Abstract
The efficacy of chemotherapeutic agents may be determined by a number of different factors, including the genotype of the tumor cell. The p53 tumor suppressor gene frequently is mutated in human tumors, and this may contribute to chemotherapeutic resistance. We tested the requirement for wild-type p53 in the response of tumor cells to treatment with paclitaxel (trade name Taxol), an antineoplastic agent that stabilizes cellular microtubules. Although paclitaxel is broadly effective against human tumor xenografts in mice, including some known to carry p53 mutations, we found that p53-containing mouse tumor cells were significantly more sensitive to direct treatment with this drug than were p53-deficient tumor cells. In an attempt to reconcile this apparent discrepancy, we examined the requirement for p53 in the cytotoxic effects of tumor necrosis factor α (TNF-α), a cytokine released from murine macrophages upon paclitaxel treatment. Conditioned medium from paclitaxel-treated macrophages was capable of inducing p53-independent apoptosis when applied to transformed mouse embryonic fibroblasts and was inhibitable by antibodies against TNF-α. Furthermore, in response to direct treatment with TNF-α, both wild-type and p53-deficient tumor cells underwent apoptosis to similar extents and with similar kinetics. Our results suggest that the efficacy of paclitaxel in vivo may be due not only to its microtubule-stabilizing activity, but its ability to activate local release of an apoptosis-inducing cytokine.
Most chemotherapeutic agents were identified by virtue of their cytotoxicity against tumor cell lines. The basis for their mechanism of action remains poorly understood, but has been thought to be due to inhibiting tumor cell growth. Recently, the realization that many of these agents induce apoptosis, a genetically determined form of cell death, has forced a reevaluation of the mechanisms by which cytotoxic agents inhibit tumor growth. These studies have identified correlations between drug responsiveness and tumor genotype (refs. 1 and 2; reviewed in refs. 3 and 4). Consequently, a further understanding of how tumor-specific mutations affect treatment efficacy ultimately may provide a more rational basis for choice of anticancer regimen.
Mutations in the p53 tumor suppressor gene recently have been shown to have an impact on the clinical course of human tumors. Indeed, patients harboring tumors with p53 mutations often have a worse prognosis than those harboring tumors with wild-type p53 (reviewed in ref. 5), and in several instances p53 mutations have been associated with drug-resistant tumors (6–9). Consistent with these clinical findings, inactivation of p53 can promote tumorigenesis and lead to drug resistance in experimental settings. The p53 gene encodes a transcription factor that can regulate cell proliferation and survival by modulating transcription of downstream target genes, inducing either G1 arrest or apoptosis (1, 10–13). p53 is activated to promote G1 arrest or apoptosis by several stimuli, the most well characterized being DNA damage. Many known anticancer agents cause DNA damage, presumably leading to p53-dependent apoptosis. Inactivation of the apoptotic response provides an attractive explanation to account for the poor responsiveness of p53 mutant tumors to these agents. Thus, identifying chemotherapeutic agents that act independently of the p53 pathway is of fundamental importance.
Paclitaxel, a drug used for cancer therapy, is derived from the Pacific yew tree (Taxus brevifolia) and initially was considered promising because of its cytotoxic activity against several tumor cell lines (14, 15). Subsequent studies demonstrated that paclitaxel was effective against a variety of murine tumors and human xenografts (16–18), as well as advanced human carcinomas refractory to traditional chemotherapy (reviewed in ref. 19). Paclitaxel promotes the assembly of microtubules in vitro (20). Consequently, the cytotoxic effect of the drug in tissue culture can be attributed to its ability to stabilize cellular microtubules and thus inhibit formation of the mitotic spindle (21). However, in addition to its microtubule-stabilizing activity, paclitaxel also stimulates the lipopolysaccharide (LPS) signaling pathway in murine macrophages, resulting in secretion of the cytokines interleukin 1β and tumor necrosis factor α (TNF-α) (22–25). This effect is independent of its ability to stabilize microtubules, because some derivatives of paclitaxel retain microtubule-stabilizing activity but do not stimulate cytokine secretion (26, 27). Interestingly, TNF-α itself can induce apoptosis and has well documented anticancer activity (reviewed in refs. 28 and 29). In human macrophages, paclitaxel alone has not been shown to induce TNF-α or interleukin-1β secretion, but it does enhance production of these cytokines when applied in conjunction with LPS (30).
