, in a standardized fashion, the spatial and temporal relationship between centrosome position and the direction of cell movement in actively migrating cells. The method of analysis is illustrated in Fig. 2 a and b and explained in the figure legend. This assay allows one to detect even a subtle shift of the centrosome toward a new site of pseudopod extension.
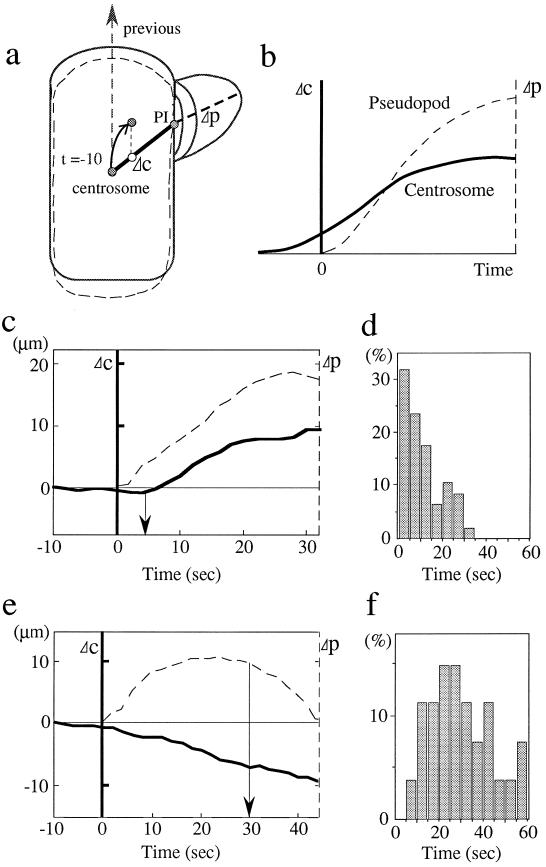
Figure 2.
Spatial and temporal relationship of pseudopod extension and centrosome position in randomly migrating amoebae. (a) Method of analysis. A cell moving toward the top (previous direction of movement) extends a new pseudopod at t = 0 whose extension over time (Δp) can be measured (broken line). A line is drawn between the site of initiation of the pseudopod (PI) and the position of the centrosome at t = −10 sec prior to pseudopod initiation, specifying an axis c. t = −10 sec has proven empirically to be a good time point at which centrosome position can still be related spatially to the future site of initiation of a pseudopod in the majority of cells. In this way the history of centrosome position prior to pseudopod initiation (at t = 0) is included in the analysis. Even earlier time points (more than 10 sec prior to pseudopod initiation) cannot be used due to the rapid shape changes of moving amoebae. Any shift in the position of the centrosome toward the PI possesses a component of movement, Δc, that can be determined and plotted. Should the centrosome remain stationary, continue to move in the previous direction of migration, or even shift away from the new pseudopod, its effective movement along c is zero or negative. Criteria for a true shift of the centrosome were a movement of 1 μm or more that lasted for at least 5 sec. This is necessary because of the basal motile activity of the centrosome that leads to small, erratic excursions, including movements along the axis c. In the hypothetical case shown in b, the centrosome reorients prior to the beginning of extension of the pseudopod. Note that Δc, Δp = 0 for the pseudopod and the centrosome, respectively, are different positions within the framework of the cell. (c–f) Correlation between pseudopod extension and centrosome position. Centrosome reorientation invariably follows pseudopod extension. In the example shown in c, the lag time is 3 sec. A summary of the lag times in histogram form of 47 cells is shown in d. If the centrosome does not reorient, a pseudopod is retracted (e). Retraction occurs after 31 sec on average (n = 27) (f).
