Abstract
Rer1p, a Golgi membrane protein, is required for the correct localization of an endoplasmic reticulum (ER) membrane protein, Sec12p, by a retrieval mechanism from the cis-Golgi to the ER. To test whether or not the role of Rer1p is common to multiple ER membrane proteins, we examined the localization of two other ER membrane proteins, Sec71p and Sec63p, in the wild-type and rer1 mutant yeast cells, using their fusions with an α-mating factor precursor (Mfα1p). Although Sec71p and Sec63p have completely different topology from Sec12p, their Mfα1p fusion proteins were also mislocalized to the trans-Golgi in the rer1 mutant. Overexpression of these fusions caused their mislocalization to the trans-Golgi even in the wild-type cells, and this mislocalization was partially suppressed by the co-overexpression of Rer1p. Either Sec71p or an artificial chimeric protein whose ER localization depends on Rer1p gave a competitive effect on the localization of the Mfα1-Sec71p fusion, which was abolished in rer1. Thus, Rer1p appears to be one of the common limiting components in the retrieval machinery for ER membrane proteins. The results also suggest that Sec71p and Sec63p depend on ER-Golgi recycling, at least partly, for ER localization. We also examined the effect of a mutation in α-COP, a subunit of yeast coatomer, on the localization of these ER membrane proteins. The Mfα1p fusions of Sec12p, Sec71p, and Sec63p were all more or less mislocalized in ret1–1. These observations imply that the roles of Rer1p and coatomer are much more general than thought before.
Keywords: retrieval, vesicle recycling, Golgi apparatus, coatomer, Saccharomyces cerevisiae
Organelles in the secretory pathway are connected by dynamic membrane traffic. Each protein that functions in a particular organelle must have a specific signal for its localization. To date, several signals have been identified for the localization of proteins in the endoplasmic reticulum (ER). Many ER lumenal proteins have a KDEL or similar signal at their C terminus (1, 2). This signal is recognized by its receptor in the Golgi apparatus, which results in the recycling of KDEL proteins back to the ER (3–5). Some ER membrane proteins carry a dilysine or diarginine motif at their cytoplasmic tail, which is also believed to act as a retrieval signal (6, 7). It has been shown that coatomer (COP I) binds to the dilysine motif (8), and mutations in COP I subunits cause mislocalization of dilysine-tagged marker proteins (9, 10). However, there are many ER membrane proteins that contain none of these signals. The mechanisms to localize these proteins to the ER remain largely unknown.
Yeast Sec12p is a type II transmembrane glycoprotein required for the formation of transport vesicles from the ER and does not harbor any signals described above (11, 12). To clarify the molecular mechanisms of ER localization of Sec12p, we isolated two recessive mutants, rer1 and rer2, which have a defect in the proper localization of Sec12-Mfα1 fusion protein (S12Mp) (13). The rer1 mutant has been demonstrated to mislocalize not only S12Mp but the authentic Sec12p as well. The RER1 gene encodes a protein of 188 amino acid residues containing four membrane-spanning domains (14, 15). Rer1p is localized in the early region of Golgi apparatus and, thus, should be involved in dynamic retrieval of Sec12p from the Golgi apparatus. By systematic analyses using various chimeras with a vacuolar membrane protein, Dap2p, we have demonstrated that the cytoplasmic and transmembrane domains of Sec12p contain an RER1-independent signal for static retention and an RER1-dependent retrieval signal, respectively (16).
In this study, we focused on two different types of ER membrane proteins and applied our method using Mfα1p fusions to monitor their behaviors in vivo. One is Sec71p, a type III membrane glycoprotein (17), and the other is Sec63p, which spans the ER membrane three times. Both of these molecules have quite different topology from Sec12p; their N termini are facing the ER lumen and the C termini are cytoplasmic (18–20). They form a multimeric complex required for the posttranslational translocation of newly synthesized secretory proteins (21–28). Here, we show that Rer1p is required for the correct localization of Sec71p and Sec63p as well. Furthermore, there is an apparent competition for Rer1p between these proteins, suggesting that Rer1p is a limiting component of the cellular machinery involved in retrieval. The role of Rer1p will be discussed from the viewpoint of localization mechanisms for various types of ER membrane proteins.