Because p53 promotes apoptosis after DNA damage, it might be expected that a microtubule-stabilizing drug such as paclitaxel would have p53-independent effects. Indeed, several of the human xenografts that responded to paclitaxel in preclinical trials are documented to have p53 mutations (16, 31–34). However, paclitaxel-induced cell cycle arrest is compromised in murine fibroblasts lacking p53, suggesting that p53 may, in fact, contribute to paclitaxel’s biological effects (35). Furthermore, in an ovarian teratocarcinoma cell line, paclitaxel induced apoptosis to a greater extent in cells with intact p53 function than in cells in which p53 was inactivated through expression of human papilloma virus E6 protein (36). Thus, there is an apparent discrepancy between the effects of p53 status on the response of cells to paclitaxel in vivo versus in vitro. To characterize the effectiveness of paclitaxel against transformed cells, we sought to determine the relationship between paclitaxel response and p53 status.
MATERIALS AND METHODS
Cells and Cell Culture.
p53 +/+ and p53 −/− mouse embryonic fibroblasts (MEFs) were derived from 13.5-day-old embryos and used between passages 3 and 8 (54). p53 +/+ and p53 −/− MEF clones stably expressing E1A and T24 H-ras were generated by calcium phosphate coprecipitation as previously described (1). The RAW 264.7 cell line was a gift from Gerald Wogan (Massachusetts Institute of Technology, Division of Toxicology, Cambridge, MA). All cells were maintained in DMEM containing 10% fetal bovine serum supplemented with penicillin and streptomycin.
Dose-Response Assays.
Exponentially growing fibroblasts were plated at a density of 8 × 104 cells per 34-mm well for untransformed MEFs and 1.5 × 105 cells per 34-mm well for transformed MEFs. Twenty-four hours after plating, growth medium was replaced with fresh medium containing the appropriate concentration of recombinant murine tumor necrosis factor α (TNF-α) (Boehringer Mannheim), paclitaxel or cephalomannine (both provided by the National Cancer Institute, Drug Synthesis and Chemistry Branch). Cell viability was determined after a 48-hr treatment by pooling adherent and nonadherent cells and measuring uptake of propidium iodide as determined by FACScan.
Conditioned Medium Assay.
RAW 264.7 murine macrophages were plated at a density of 7.5 × 106 cells per 100-mm dish, allowed to adhere, and treated with 30 μM paclitaxel or cephalomannine for 24 hr. The macrophage growth medium was collected, pushed through a syringe attached to a .45 μM filter, and applied to wells of fibroblasts (8 × 104 cells per 34-mm well for untransformed MEFs; 1.5 × 105 cells per 34-mm well for E1A-Ras-expressing MEFs). Fibroblasts were grown in the conditioned medium for 24 hr and then analyzed for cell viability by propidium iodide uptake. For anti-TNF-α experiments, macrophage growth medium was incubated with monoclonal hamster anti-mouse TNF-α (Genzyme) at concentrations of 0.1, 0.2, and 1 μg/ml of conditioned media, for 2 hr at 37°C before addition to fibroblasts. The control antibody was monoclonal hamster anti-mouse CD3 used at a concentration of 1 μg/ml of conditioned media (PharMingen).
Apoptosis Assay.
E1A-Ras-expressing MEFs were plated onto poly-d-lysine coated coverslips at a density of 1.5 × 105 cells per 34-mm well. Twenty-four hours after plating, growth medium was replaced with fresh medium containing 10 ng/ml recombinant TNF-α. After 15 hr of treatment, cells were fixed in 2% paraformaldehyde/PBS for 15 min, washed with PBS, permeabilized for 15 min in 0.25% Triton X-100/PBS, and washed again with PBS. Cells were then stained for 5 min with 4,6-diamidino-2-phenylindole (DAPI) (Sigma) at a concentration of 1 μg/ml PBS. Coverslips were mounted on glass slides with Mowiol and analyzed via fluorescence microscopy.
RESULTS
Induction of p53-Dependent Apoptosis by Paclitaxel.
We chose to examine paclitaxel response in a well characterized system consisting of wild-type and p53-deficient primary MEFs transformed with the adenovirus-5-E1A oncogene and the T24-activated-H-ras allele (1). These highly transformed cells differ in p53 genotype but are otherwise genetically similar, thus constituting a good model system in which to determine how p53 status affects response to chemotherapeutic agents (e.g., see ref. 1).