We analyzed 47 cells during their random migration on glass coverslips. A typical example of a migrating cell analyzed in this way is shown in Fig. 1a. The initiation of a new direction in the course of cell movement was never preceded by a reorientation of the centrosome in that direction (Fig. 2 c and d). In two cells pseudopod extension and centrosome repositioning occurred more or less simultaneously (i.e., within 1–2 sec). In the other 45 cells centrosome repositioning lagged behind pseudopod extension by, on average, 12 sec (range 3–30 sec). Five of these even showed significant (at least 2 μm) and persistent (lasting 8–13 sec) movement of the centrosome away from the new pseudopod prior to its extension. Once a shift has occurred, the centrosome shows a remarkable persistence in following the extending pseudopod. Conversely, if the centrosome did not reorient toward a new pseudopod, as determined in 27 cells, that pseudopod was retracted after 31 sec on average (range 8–60 sec) (Fig. 2 e and f). This observation suggests a role of the microtubule system in pseudopod stabilization. It is also conceivable that pseudopods not stabilized by a reorienting centrosome differ qualitatively from those into which the centrosome does reorient.
Dictyostelium amoebae respond chemotactically to a gradient of cAMP (30). To determine centrosome behavior under conditions of chemotactic stimulation, a micropipette filled with cAMP was placed in the vicinity of a cell in a culture dish, causing the cell to migrate toward the pipette tip. Twenty-one cells were analyzed in this way, and one example is shown in Fig. 3. The cell was migrating toward the bottom before the micropipette was placed at the opposite end. About 20 sec later the cell started to extend toward the pipette tip, whereas the centrosome shifted its position toward the new cell anterior after 26 sec. On average, the shift in centrosome position lagged behind the initiation of pseudopod extension by 9.0 ± 5.0 sec (n = 21). Thus also under conditions of chemotactic stimulation pseudopod extension preceded centrosome repositioning.
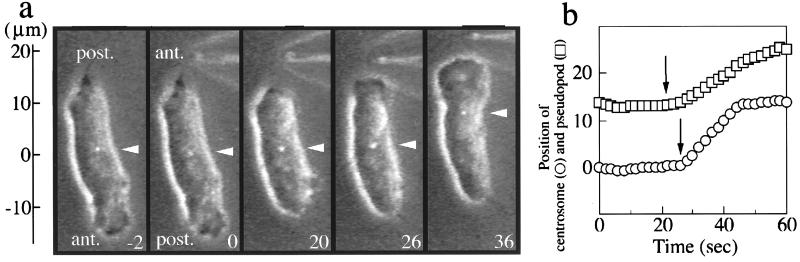
Figure 3.
Centrosome behavior in a chemotactically stimulated cell. (a) The amoeba shown in the first first panel (−2 sec) is moving toward a microneedle filled with cAMP placed at the bottom. (×1100.) At t = 0 the microneedle is moved to the top and pseudopod extension and centrosome position are monitored (b). Pseudopod extension toward the top begins about 6 sec before centrosome repositioning.
Cell polarity and centrosome position in migrating cells has been studied in various cell types by using a spectrum of different approaches (8–13, 31, 32), but a consistent picture of the causal relationship between the initiation of directional changes during cell locomotion and the positioning of the microtubule-organizing center has not yet emerged (33). Labeling the centrosome of Dictyostelium with GFP-tagged γ-tubulin has allowed us to study its behavior in real time during rapid changes in the direction of cell movement. Two straightforward conclusions can be drawn from the experiments reported here: (i) Pseudopod extension clearly precedes centrosome repositioning, demonstrating that the centrosome “follows” a change in the direction of cell movement initiated in the cell cortex. (ii) If the centrosome does not reposition, the pseudopod is retracted, suggesting that the centrosome, and the microtubule system it organizes, is required for stabilizing an extending pseudopod and hence for reinforcing persistence of locomotion. For example, if Dictyostelium amoebae are separated into a nucleated and an anucleated cell fragment by microsurgery, only the nucleated, centrosome-bearing cell fragment is capable of persistent locomotion (34). Thus the centrosome does not determine the direction of cell migration, but it may contribute to the stabilization of directional movement (27, 33, 35, 36). The cellular basis of such a stabilizing effect is not understood, but it seems plausible that the microtubules extending from the centrosome are required, presumably by maintaining the centrosome in a near-centroid position (27). Directional changes initiated (and executed) in the cell periphery may lead to a gradual repositioning of the microtubule-organizing center by virtue of dynamic length changes of microtubules, as suggested by the elegant experiments of Hamaguchi and Hiramoto (37). Thus microtubules may be considered as sensors of directional changes that help maintain the centrosome in a position near the cell center where the microtubule system can respond quickly to rapid and sometimes gross changes in cell morphology in the course of cell movement. This may be important for the transport of secretory vesicles from the pericentrosomal Golgi apparatus to the leading edge (8, 38–40). Microtubules do not seem to serve, however, as effectors of directional cues generated by centrosomal repositioning.