MATERIALS AND METHODS
Yeast Strains and Culture Condition.
Saccharomyces cerevisiae strains used in this study are listed in Table 1. Yeast cells were grown in YPD [1% (wt/vol) Bacto yeast extract (Difco), 2% (wt/vol) polypeptone (Nihon Seiyaku, Tokyo) and 2% (wt/vol) glucose] or in MVD [0.67% yeast nitrogen base without amino acids (Difco) and 2% glucose] medium supplemented appropriately. MCD medium is MVD containing 0.5% casamino acids (Difco).
Table 1.
Yeast strains used in this study
| Strain | Genotype |
|---|---|
| EGY101* | MATa ret1-1 ura3 leu2 his3 trp1 suc2 |
| RSY926† | MATα sec71::LEU2 ura3 leu2 his3 ade2 trp1 lys2 |
| RSY151† | MATα sec63-1 ura3 leu2 pep4 |
| SNY9‡ | MATα mfα1::ADE2 mfα2::TRP1 bar1::HIS3 ura3 leu2 trp1 his3 lys2 ade2 |
| SNY22‡ | MATα mfα1::ADE2 mfα2::TRP1 bar1::HIS3 pep4:LEU2 ura3 leu2 trp1 his3 lys2 ade2 |
| SKY1§ | MATα mfα1::ADE2 mfα2::LEU2 bar1::HIS3 ura3 leu2 trp1 his3 lys2 ade2 |
| SKY7§ | MATα rer1::LEU2 mfα1::ADE2 mfα2::TRP1 bar1::HIS3 ura3 leu2 trp1 his3 lys2 ade2 |
| SKY15§ | MATα rer1::LEU2 pep4::ADE2 ura3 trp1 his3 leu2 |
| SKY27¶ | MATα ret1-1 mfα1::ADE2 mfα2::TRP1 bar1::HIS3 ura3 leu2 trp1 his3 lys2 ade2 |
| SKY30¶ | MATα mfα1::ADE2 mfα2::TRP1 bar1::HIS3 ura3 trp1 his3 lys2 ade2 leu2::GAP-MFα1-SEC71-3HA::LEU2 |
| SKY33¶ | MATα rer1::LEU2 mfα1::ADE2 mfα2::TRP1 bar1::HIS3 ura3 trp1 his3 lys2 ade2 leu2::GAP-MFα1-SEC71-3HA::LEU2 |
Plasmid Construction.
SEC71 and SEC63-myc genes (19, 20) were kindly provided by D. Feldheim and R. Schekman of the University of California at Berkeley. SEC63-myc contains a DNA fragment encoding the human c-myc epitope (N-LEEQKLISEEDLLRKR-C) just before the stop codon. To insert the Mfα1p peptide at the N termini of Sec71p and Sec63-mycp, a BstBI site was introduced just after the start codon by site-directed mutagenesis with a synthetic oligonucleotide 5′-GAGTTTGCCAATATGTTCGAATTTAATGAAACA-3′ or by PCR mutagenesis with synthetic oligonucleotides: M1320, 5′-GTAAAACGACGGCCAGTGAG-3′; SEC631, 5′-CATACTCGTAATTTGTTTCGAAAGGCATTGTGCTGTA-3′; SEC632, 5′-TACAGCACAATGCCTTTCGAAACAAATTACGAGTATG-3′; and M13R, 5′-GGAAACAGCTATGACCATG-3′. The resulting genes were named SEC71-B and SEC63-myc-B, respectively. In SEC71-B, a NheI site was also introduced just before the stop codon by PCR mutagenesis with M1320, M13R, SEC71C3 (5′-AATAATGATGGAAGATTAGCTAGCTAGTGCCTACTGTGTGCA-3′), and SEC71C4 (5′-TGCACACAGTAGGCACTAGCTAGCTAATCTTCCATCATTATT-3′). A DNA fragment encoding three copies of the HA epitope (YPYDVPDYA) (14) was inserted into this NheI site of SEC71-B. This tagged gene was named SEC71B-3HA. Various-length MFα1 DNA fragments were synthesized by PCR with the following primers: MFα1L, 5′-CCCATCGATAGATTTCCTTCAATTTTT-3′ and 5′-GGGTTCGAAGGGTTTTAACTGCAACCA-3′; MFα1M, 5′-CCATCGATGAAACGGCACAAATT-3′ and 5′-GGATCGATGGGTTTTAACTGCAACCA-3′; and MFα1S, 5′-CCTTCGAAGTACATTGGTTGGCCGGGTTT-3′ and 5′-CCATCGATAAAAGAGAGGCTGAAGCTTGG-3′. The ClaI-digested MFα1M fragment was inserted into the BstBI-digested SEC71-B-3HA and the resulting fusion gene was named MS71H. The ClaI-BstBI-digested MFα1L and MFα1S and the ClaI-digested MFα1M fragment were inserted into the BstBI-digested SEC63-myc-B to produce MS63L, MS63S, and MS63M, respectively. These fusion genes were expressed on a single-copy plasmid pRS316 (29) or a multicopy plasmid pYO326 (30). For the construction of GAP-MS71H, the 0.8-kb SpeI-EcoRI promoter region of the glyceraldehyde-3-phosphate dehydrogenase gene (TDH2) was inserted in place of the SpeI-EcoRI fragment of the SEC71 promoter in MS71H. For the construction of 3HA-SEC71, a DNA linker (CGCTAG) was inserted in the BstBI site of SEC71-B to create a NheI site. The DNA fragment encoding the 3HA epitope was integrated at this NheI site. The resulting gene (3HA-SEC71) complemented the temperature-sensitive growth of Δsec71 completely and was thus used as a functional SEC71.
DNA manipulations, including restriction enzyme digestions, ligations, plasmids isolation, and E. coli transformation, were carried out by standard methods. Yeast transformation was performed by a lithium thiocyanate method (31). DNA fragments were purified from agarose gel pieces using the DNA PREP kit (Asahi, Tokyo). DNA nucleotide sequences were determined by the dideoxy method (32) using a DNA sequencer (Model 373A; Applied Biosystems, Japan).
Immunofluorescence Microscopy.
Indirect immunofluorescence was performed as described previously (14, 33). The staining of the HA-tagged MS71Hp and myc-tagged MS63Lp were observed by using the 12CA5 and 9E10 monoclonal antibodies, respectively. Decoration of these antibodies was performed by the addition of 5 μg/ml biotinylated goat anti-mouse antibody followed by 5 μg/ml streptavidin-fluorescein.
Halo Assay.
Halo assay was performed on MCD plates with a tester MATa sst2 strain as described previously (13). Eight independent spots were examined to quantify the amount of α-factor secreted in the all experiments.
Pulse–Chase Experiments.
Metabolic labeling of yeast cells, preparation of cell extracts and immunoprecipitation were performed as described previously (33). A monoclonal anti-HA antibody (12CA5) was used to immunoprecipitate MS71Hp from cell extracts. This immunoprecipitate was dissolved in 1% SDS, divided into three aliquots, diluted with 10× volume of 2% Triton X-100, and subjected to the second immunoprecipitation with the anti-HA antibody, anti-α1→6 mannose antiserum or anti-α1→3 mannose antiserum. Endoglycosidase H treatment and analysis by SDS/PAGE and fluorography were performed as described (34). Radioimage was observed and quantified with a Fuji Film image analyzer BAS-1000.
RESULTS
Construction of Fusions Between Mfα1p and ER Membrane Proteins.