The viability of E1A-Ras-transformed, wild-type, and p53-deficient MEFs treated with paclitaxel is summarized in Fig. 1, along with untransformed control MEFs. Cells were treated with the indicated concentration of paclitaxel for 48 hr, then analyzed for viability by propidium iodide exclusion. In the transformed fibroblast populations, paclitaxel treatment led to preferential killing of cells containing wild-type p53. p53 +/+, E1A-Ras-transformed MEFs had an IC50 of 75 nM, whereas the IC50 of their p53 −/− counterparts was >30 μM. Death occurred by induction of the apoptotic pathway, as determined by condensed chromatin and nuclear fragmentation visible in cells stained with 4,6-diamidino-2-phenylindole (DAPI) (data not shown). The viability of untransformed MEFs was largely unaffected by exposure to paclitaxel at the concentrations tested, although p53 −/− MEFs did show a slight decrease in viability, as has been observed previously (35, 37).
Figure 1.
Cytotoxicity of paclitaxel is affected by p53 genotype and transformation status. Viability of p53 +/+ untransformed fibroblasts (□), p53 −/− untransformed fibroblasts (▪), p53 +/+ E1A-Ras-expressing fibroblasts (▵), and p53 −/− E1A-Ras-expressing fibroblasts (▴) after paclitaxel treatment. Cells were grown in paclitaxel-containing media at the indicated concentrations for 48 hr, harvested, and analyzed for viability as determined by propidium iodide exclusion using flow cytometry. Treatment of cells with equal amounts of carrier alone (ethanol) had no effect on viability (not shown). Data shown represent the analysis of three experiments with error bars indicated.
Macrophage-Mediated Killing of p53-Deficient Tumor Cells.
The observation that paclitaxel induced p53-dependent apoptosis in transformed cells was unexpected, because paclitaxel was effective against p53 mutant tumors in preclinical studies (16, 31–34). This discrepancy could be resolved if, in addition to its ability to directly induce p53-dependent apoptosis, paclitaxel acted through an indirect mechanism that required a tumor microenvironment. In this regard, the ability of paclitaxel to stimulate release of cytokines from tumor-associated macrophages provides a potential mechanism.
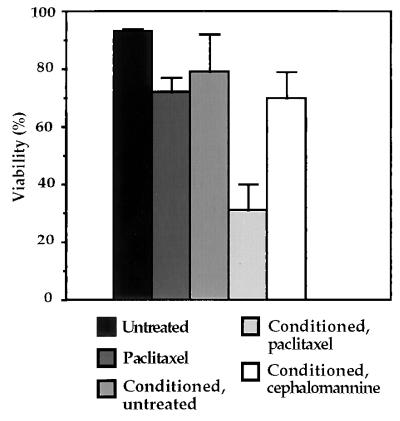
To determine whether paclitaxel could induce secretion of a cytotoxic cytokine from macrophages, we used an in vitro tissue culture assay that recapitulated events that might occur in an in vivo tumor setting. We included as a control the paclitaxel derivative cephalomannine, which retains the ability to stabilize microtubules but does not induce cytokine secretion (27). Cells from the murine macrophage cell line RAW 264.7 were exposed to 30 μM paclitaxel or 30 μM cephalomannine for 24 hr. This concentration of paclitaxel is comparable to plasma levels of paclitaxel after a standard therapeutic dose (38). The media from the treated macrophages then was collected, filtered, and applied to p53 −/− E1A-Ras-expressing MEFs. After 24 hr of incubation with the macrophage-conditioned media, the transformed MEFs were assayed for viability (Fig. 2). As would be predicted from the results presented in Fig. 1, treating E1A-Ras p53 −/− cells directly with 30 μM paclitaxel led to only a modest loss of viability. However, exposing these cells to conditioned media from macrophages treated with 30 μM paclitaxel caused a sharp decrease in viability. Importantly, incubation of the cells in media from macrophages treated with cephalomannine did not cause a decrease in viability beyond that observed after direct paclitaxel treatment. These data suggest that macrophages exposed to paclitaxel release a soluble factor into the media, which induces apoptosis in p53 −/− E1A-Ras-transformed fibroblasts.
Figure 2.
Conditioned media from paclitaxel-treated macrophages induces apoptosis in p53-deficient, E1A-Ras-transformed fibroblasts. Murine macrophages (RAW 264.7) were treated with 30 μM paclitaxel or 30 μM cephalomannine for 24 hr. Macrophage-conditioned medium was collected, filtered, and applied to E1A-Ras p53 −/− fibroblasts. After 24 hr, viability of fibroblasts was determined using propidium iodide exclusion and flow cytometry. Viability of E1A-Ras p53-1-fibroblasts was also determined after treatment with 30 μM paclitaxel directly, and after incubation in conditioned medium from untreated RAW 264.7 cells. Data shown represent the analysis of three experiments with error bars indicated.
