Abstract
Cross-linked antigens on the surface of a motile cell cap at the trailing end of the cell. In Dictyostelium discoideum, myosin II null mutants have previously been reported to be unable to cap Con A receptors, although they are able to locomote. This finding implicated myosin II as an essential component of the capping mechanism, although not of the machinery for locomotion. Here we show that myosin II null mutants do cap Con A receptors, albeit less efficiently than does wild type. This shows that cap formation is not absolutely dependent on myosin II and that a close mechanistic relationship between capping, particle movement, and cell migration may still exist.
When cell surface receptors on motile cells are cross-linked by an appropriate antibody or lectin, they move away from the front of the cell and collect at its tail to form a “cap” (1). Capping is not induced by the binding of ligands to cell surface proteins per se—non-cross-linking antibodies or lectins do not work—but rather results when surface proteins are cross-linked to form two-dimensional precipitates or patches. The molecular mechanism underlying the process in which patches are driven toward the tail of the cell is uncertain, but two general classes of model exist: the motive force for capping is provided either by the cytoskeleton or by membrane flow (see, for example, refs. 2–6).
A similar rearward movement of surface proteins occurs during cell migration. The feet of the cell are surface proteins—usually integrins—which bind to the substratum and are effectively cross-linked by it. When the cell migrates, these attached receptors are driven toward the rear of the cell. If the cell’s feet behave as do other cross-linked surface receptors, they would undergo capping and then drive the cell forward because they are immobilized by the substratum. Thus, in both capping and cell locomotion there is a movement of cross-linked proteins toward the rear of the cell. This common feature implies that movement and capping may share a common mechanism; if so, this would allow that mechanism to be investigated through experiments on capping, which are in some cases much easier and more revealing.
The connection between capping and cell locomotion has, however, been challenged (7, 8). It had previously been found that there is only a single myosin II heavy chain gene in Dictyostelium discoideum (9). Cells lacking this myosin II gene, mhcA−, are still able to migrate (10), yet they are reported to be unable to cap cell surface Con A receptors (7, 8). These results have been interpreted to demonstrate that capping and cell locomotion are mechanistically distinct processes.
Here we show that mhcA− Dictyostelium cells in fact retain some capping activity. Therefore models invoking a relationship between capping and the rearward movement of a cell’s feet as it migrates remain tenable.
METHODS
mhcA− Dictyostelium [strain HS2206, from D. Manstein and J. Spudich (11)], its parent Ax2, and a new mhcA− line derived from DH1 (strain AC12, kindly provided by Terry O’Halloran and Arturo De Lozanne, Duke University Medical Center, Durham, NC) were grown in HL5 medium (1% proteose peptone/0.5% yeast extract/1% glucose/2.4 mM Na2HPO4/8.8 mM KH2PO4, pH 6.5) on plates. Cells were washed and allowed to attach to wells on glass slides (precoated with 0.1 mg/ml BSA) for 10 min in KK2 buffer (20 mM potassium phosphate, pH 6.2, 2 mM MgSO4) supplemented with 1 mM CaCl2 (KC buffer). They were then either (i) incubated in 25 μg/ml fluorescent Con A (Vector) in KC containing 1 mg/ml BSA (KCB) for 1 min, washed, and held in KC for various times before fixation in 2% formaldehyde in KK2 buffer for 30 min, or (ii) incubated in 25 μg/ml biotinylated Con A (Vector) in KCB for 1 min, washed, incubated in rabbit anti-biotin antiserum (1:500, kindly provided by Tim Mitchison, University of California, San Francisco) in KCB for 3 min, washed, labeled in 1:50 fluoresceinated donkey anti-rabbit Ig (Amersham) in KCB for 3 min, and finally washed in KCB before incubation in KC, all operations being carried out at about 23°C. Fixed cells were mounted in diazabicyclo[2.2.2]octane (DABCO) and viewed in a Zeiss microscope (40× objective) and photographed using Kodak Technical Pan film (for Nomarski) or T-Max 400 film (for fluorescence).