In our previous study, we devised a method using a fusion protein between Sec12p and a yeast α-mating factor precursor (Mfα1p) to monitor its localization in vivo (13). When this fusion protein is transported to the trans region of the Golgi apparatus, three processing proteases act consecutively on the Mfα1p moiety and produce the mature α-factor. The secretion of α-factor can be easily detected by the halo assay. This method has been successfully applied to the analysis of the rer1 mutant (13, 14) and identification of the ER localization signals of Sec12p (16). In the present study, we employed the same strategy to test the generality of RER1 function for ER protein localization. The ER membrane proteins chosen as markers are Sec71p and Sec63p. The constructs we made as fusions with Mfα1p are shown in Fig. 1.
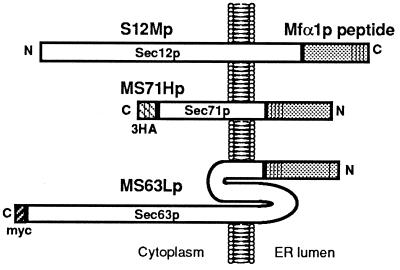
Figure 1.
Fusion proteins used in this study. S12Mp, Sec12-Mfα1p; MS71Hp, Mfα1-Sec71p with 3HA tag; MS63Lp, Mfα1-Sec63p with c-myc tag. S12Mp and MS71Hp could complement Δsec12 and Δsec71 cells, but MS63Lp did not complement sec63–1.
SEC71 encodes a 31.5-kDa ER membrane glycoprotein of type III topology, which is completely opposite to Sec12p. The SEC71-disrupted mutant shows temperature-sensitive growth. MS71Hp was designed to contain an Mfα1p derivative without the signal sequence at the N terminus of Sec71–3HAp, because the authentic SEC71 product does not carry a cleavable signal sequence at the N terminus. The MS71H gene complemented the temperature-sensitive growth of Δsec71 (RSY926) at 37°C on either a single-copy or multicopy plasmid as well as the bona fide SEC71 (data not shown). Immunofluorescence microscopy showed that MS71Hp was correctly localized to the ER at steady state (Fig. 2A–C). These results indicate that MS71Hp remains fully functional as Sec71p in the ER.
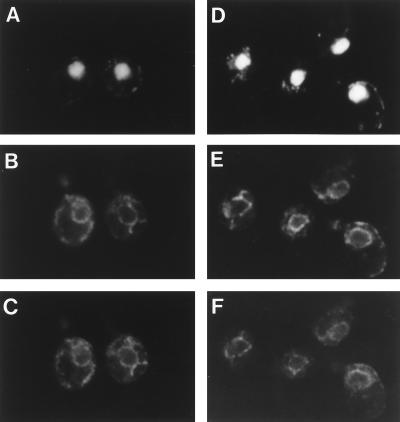
Figure 2.
Subcellular localization of MS71Hp and MS63Lp. Wild-type cells (SNY9) expressing MS71Hp (A–C) or MS63Lp (D–F) on a single-copy plasmid were fixed and prepared for immunofluorescence microscopy. Both strains were stained with DAPI (A and D), anti-BiP polyclonal antibody (B and E), and anti-HA (12CA5) monoclonal antibody (C) or anti-myc (9E10) monoclonal antibody (F). Note the colocalization of MS71Hp and MS63Lp with BiP, the ER marker.
SEC63 encodes a 73-kDa ER membrane protein, which spans the ER membrane three times (see Fig. 1). First, we inserted an Mfα1p peptide without the signal sequence at the N terminus of Sec63p, but this fusion failed to complement the temperature sensitivity of the sec63–1 mutant (RSY151). We constructed two more fusions, MS63Sp and MS63Lp, which contained four repeats of the mature α-factor peptide only or the whole polypeptide of prepro-α-factor, respectively. Unfortunately, these fusions did not complement sec63–1 either. Nevertheless, because we could observe clear ER localization of MS63Lp (Fig. 2 D–F), we decided to analyze its behavior in terms of Rer1p dependency of localization.
RER1 Is Important for ER Localization of Multiple Membrane Proteins.