TNF-α Release from Macrophages.
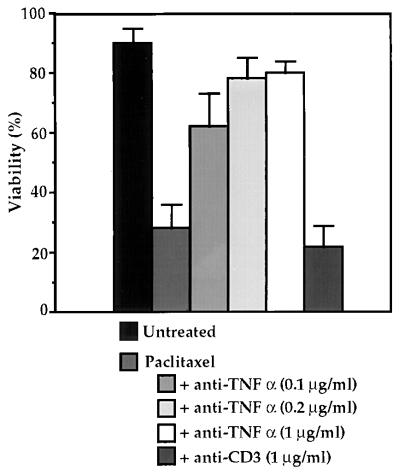
TNF-α is a likely candidate for the cytotoxic factor secreted by paclitaxel-treated macrophages, because earlier studies have shown both that paclitaxel induces secretion of TNF-α (22) and that TNF-α can promote apoptosis (28). To test whether TNF-α was the cytotoxic factor present in the conditioned media, RAW 264.7 macrophages were treated with paclitaxel for 24 hr, and the conditioned media then was collected and incubated for 1 hr with a mAb to TNF-α. The MEFs then were grown in the macrophage-conditioned media for 24 hr and subsequently assayed for viability. As shown in Fig. 3, preincubation of the conditioned media with 0.1 μg/ml of an anti-TNF-α mAb protected a large portion of the cell population from death. Incubation with higher concentrations of antibody restored the viability of the p53 −/− E1A-Ras-transformed MEFs to near untreated levels. However, preincubation of the conditioned media with a control mAb against CD3 had no effect on viability. These data strongly suggest that the cytotoxic factor released by macrophages upon paclitaxel treatment is TNF-α.
Figure 3.
Addition of anti-TNF-α antibody prevents cell death in macrophage-conditioned medium. Conditioned medium from paclitaxel-treated macrophages was incubated with monoclonal anti-TNF-α or anti-CD3 antibody, then applied to E1A-Ras p53 −/− fibroblasts. Viability of fibroblasts was determined after 24 hr using propidium iodide staining and flow cytometry. Data shown represent the analysis of three experiments with error bars indicated.
Direct Induction of Apoptosis by TNF-α.
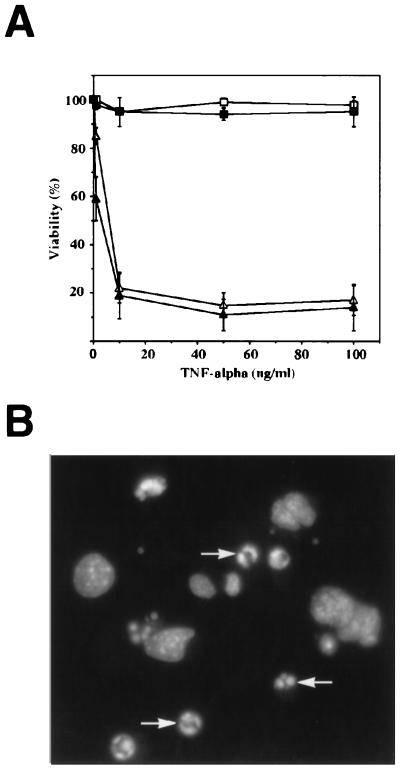
To prove that TNF-α is capable of inducing p53-independent apoptosis, we examined the response of E1A-Ras-transformed MEFs when treated directly with TNF-α (Fig. 4A). Untransformed and E1A-Ras-expressing p53 +/+ and p53 −/− MEFs were treated with TNF-α at various concentrations for a 48-hr period and then assayed for viability. Exposure to TNF-α led to significant cytotoxicity in all E1A-Ras-expressing cells, independent of their p53 status. Cells died by apoptosis, as determined morphologically and by nuclear staining with 4,6-diamidino-2-phenylindole (DAPI) (Fig. 4B). The viability of untransformed MEFs was unaffected by TNF-α treatment (Fig. 4A). The ability of TNF-α to induce death in E1A-Ras p53 −/− tumor cells supports the idea that paclitaxel acts via TNF-α secretion in vivo.
Figure 4.