Acknowledgments
M.U. is supported by a Japan Society for the Promotion of Science Postdoctoral Fellowship for Research Abroad. This work was supported by the Deutsche Forschungsgemeinschaft (Sonderforschungsbereich 184) and a grant from the Human Capital and Mobility Programme of the European Community.
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
Abbreviations: GFP, green fluorescent protein; γtub-GFP, γ-tubulin-GFP fusion protein.
Data deposition: The sequence reported in this paper has been deposited in the GenBank database (accession no. AJ000492).
References
- 1.Bray D. Cell Movements. New York: Garland; 1992. [Google Scholar]
- 2.Vasiliev J M, Gelfand I M, Domnina L V, Ivanova L Y, Komm S G, Olshevskaya L V. J Embryol Exp Morphol. 1970;24:625–640. [PubMed] [Google Scholar]
- 3.Goldman R D. J Cell Biol. 1971;51:752–762. doi: 10.1083/jcb.51.3.752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Trinkaus J P. Exp Biol Med. 1985;10:130–173. [Google Scholar]
- 5.Singer S J, Kupfer A. Annu Rev Cell Biol. 1986;2:337–365. doi: 10.1146/annurev.cb.02.110186.002005. [DOI] [PubMed] [Google Scholar]
- 6.Kalnins V I. The Centrosome. New York: Academic; 1992. [Google Scholar]
- 7.Gotlieb A I, May L M, Subrahmanyan L, Kalnins V I. J Cell Biol. 1981;91:589–594. doi: 10.1083/jcb.91.2.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kupfer A, Louvard D, Singer S J. Proc Natl Acad Sci USA. 1982;79:2603–2607. doi: 10.1073/pnas.79.8.2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Koonce M P, Cloney R A, Berns M W. J Cell Biol. 1984;98:1999–2010. doi: 10.1083/jcb.98.6.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lewis L, Albrecht-Buehler G. Cell Motil Cytoskeleton. 1987;7:282–290. doi: 10.1002/cm.970070310. [DOI] [PubMed] [Google Scholar]
- 11.Middleton C A, Brown A F, Brown R M, Karavanova I D, Roberts D J H, Vasiliev J M. J Cell Sci. 1989;94:25–32. doi: 10.1242/jcs.94.1.25. [DOI] [PubMed] [Google Scholar]
- 12.Keller H U, Bessis M. Nature (London) 1975;258:73–74. doi: 10.1038/258723a0. [DOI] [PubMed] [Google Scholar]
- 13.Euteneuer U, Schliwa M. Nature (London) 1984;310:58–61. doi: 10.1038/310058a0. [DOI] [PubMed] [Google Scholar]
- 14.Malawista S E, de Boisfleury A C. J Cell Biol. 1982;95:960–973. doi: 10.1083/jcb.95.3.960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Anderson D C, Wible L J, Hughes B J, Smith C W, Brinkley B R. Cell. 1982;31:719–729. doi: 10.1016/0092-8674(82)90326-9. [DOI] [PubMed] [Google Scholar]
- 16.Schliwa M, Pryzwanski K B, Euteneuer U. Cell. 1982;31:705–717. doi: 10.1016/0092-8674(82)90325-7. [DOI] [PubMed] [Google Scholar]
- 17.Sameshima M, Imai Y, Hashimoto Y. Cell Motil Cytoskeleton. 1988;9:111–116. doi: 10.1002/cm.970090203. [DOI] [PubMed] [Google Scholar]
- 18.Chalfie M, Tu Y, Euskirchen G, Ward W W, Prasher D. Science. 1994;263:802–805. doi: 10.1126/science.8303295. [DOI] [PubMed] [Google Scholar]