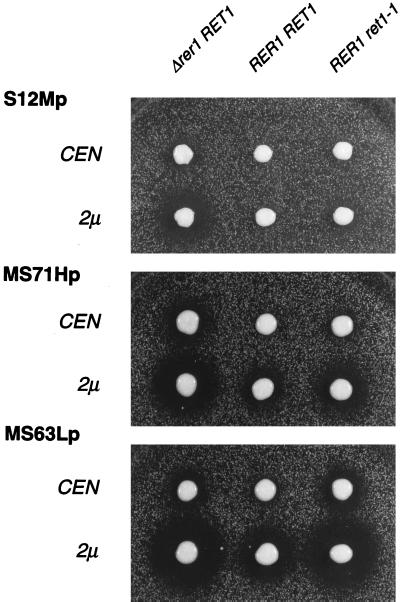
We examined the localization of these fusion proteins by measuring α-factor secretion by the halo assay. As described previously (16), the size of the halo is a quantitative measure of the amount of the fusion protein that has arrived at the trans-Golgi. The three fusions, S12Mp, MS71Hp, and MS63Lp, were introduced into RER1 (SNY9) and Δrer1 (SKY7) cells on a single-copy (CEN) or multicopy (2μ) plasmid and subjected to the halo assay (Fig. 3). As shown in our previous studies (13, 14), S12Mp produced no halo in RER1 but formed a halo in Δrer1 in a dose-dependent manner. In contrast, MS71Hp and MS63Lp made a large halo even in the wild-type cells (RER1 RET1) when they were on the multicopy plasmid. This suggests that the capacities of their ER localization mechanisms are saturable. Surprisingly, the α-factor secretion from MS71Hp and MS63Lp showed clear RER1 dependency like the case of S12Mp (compare RER1 RET1 and Δrer1 RET1). This result would indicate that RER1 is involved in the ER localization of not only Sec12p but also Sec71p and Sec63p. It also implies that the ER localization of these proteins is fulfilled, at least partly, by dynamic retrieval from the Golgi apparatus.
Figure 3.
Halo assay of the Δrer1 and ret1–1 mutants expressing Mfα1p fusion proteins. Cells of SNY9 (RER1: Center), SKY7 (Δrer1: Left), and SKY27 (ret1–1: Right) expressing either S12Mp, MS71Hp, or MS63Lp on a single-copy (CEN) or multicopy (2 μ) plasmid were examined for α-factor secretion by the halo assay. The plates were incubated at 23°C for 2 days.
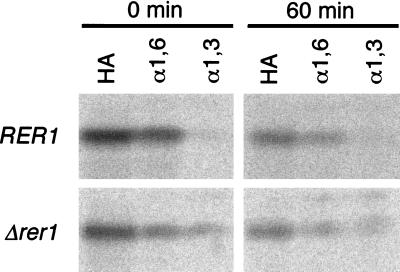
To rule out the possibility that the α-factor moiety might have affected the localization, we examined the behavior of Sec71p without the Mfα1 fusion. The HA-tagged Sec71p (3HA-Sec71p) was introduced on a multicopy plasmid into the wild-type and Δrer1 cells, and the ER localization was confirmed by immunofluorescence (not shown). The cells were then labeled with Tran35S-label and subjected to immunoprecipitation analysis. As shown in Fig. 4, a large portion (38%) of 3HA-Sec71p received α1→6 mannosyl linkages (cis-Golgi modification) during a 20-min pulse, but the acquisition of the α1→3 mannosyl linkages (medial-Golgi modification) was very little in the wild-type cells (1% at 0 min and 6% at 60 min). In the Δrer1 cells, by contrast, a significant fraction of 3HA-Sec71p (8% at 0 min and 17% at 60 min) underwent α1→3 mannosyl modification. These observations indicate that the authentic Sec71p indeed utilizes retrieval from the cis-Golgi for ER localization and that Rer1p is important for this process.
Figure 4.