Cytotoxicity of TNF-α is independent of p53 genotype. (A) Viability of p53 +/+ untransformed fibroblasts (□), p53 −/− untransformed fibroblasts (▪), p53 +/+ E1A-Ras-expressing fibroblasts (▵), and p53 −/− E1A-Ras-expressing fibroblasts (▴) after TNF-α treatment. Cells were grown in media containing TNF-α at the indicated concentrations for 48 hr, harvested, and analyzed for viability as determined by propidium iodide exclusion using flow cytometry. Data shown represent the analysis of three experiments with error bars indicated. (B) Induction of apoptosis by TNF-α in E1A-Ras p53 −/− fibroblasts. Cells were treated with TNF-α (10 ng/ml) for 15 hr, at which time a significant fraction had undergone apoptosis. Cells then were fixed, stained with the nuclear stain 4,6-diamidino-2-phenylindole (DAPI), and examined using fluorescence microscopy. Arrows indicate apoptotic nuclei, with condensed, brightly staining chromatin.
DISCUSSION
The present study describes how the chemotherapeutic agent paclitaxel may use a novel mode of action against tumors. Like traditional antineoplastic therapies, such as adriamycin and γ-irradiation (1, 2), paclitaxel can act directly on the target tumor cell and induce apoptosis via a p53-dependent pathway. However, unlike these other treatments, paclitaxel also has the potential to act indirectly against tumor cells by stimulating macrophages to secrete the cytokine TNF-α. This secondary effect could circumvent the issue of the p53 genotype of the target cell, because we have demonstrated that TNF-α induces apoptosis in transformed cells irrespective of their p53 status.
Given that p53’s function is understood primarily from its role in the DNA damage response pathway, it is interesting that paclitaxel, a microtubule-stabilizing drug, should elicit a p53-dependent response. Data from untransformed fibroblasts also suggest a p53 connection, as MEFs lacking p53 have decreased viability after paclitaxel treatment (35, 37). Also, experiments in which untransformed fibroblasts were treated with the microtubule-destabilizing agents nocodazole and colcemid indicate a possible role for p53 as a mitotic spindle checkpoint (39). It is presently unclear, however, how the state of cellular microtubules influences the p53 pathway.
The extreme sensitivity of p53 +/+ E1A-Ras-transformed cells to paclitaxel is striking, with apoptotic cells observed at paclitaxel concentrations as low as 10 nM. In untransformed fibroblasts, low concentrations of paclitaxel induce mitotic block, not by increasing tubulin polymerization, but by suppressing the dynamic growth and shrinkage of the mitotic spindle (40). Interestingly, this mechanism is identical to that of the microtubule-destabilizing agent vinblastine, suggesting that in untransformed cells, both classes of antimitotic drugs share a common antiproliferative mechanism (40, 41). A common pathway may be functioning in transformed cells treated with antimitotic drugs, because the microtubule-destabilizing agent vincristine, which is structurally similar to vinblastine, also induces p53-dependent apoptosis in E1A-Ras-transformed fibroblasts (data not shown). Thus, drugs that stabilize or destabilize microtubules may lead to p53-dependent cell cycle arrest or apoptosis, depending on the cellular context.
Paclitaxel also has the ability to induce TNF-α release from murine macrophages (22). Other studies suggest that TNF-α-induced apoptosis proceeds through a complex of molecules that link the trimeric TNF-α receptor to several downstream effectors (42). In this study, we observe that p53 is dispensable for TNF-α induced apoptosis, because TNF-α treatment triggered apoptosis in E1A-Ras-transformed fibroblasts in a p53-independent manner. Therefore, our data suggest that the p53 protein is not a component of the TNF-α apoptotic pathway.
The observation that TNF-α can induce p53-independent apoptosis in transformed cells would suggest that TNF-α itself could be an effective antineoplastic treatment, particularly against tumors that have sustained p53 mutations. TNF-α has long been recognized as an agent capable of inducing marked tumor regression and has undergone considerable testing against tumors in patients and animal models. Systemic administration of TNF-α in humans produced severe side effects and was ineffective against tumors (reviewed in ref. 43). More recently, however, local administration of TNF-α alone, or in combination with other drugs or cytokines, was effective at inhibiting tumor growth with minimal side effects in both tumor models and human patients (44–48). These results lend support to further testing of TNF-α as an anti-cancer agent.
This study supports previous observations that paclitaxel can promote the release of TNF-α from murine macrophages, and additionally shows that the secreted cytokine can efficiently induce apoptosis in p53-deficient E1A-Ras-transformed fibroblasts. We suggest that this mode of action contributes to the efficacy of paclitaxel in murine systems, as solid tumors often are infiltrated by macrophages (49). Accordingly, our study predicts that paclitaxel should be effective against tumors with inactivated p53. We have attempted to demonstrate this by treating tumors generated in nude mice from E1A-Ras-transformed cells; however, we found that paclitaxel was not measurably active against such tumors, regardless of their p53 genotype (data not shown). However, data obtained from testing of paclitaxel in other tumor models support our prediction: paclitaxel was effective in preclinical testing against the human tumors CX-1 (colon), A431 (vulva), and FaDu (hypopharynx), when xenografted into nude mice (16, 31, 32), all of which are documented to contain p53 mutations (33, 34). Additionally, paclitaxel was effective against numerous other tumor xenografts (31, 32, 50, 51), few of which have been screened for their p53 status but at least some of which are likely to contain mutated p53.