RESULTS
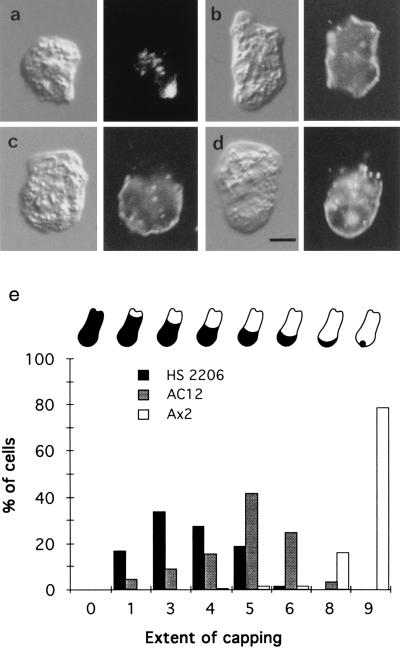
Wild-type (Ax2) and mutant (HS2206 or AC12) amoebae were placed on glass slides, briefly labeled with fluorescein-labeled Con A, washed, and incubated at room temperature (about 23°C) for different times; they were then fixed prior to observation. This showed that, after 30 min at 23°C, many wild-type cells had formed a cap of fluorescent Con A, whereas mhcA− cells had not, as reported (7, 8). However, careful inspection of the surface fluorescence of individual mhcA− cells, compared with their Nomarski images, showed that most of them had cleared the Con A receptors from at least a part of the cell surface. To quantitate these effects, fields of cells were photographed and the fluorescence and Nomarski images of each cell were compared: the proportion of each cell surface cleared of Con A receptors was judged by eye. Fig. 1a shows a typical capped wild-type amoeba; Fig. 1 b–d shows mhcA− cells in which regions of ≈10%, ≈30%, and ≈50% of the cell surface, respectively, have been cleared of Con A receptors after a 30-min incubation; mhcA− and wild-type cells that were fixed before incubation with Con A displayed only uniform fluorescence (not shown). A histogram of the proportion of cells displaying a particular degree of clearance is presented in Fig. 1e, which also shows that the two independent mhcA− amoeba strains behave similarly: both are able to cap their Con A receptors, but do so poorly compared with wild type. We also examined those cells which had been labeled with Con A, but incubated for different times before fixation. This showed that the extent of clearance of Con A increased with time up till about 20 min. Thereafter, the degree of this clearance (averaged over a field of cells) changed little, indicating that an endpoint had been reached.
Figure 1.
Con A distribution on the surfaces of amoebae after a 30-min incubation (Nomarski images on left, Con A fluorescence on right). (a) Wild-type Ax2 cell. (b–d) mhcA− (HS2206) cells showing ≈10% clearance from two regions at opposite ends of the cell (b), ≈30% clearance (c) and ≈50% clearance (d). (e) Histogram of the extent of capping Con A receptors in which all the cells (about 200) in a field were scored. The cartooned amoebae above the histogram indicate the extent of capping in each case: 0 signifies an uncapped amoeba, 1 ≈ 10% capped, 3 ≈ 30% capped, and so on. Note also that the cells in a–d all have some internalized fluorescent Con A. (Scale bar in d is 5 μm.)
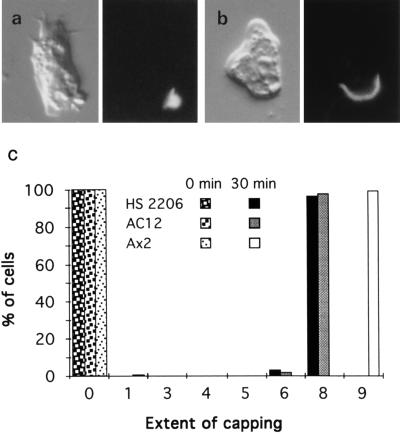
Because it is known from previous work on the capping process in animal cells that cap formation requires efficient cross-linking of surface proteins, we tried to see whether mhcA− cells could be induced to cap Con A receptors more efficiently by increasing the degree of cross-linkage. Amoebae on glass slides were labeled briefly with biotinylated Con A, then with rabbit anti-biotin antiserum, and finally with fluoresceinated donkey anti-rabbit antibody. After 30 min at 23°C, the Con A was seen to have capped as a crescent on the majority of mhcA− cells (Fig. 2b); wild-type cells, under these conditions, had a tighter cap—almost a button on the end of the cell (Fig. 2a). A histogram (Fig. 2c) shows this result more graphically.
Figure 2.
Con A is capped more efficiently when two additional layers of cross-linking are added. Micrographs are as in Fig. 1. (a) Wild-type Ax2 cell. (b) mhcA− (HS2206) cell. (c) Histogram showing the extent of capping Con A receptors (as defined in Fig. 1e; about 300 of each cell type were scored). Note that no substantial endocytosis of fluorescence occurs in these experiments.
There is an additional point which may be worth making. When Con A alone is employed as the cross-linking agent, the amount of label seen in intracellular structures increases with time; this is true of both wild-type and mhcA− cells. However, no intracellular label is seen when three layers are used, even after an extended incubation of an hour.
DISCUSSION
Our studies, which have been carried out with two independent mhcA− cell lines with similar results, show that mhcA− cells are able to cap Con A receptors. Previous experiments, using Con A alone, had detected a large difference between wild-type and mhcA− amoebae in their abilities to cap Con A—which we confirm. However, the difference is a quantitative one, not a qualitative one. If Con A receptors are adequately cross-linked, then having the mhcA gene product there or not does not grossly affect the outcome (Fig. 2). We do not know why mhcA− cells are less efficient at capping surface-bound Con A than wild type; however, it may simply be an indirect effect. mhcA− cells exhibit pleiotropic defects: they are flatter (12), cannot divide unless provided with a solid substratum, are less proficient at extending pseudopods, and, indeed, grow more slowly (10). We noticed a further difference between them and wild-type cells: occasional mhcA− cells cap the Con A away from both ends of the cell, leaving the Con A as a band around the cell’s middle as shown in Fig. 1b. This may result from the altered behavior of these cells, as a detailed analysis of how mhcA− cells locomote has shown that, compared with wild type, their persistence in moving in a particular direction is diminished (10): they seem less certain about where they are going. This can result in an mhcA− cell having more than one growing pseudopod at a given moment, which would occasionally be expected to lead to bipolar cells labeled in the way we observe (Fig. 1b). It is possible that the loss of many different gene products involved in intracellular organization would give rise to cells with similar defects.