Pulse–chase analysis of 3HA-Sec71p. Wild-type (SNY22) and Δrer1 (SKY15) cells expressing 3HA-Sec71p on a multicopy plasmid were labeled with tran35S-label at 30°C for 20 min and chased for the indicated times. 3HA-Sec71p was first immunoprecipitated with the anti-HA antibody and then subjected to the second immunoprecipitation with antibodies against HA, α1→6 (α1, 6), or α1→3 (α1, 3) mannosyl linkages. All samples were treated with endo H, and analyzed by SDS/PAGE and radioimaging.
Rer1p Is a Common Limiting Factor Involved in ER Localization of Sec12p and Sec71p.
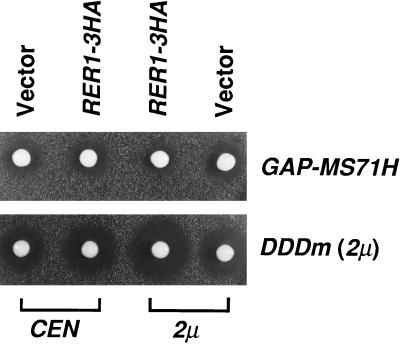
The seemingly saturable nature of ER localization of MS71Hp and MS63Lp suggests that limiting component(s) exist for this mechanism. To test the possibility that Rer1p may be such a component, we examined the effect of Rer1p overproduction on the size of halo produced by MS71Hp. To make a stable strain that overexpresses MS71Hp, we constructed a glyceraldehyde-3-phosphate dehydrogenase (GAP) promoter-driven MS71H (GAP-MS71H) and integrated it at the leu2 locus of SNY9. This integrant (SKY30) was transformed with RER1-3HA on a single-copy (CEN) or multicopy (2 μ) plasmid and examined by the halo assay (Fig. 5 Upper). As is the case of the cells expressing MS71H from a multicopy plasmid, GAP-MS71H in SKY30 cells caused halo formation (see Vector). The expression of RER1–3HA from the plasmid made the halo significantly smaller. By quantitative analysis of the halo size, the amounts of secreted α-factor were calculated to be 78 ± 8% and 52 ± 5% of the vector controls for the single-copy and multicopy plasmids of RER1–3HA, respectively. Western blot analysis using the anti-HA antibody indicated that this effect was not due to the decrease of the expression of MS71Hp (data not shown). A similar result was also obtained for MS63Lp (not shown). Thus, the raised dosage of Rer1p apparently remedies the mislocalization of the overproduced fusion proteins.
Figure 5.
Suppression of the halo formation from MS71Hp by RER1 overexpression. The wild-type cells (SKY30) expressing the GAP promoter-driven MS71H (GAP-MS71H) and the wild-type cells (SKY1) expressing DDDm were transformed with pRS316 (CEN), pRS316/RER1–3HA, pYO326 (2 μ), and pYO326/RER1–3HA and examined for the α-factor secretion by the halo assay. The plates were incubated at 30°C for 2 days.
We also examined the effect of the increase of Rer1p on the halo formation by DDDm, which is a fusion protein between Mfα1p and a type II vacuolar membrane protein, Dap2p. This fusion has been shown to be quickly transported to the vacuole in the presence or absence of Rer1p (16). In contrast to the case of MS71Hp, RER1–3HA increased the secretion of α-factor from the wild-type cells expressing DDDm (see Fig. 5 Lower). This may suggest that Rer1p has opposite effects on retrograde and anterograde protein transport.
If Rer1p is in fact a limiting component commonly required for the retrieval of Sec12p and Sec71p, competition may be observed when either of them is overproduced. We have recently reported that the transmembrane domain (TMD) of Sec12p is an RER1-dependent ER localization signal, and the transplantation of this domain makes Dap2p efficiently localized to the ER. So we examined the effects of the co-overproduction of Sec71p, Dap2p (DDD), and the Dap2p derivative (DSD) containing the TMD of Sec12p on the halo formation by the MS71Hp-overexpressing strain (SKY30). If the overproduction of any of these proteins competes for Rer1p with MS71Hp, the retrieval of MS71Hp would become less efficient, which will lead to an increase of α-factor secretion. This was indeed the case for Sec71p and DSD. The result of the quantitative analysis of halos is summarized in Table 2. The co-overexpression of Sec71p or DSD increased the α-factor secretion of SKY30 by 1.7- and 1.9-fold, respectively. DDD showed no effect in this experiment. Importantly, such a competitive effect was abolished when the RER1 gene was disrupted (Table 2). Furthermore, the competition was partly suppressed by the co-overexpression of Rer1p as well (not shown). These observations indicate that Rer1p is responsible for the dosage-dependent overflow of ER membrane proteins, which is consistent with the idea that it is Rer1p that is limiting in the retrieval of these ER membrane proteins.