It will be important to establish whether in human patients, paclitaxel’s activity is contingent upon macrophage stimulation and subsequent cytokine release. The activation of the LPS pathway by paclitaxel alone has been documented only in mouse macrophages and could represent a species difference (30, 52). The observation that paclitaxel can enhance TNF-α and interleukin-1β gene expression in the presence of LPS might suggest that paclitaxel does activate the LPS pathway in human macrophages, but only in conjunction with a second signal (30). How the p53 status of human tumors determines their responsiveness to paclitaxel is unclear, but it is noteworthy that paclitaxel is effective in treating breast cancer, a tumor type with a high frequency of p53 mutations (53). Left unexplained is the apparent selectivity of paclitaxel, which is most effectively used to treat ovarian and breast cancer. One might expect that neither the microtubule-based nor the cytokine-based effects would be specific to tumor tissue type. Based on the present work, one possible explanation for this apparent selectivity would be that different tumor types have different degrees of macrophage infiltration.
If paclitaxel acts through a cellular intermediate, then it represents a new mode of action for chemotherapeutic agents. Moreover, these data suggest that the critical feature of paclitaxel as an anti-tumor agent may not be its microtubule-stabilizing activity, but its ability to activate components of the immune system. Therefore, this aspect of the drug’s action should be a focus of efforts to improve its effectiveness through structural modification.
Acknowledgments
We are grateful to the National Cancer Institute, Drug Synthesis and Chemistry Branch for providing paclitaxel and cephalomannine. We thank J. Lees for helpful discussions. J.S.L. was supported by a predoctoral fellowship from the Office of Naval Research. T.J. is an Associate Investigator of the Howard Hughes Medical Institute. This work was supported in part by grants from the National Institutes of Health.
ABBREVIATIONS
- TNF-α
tumor necrosis factor type α
- LPS
lipopolysaccharide
- MEF
mouse embryonic fibroblast
References
- 1.Lowe S W, Ruley H E, Jacks T, Housman D E. Cell. 1993;74:957–967. doi: 10.1016/0092-8674(93)90719-7. [DOI] [PubMed] [Google Scholar]
- 2.Lowe S W, Bodis S, McClatchey A, Remington L, Ruley H E, Fisher D E, Housman D E, Jacks T. Science. 1994;266:807–810. doi: 10.1126/science.7973635. [DOI] [PubMed] [Google Scholar]
- 3.Biedler J L. Cancer. 1992;70:1799–1809. doi: 10.1002/1097-0142(19920915)70:4+<1799::aid-cncr2820701623>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 4.Reed J C. Curr Opin Oncol. 1995;7:541–546. doi: 10.1097/00001622-199511000-00012. [DOI] [PubMed] [Google Scholar]
- 5.Lowe S W. Curr Opin Oncol. 1995;7:547–553. doi: 10.1097/00001622-199511000-00013. [DOI] [PubMed] [Google Scholar]
- 6.Aas T, Borreson A-L, Geisler S, Smith-Sorenson B, Johnsen H, Varhaug J E, Akslen L A, Lonning P. Nat Med. 1996;2:811–814. doi: 10.1038/nm0796-811. [DOI] [PubMed] [Google Scholar]
- 7.Bergh J, Norberg T, Slogren S, Lindgren A, Holmberg L. Nat Med. 1995;1:1029–1034. doi: 10.1038/nm1095-1029. [DOI] [PubMed] [Google Scholar]