- 19.Oakley C E, Oakley B R. Nature (London) 1989;338:662–664. doi: 10.1038/338662a0. [DOI] [PubMed] [Google Scholar]
- 20.Joshi H C, Palacios M J, McNamara L, Cleveland D W. Nature (London) 1992;356:80–83. doi: 10.1038/356080a0. [DOI] [PubMed] [Google Scholar]
- 21.Marshall L G, Jeng R L, Mulholland J, Stearns T. J Cell Biol. 1996;134:443–454. doi: 10.1083/jcb.134.2.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zheng Y, Jung M K, Oakley B R. Cell. 1991;65:817–823. doi: 10.1016/0092-8674(91)90389-g. [DOI] [PubMed] [Google Scholar]
- 23.Spang A, Geissler S, Grein K, Schiebel E. J Cell Biol. 1996;134:429–441. doi: 10.1083/jcb.134.2.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Heim R, Cubitt K B, Tsien R. Nature (London) 1995;373:663–664. doi: 10.1038/373663b0. [DOI] [PubMed] [Google Scholar]
- 25.Nellen W, Silan C, Firtel R A. Mol Cell Biol. 1984;4:2890–2898. doi: 10.1128/mcb.4.12.2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kalt A, Schliwa M. J Cell Sci. 1996;109:3103–3112. doi: 10.1242/jcs.109.13.3103. [DOI] [PubMed] [Google Scholar]
- 27.Euteneuer U, Schliwa M. J Cell Biol. 1992;116:1157–1166. doi: 10.1083/jcb.116.5.1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Omura F, Fukui Y. Protoplasma. 1985;127:212–221. [Google Scholar]
- 29.Alt W. J Math Biol. 1980;9:147–177. doi: 10.1007/BF00275919. [DOI] [PubMed] [Google Scholar]
- 30.Konijn T M, van Haastert P J M. Methods Cell Biol. 1987;28:283–298. doi: 10.1016/s0091-679x(08)61652-0. [DOI] [PubMed] [Google Scholar]
- 31.Schütze K, Maniotis A, Schliwa M. Proc Natl Acad Sci USA. 1991;88:8367–8371. doi: 10.1073/pnas.88.19.8367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rodionov V I, Gyoeva T K, Tanaka E, Bershadsky A D, Vasiliev J M, Gelfand V I. J Cell Biol. 1993;123:1811–1829. doi: 10.1083/jcb.123.6.1811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schliwa M, Höner B. Trends Cell Biol. 1993;3:377–380. doi: 10.1016/0962-8924(93)90086-g. [DOI] [PubMed] [Google Scholar]
- 34.Swanson J A, Taylor D L. Cell. 1982;28:225–232. doi: 10.1016/0092-8674(82)90340-3. [DOI] [PubMed] [Google Scholar]
- 35.Ueda M, Ogihara S. J Cell Sci. 1994;107:2071–2079. doi: 10.1242/jcs.107.8.2071. [DOI] [PubMed] [Google Scholar]
- 36.Niu M Y, Mills J C, Nachmias V T. Cell Motil Cytoskeleton. 1997;36:203–215. doi: 10.1002/(SICI)1097-0169(1997)36:3<203::AID-CM1>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 37.Hamaguchi M S, Hiramoto Y. Dev Growth Differ. 1986;28:143–156. doi: 10.1111/j.1440-169X.1986.00143.x. [DOI] [PubMed] [Google Scholar]
- 38.Brundage R A, Fogarty K, Tuft R A, Fay F S. Science. 1991;254:703–706. doi: 10.1126/science.1948048. [DOI] [PubMed] [Google Scholar]
- 39.Bershadsky A D, Futerman A H. Proc Natl Acad Sci USA. 1994;91:5686–5691. doi: 10.1073/pnas.91.12.5686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hyman A A, Karsenti E. Cell. 1996;84:401–410. doi: 10.1016/s0092-8674(00)81285-4. [DOI] [PubMed] [Google Scholar]