Table 2.
Competitive effect of Sec71p and DSD on the mislocalization of MS71Hp
| Competitor | Relative amount of α-factor secreted, %
|
|
|---|---|---|
| RER1 | Δrer1 | |
| Vector | 100 | 100 |
| 3HA-Sec71p | 168 ± 37 | 98 ± 17 |
| DDD | 106 ± 17 | 96 ± 11 |
| DSD | 188 ± 33 | 92 ± 6 |
SKY30 (RER1) and SKY33 (Δrer1) cells were transformed with a multicopy plasmid harboring a gene encoding either 3HA-Sec71p, DDD, or DSD and examined for α-factor secretion by the halo assay. Halo sizes of eight spots were measured to quantitate the amount of α-factor secretion. The values (average ± standard deviation) relative to the vector control are shown.
RET1 Is Also Involved in ER Localization of Membrane Proteins Without the Dilysine Signal.
Evidence has been presented that components of coatomer (COP I) are required for the Golgi-to-ER retrieval of membrane proteins containing the dilysine motif (9, 10). RET1 encodes the yeast counterpart of α-COP, a subunit of COP I. A mutation of RET1 causes temperature-sensitive growth and mislocalization of Ste2-Wbp1 fusion protein, which harbors the dilysine signal, from the ER to the plasma membrane (9). We examined the effect of this ret1 mutation on the localization of our fusions, S12Mp, MS71Hp, and MS63Lp, which do not have any dilysine or related motifs at their cytoplasmic ends. To use our tools, we constructed a ret1–1 Δmfα1 Δmfα2 Δbar1 strain (SKY27) by crossing SKY7 (MATα Δrer1::LEU2 Δmfα1 Δmfα2 Δbar1) and EGY101 (MATa ret1–1). In the course of construction of this strain, we dissected 20 tetrads after mating and sporulation, and 13 of them gave rise to four viable spores at 23°C, including 9 Leu+ Ts− spores. This indicates that the Δrer1 ret1–1 double mutant is viable. However, the Δrer1 ret1–1 cells did not grow at 35°C, at which temperature either single mutant can grow (data not shown), indicating some synthetic effect of these mutations. The obtained ret1–1 cells (SKY27) were transformed with a single-copy or multicopy plasmid harboring S12Mp, MS71Hp, or MS63Lp. As shown in Fig. 3, S12Mp on a multicopy plasmid in ret1–1 formed a small but visible halo, suggesting that COP I is also involved in the retrieval of Sec12p. In the cases of MS71Hp and MS63Lp, the effect of the ret1–1 mutation was more obvious. Halos were observed even when they were expressed from a single-copy plasmid. On the multicopy plasmids, the dependency on RET1 is seen for all the fusions examined. This result might argue that the role of COP I in the Golgi-to-ER retrieval is not restricted to the proteins that contain the dilysine motif. It may be also worth mentioning that the dependency on Rer1p and Ret1p varies among the three fusions. In the cases of S12Mp and MS71Hp, the disruption of RER1 showed a more severe effect than ret1–1. With MS63Lp, on the other hand, the halo size in ret1–1 is comparable to or even larger than that in the RER1 knock out.