- 8.Goh H, Yao J, Smith D. Cancer Res. 1995;55:5217–5221. [PubMed] [Google Scholar]
- 9.Rusch V, Klimstra D, Venkatraman J, Martini N, Gralla R, Kris M, Dmitrovsky E. Cancer Res. 1995;55:5038–5042. [PubMed] [Google Scholar]
- 10.Harper J W, Adami G R, Wei N, Keyomarsi K, Elledge S J. Cell. 1993;75:805–816. doi: 10.1016/0092-8674(93)90499-g. [DOI] [PubMed] [Google Scholar]
- 11.El-Deiry W S, Tokino T, Velculescu V E, Levy D B, Parsons R, Trent J M, Lin D, Mercer W E, Kinzler K, Vogelstein B. Cell. 1993;75:817–825. doi: 10.1016/0092-8674(93)90500-p. [DOI] [PubMed] [Google Scholar]
- 12.Clarke A R, Purdie C A, Harrison D J, Morris R G, Bird C C, Hooper M L, Wyllie A H. Nature (London) 1993;362:849–852. doi: 10.1038/362849a0. [DOI] [PubMed] [Google Scholar]
- 13.Lowe S W, Schmitt E, Smith S W, Osborne B A, Jacks T. Nature (London) 1993;362:847–849. doi: 10.1038/362847a0. [DOI] [PubMed] [Google Scholar]
- 14.Wani M C, Taylor H L, Wall M E, Coggon P, McPhail A T. J Am Chem Soc. 1971;93:2325–2327. doi: 10.1021/ja00738a045. [DOI] [PubMed] [Google Scholar]
- 15.Douros J, Suffness M. Cancer Chemother Pharmacol. 1978;1:91–100. doi: 10.1007/BF00254042. [DOI] [PubMed] [Google Scholar]
- 16.National Cancer Institute. Clinical Brochure: Taxol (NSC 125973) Bethesda, MD: National Division of Cancer Treatment; 1983. pp. 6–12. [Google Scholar]
- 17.Riondel J, Jacrot M, Picot F, Beriel H, Mouriquand C, Potier P. Cancer Chemother Pharmacol. 1986;17:137–142. doi: 10.1007/BF00306742. [DOI] [PubMed] [Google Scholar]
- 18.Riondel J, Jacrot M, Nissou M F, Picot F, Beriel H, Mouriquand C, Potier P. Anticancer Res. 1988;8:387–390. [PubMed] [Google Scholar]
- 19.Rowinsky E, Donehower R. N Engl J Med. 1995;332:1004–1014. doi: 10.1056/NEJM199504133321507. [DOI] [PubMed] [Google Scholar]
- 20.Schiff P B, Fant J, Horwitz S B. Nature (London) 1979;277:665–667. doi: 10.1038/277665a0. [DOI] [PubMed] [Google Scholar]
- 21.Schiff P B, Horwitz S B. Proc Natl Acad Sci USA. 1980;77:1561–1565. doi: 10.1073/pnas.77.3.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ding A H, Porteau F, Sanchez E, Nathan C F. Science. 1990;248:370–372. doi: 10.1126/science.1970196. [DOI] [PubMed] [Google Scholar]
- 23.Bogdan C, Ding A. J Leukocyte Biol. 1992;52:119–121. doi: 10.1002/jlb.52.1.119. [DOI] [PubMed] [Google Scholar]
- 24.Manthey C, Brandes M, Perera P, Vogel S. J Immunol. 1992;149:2459–2465. [PubMed] [Google Scholar]
- 25.Carboni J, Singh C, Tepper M. Natl Cancer Inst Monogr. 1993;15:95–101. [PubMed] [Google Scholar]
- 26.Manthey C, Qureshi N, Stutz P, Vogel S. J Exp Med. 1993;178:695–702. doi: 10.1084/jem.178.2.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Burkhart C, Berman J, Swindell C, Horwitz S. Cancer Res. 1994;54:5779–5782. [PubMed] [Google Scholar]
- 28.Beyaert R, Fiers W. FEBS Lett. 1994;340:9–16. doi: 10.1016/0014-5793(94)80163-0. [DOI] [PubMed] [Google Scholar]
- 29.Hieber U, Heim M. Oncology. 1994;51:142–153. doi: 10.1159/000227329. [DOI] [PubMed] [Google Scholar]
- 30.Allen J, Moore S A, Wewers M D. J Lab Clin Med. 1993;122:374–381. [PubMed] [Google Scholar]
- 31.Joschko M A, Weber L K, Groves J, Ball D L, Bishop J F. Proc Am Assoc Cancer Res. 1994;35:647. [Google Scholar]
- 32.Rose W C. Natl Cancer Inst Monogr. 1993;15:47–53. [PubMed] [Google Scholar]