Inefficient capping, the clearance of a cross-linked antigen from only a small portion of the cell surface, as shown in our Fig. 1 b–e, has not, to our knowledge, been reported before. This observation itself is of interest and may most easily be explained by a mechanism for cap formation which depends on a decreasing rate of membrane flow from the leading edge of the cell (6). If the lack of myosin II reduces the rate of membrane flow compared with wild type (for whatever reason), poorly cross-linked antigens would be expected to be only poorly capped—that is, cleared away from a small region at the front of the cell. More extensive cross-linkage leading to the formation of larger patches would be expected to cause a greater extent of capping, as we find. However, alternative interpretations in which the actin cytoskeleton itself effects capping might also explain our observations. For example, if the cytoskeleton continuously rakes the cell surface, as originally proposed by de Petris (2), then increasing the size of patches of Con A receptors might also enhance cap formation (6).
Our observations remove an apparent discrepancy: an mhcA− cell can transport Con A-coated particles to its trailing end (13), yet cannot cap Con A receptors. In fact, both transport processes function. In addition, our results remove an obstacle to understanding how cells move. Since the discovery of antigen capping and particle migration it has been assumed that the forces driving these processes are the same as those that drive the rearward movement of a cell’s feet as it advances. In each case the rearwardly moving cell surface proteins are extensively cross-linked—by antibodies (or lectins), by ligands on the particle surface, or by the substratum. The fact that Dictyostelium myosin II seemed to be required for capping surface antigens, yet is clearly not required for cell locomotion, implied that the mechanism of capping surface antigens is not relevant to how cells locomote (14). As a result, the extensive knowledge of capping has become eclipsed—to many it seems irrelevant to an understanding of how cells move. The results reported here should change that position and enable those interested to view the problem of how cells move with a wider perspective.
Acknowledgments
We thank Rob Kay for his generous help and advice. C.A.-V. was supported by a Howard Hughes Medical Institute International Scholars Award to Rob Kay.
References
- 1.Taylor R B, Duffus W P H, Raff M C, de Petris S. Nat New Biol. 1971;233:225–229. doi: 10.1038/newbio233225a0. [DOI] [PubMed] [Google Scholar]
- 2.de Petris S. In: Dynamic Aspects of Cell Surface Organisation. Poste G, Nicolson G L, editors. Amsterdam: North Holland; 1977. pp. 643–728. [Google Scholar]
- 3.Condeelis J. Annu Rev Cell Biol. 1993;9:411–444. doi: 10.1146/annurev.cb.09.110193.002211. [DOI] [PubMed] [Google Scholar]
- 4.Heath J P, Holifield B F. Cell Motil Cytoskeleton. 1991;18:245–257. doi: 10.1002/cm.970180402. [DOI] [PubMed] [Google Scholar]
- 5.Bretscher M S. Science. 1984;224:681–686. doi: 10.1126/science.6719108. [DOI] [PubMed] [Google Scholar]
- 6.Bretscher M S. Cell. 1996;87:601–606. doi: 10.1016/s0092-8674(00)81380-x. [DOI] [PubMed] [Google Scholar]
- 7.Pasternak C, Spudich J A, Elson E L. Nature (London) 1989;341:549–551. doi: 10.1038/341549a0. [DOI] [PubMed] [Google Scholar]
- 8.Fukui Y, De Lozanne A, Spudich J A. J Cell Biol. 1990;110:367–378. doi: 10.1083/jcb.110.2.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Lozanne A, Lewis M, Spudich J A, Leinwand L A. Proc Natl Acad Sci USA. 1985;82:6807–6810. doi: 10.1073/pnas.82.20.6807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wessels D, Soll D R, Knecht D, Loomis W F, De Lozanne A, Spudich J A. Dev Biol. 1988;128:164–177. doi: 10.1016/0012-1606(88)90279-5. [DOI] [PubMed] [Google Scholar]
- 11.Manstein D J, Titus M A, De Lozanne A, Spudich J A. EMBO J. 1989;8:923–932. doi: 10.1002/j.1460-2075.1989.tb03453.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shelden E, Knecht D A. Cell Motil Cytoskeleton. 1996;35:59–67. doi: 10.1002/(SICI)1097-0169(1996)35:1<59::AID-CM5>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 13.Jay P Y, Elson E L. Nature (London) 1992;356:438–440. doi: 10.1038/356438a0. [DOI] [PubMed] [Google Scholar]
- 14.Egelhoff T T, Spudich J A. Trends Genet. 1991;7:161–166. doi: 10.1016/0168-9525(91)90380-9. [DOI] [PubMed] [Google Scholar]