- 33.Reiss M, Brash D E, Munoz-Antonia T, Simon J A, Ziegler A, Velluxxi V F, Zhou Z L. Oncol Res. 1992;4:349–357. [PubMed] [Google Scholar]
- 34.Baker S J, Fearon E R, Nigro J M, Hamilton S R, Preisinger A C, Jessup J M, VanTuinen P, Ledbetter D H, Barker D F, Nakamura Y, White R, Vogelstein B. Science. 1989;244:217–221. doi: 10.1126/science.2649981. [DOI] [PubMed] [Google Scholar]
- 35.Wahl A, Donaldson K, Fairchild C, Lee F, Foster S A, Demers G W, Galloway D A. Nat Med. 1996;2:72–79. doi: 10.1038/nm0196-72. [DOI] [PubMed] [Google Scholar]
- 36.Wu G S, El-Deiry W S. Nat Med. 1996;2:255–256. doi: 10.1038/nm0396-255a. [DOI] [PubMed] [Google Scholar]
- 37.Hawkins D S, Demers G W, Galloway D A. Cancer Res. 1996;56:892–898. [PubMed] [Google Scholar]
- 38.Innocenti F, Danesi R, Di Paolo A, Agen C, Nardini D, Bocci G, Del Tacca M. Drug Metab Dispos. 1995;23:713–717. [PubMed] [Google Scholar]
- 39.Cross S M, Sanchez C A, Morgan C A, Schimke M K, Ramel S, Idzerda R L, Raskind W H, Reid B J. Science. 1995;267:1353–1356. doi: 10.1126/science.7871434. [DOI] [PubMed] [Google Scholar]
- 40.Jordan M A, Toso R J, Thrower D, Wilson L. Proc Natl Acad Sci USA. 1993;90:9552–9556. doi: 10.1073/pnas.90.20.9552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jordan M A, Thrower D, Wilson L. Cancer Res. 1991;51:2212–2222. [PubMed] [Google Scholar]
- 42.Baker S J, Reddy E P. Oncogene. 1996;12:1–9. [PubMed] [Google Scholar]
- 43.Spriggs D, Yates S. In: Tumor Necrosis Factors: Their Emerging Role in Medicine. Beutler B, editor. New York: Raven; 1992. pp. 383–405. [Google Scholar]
- 44.Baisch H, Otto U, Kloppel G. Cancer Res. 1990;50:6389–6395. [PubMed] [Google Scholar]
- 45.Asher A L, Mule J J, Kasid A, Restifo N P, Salo J C, Reichert C M, Jaffe G, Fendly B, Kriegler M, Rosenberg S A. J Immunol. 1991;146:3227–3234. [PMC free article] [PubMed] [Google Scholar]
- 46.Lasek W, Sora M, Wankowicz A, Jakobisiak M. Cancer Lett. 1995;89:137–143. doi: 10.1016/0304-3835(95)90169-8. [DOI] [PubMed] [Google Scholar]
- 47.Lasek W, Wankowicz A, Kuc K, Feleszko W, Giermasz A, Jakobisiak M. Oncology. 1996;53:31–37. doi: 10.1159/000227531. [DOI] [PubMed] [Google Scholar]
- 48.Lejeune F, Lienard D, Eggermont A, Schraffordt Koops H, Rosenkaimer F, Gerain J, Klaase J, Kroon B, Vanderveken J, Schmitz P. J Cell Biochem. 1994;56:52–61. doi: 10.1002/jcb.240560110. [DOI] [PubMed] [Google Scholar]
- 49.Mantovani A, Bottazzi B, Colotta F, Sozzani S, Ruco L. Immunol Today. 1992;13:265–270. doi: 10.1016/0167-5699(92)90008-U. [DOI] [PubMed] [Google Scholar]
- 50.Nicoletti M I, Lucchini V, Massazza G, Abbott B J, D’Incalci M, Giavazzi R. Ann Oncol. 1993;4:151–155. doi: 10.1093/oxfordjournals.annonc.a058419. [DOI] [PubMed] [Google Scholar]
- 51.Milross C G, Peters L J, Hunter N R, Mason K A, Milas L. Int J Cancer. 1995;62:599–604. doi: 10.1002/ijc.2910620518. [DOI] [PubMed] [Google Scholar]
- 52.Ding A, Sanchez E, Nathan C F. J Immunol. 1993;151:5596–5602. [PubMed] [Google Scholar]
- 53.Ozbun M, Butel J S. Adv Cancer Res. 1995;66:71–141. doi: 10.1016/s0065-230x(08)60252-3. [DOI] [PubMed] [Google Scholar]
- 54.Jacks T, Remington L, Williams B O, Schmitt E, Halachmi S, Bronson R T, Weinberg R A. Curr Biol. 1994;4:1–7. doi: 10.1016/s0960-9822(00)00002-6. [DOI] [PubMed] [Google Scholar]