DISCUSSION
Retrieval from the Golgi apparatus is important for many ER proteins to attain their strict localization in the ER. Several distinct systems have been studied to date. We have shown that Rer1p is essential for the retrieval of Sec12p from the Golgi to the ER and that the TMD of Sec12p acts as a signal in this Rer1p-dependent mechanism (14, 16). Erd2p, which resides in the Golgi membrane, functions as the receptor of the HDEL signal and thus sends back HDEL-bearing proteins to the ER (3–5). Coatomer (COP I) binds to the dilysine signal on the cytoplasmic tail of membrane proteins and drives formation of COP I-coated vesicles, a retrograde carrier from the Golgi to the ER (8–10). Are these seemingly different mechanisms responsible only for retrieval of their specific cargos?
Taking advantage of the halo assay method, which we developed to detect mislocalization of α-factor fusion proteins (13), we have shown in this study that Mfα1p fusions with Sec71p (MS71Hp), Sec63p (MS63Lp), and Sec12p (S12Mp) are all mislocalized to the trans-Golgi in the Δrer1 mutant cells. The role of Rer1p has also been demonstrated for Sec71p itself by the analysis of oligosaccharide modification. Because these three membrane proteins have completely different topology and share no apparent sequence homology, this suggests that Rer1p is a component of general retrieval machinery utilized by a variety of membrane proteins.
During the analysis of these fusion proteins, we realized that the Rer1p-dependent retrieval appeared to be a saturable process. When MS71Hp and MS63Lp were overproduced, they were mislocalized even in the wild-type cells. Similar overflow was also observed when DSDm, a Dap2p/Sec12p chimera with Mfα1p fusion whose ER localization is strongly dependent on Rer1p, was overexpressed in the wild type (M.S., unpublished result). The mislocalization of MS71Hp and MS63Lp due to their overproduction is partially suppressed by the co-overproduction of Rer1p. This would argue that Rer1p is one of the limiting factors required for retrieval of these membrane proteins. This possibility was further supported by a competition experiment. When Sec71p or DSD without the Mfα1p peptides was highly expressed together with MS71Hp, the mislocalization of MS71Hp was significantly exacerbated. This can be interpreted as indicating that the capacity of the machinery to retrieve these membrane proteins is limited, and thus, the overflow of MS71Hp becomes even worse when another cargo is overproduced. Such exaggeration of mislocalization was abolished when the RER1 gene was knocked out. These observations led us to conclude that Rer1p is a limiting component of the cellular machinery commonly involved in retrieval of various ER membrane proteins.
There are several possibilities about the role of Rer1p in retrieval. It could be a receptor of retrieval signal(s) or may function as a chaperone during membrane protein sorting in the cis-Golgi. It is also conceivable that Rer1p blocks transport of ER membrane proteins to the later compartments of the Golgi apparatus. Whether Rer1p is recycled back to the ER together with cargos is an important question in this regard. Interestingly, Rer1p appears to exert opposite effects on retrograde and anterograde transport from the cis-Golgi. Although the overproduction of Rer1p suppresses the halo formation from MS71Hp, it clearly increases the size of halo produced from Dap2-Mfα1p (DDDm). On the contrary, the disruption of RER1 causes the halo from DDDm to become slightly smaller (see figure 4 of ref. 16). These observations might imply that Rer1p plays roles not only in retrieval of ER membrane proteins but also in accelerating forward transport of vacuolar and perhaps plasma membrane proteins. If so, a complex mechanism may be required for Rer1p to distinguish these two categories of proteins.
We have also shown that all three fusion proteins, MS71Hp, MS63Lp, and S12Mp, are more or less mislocalized to the trans-Golgi in ret1–1, a mutant of α-COP. The COP I complex is known to be required for the recognition of dilysine retrieval signals, but none of these fusion proteins harbors dilysine or related motifs. Then, do Rer1p and COP I cooperate on retrieval of these proteins? The combination of Δrer1 and ret1–1 mutations confers a synthetic growth defect to the cells, indicating their intimate relationship. Furthermore, we found that the overproduction of Rer1p failed to suppress the mislocalization of MS71Hp in the ret1–1 mutant (unpublished result). From these, we suggest that Rer1p and COP I function in the same pathway.
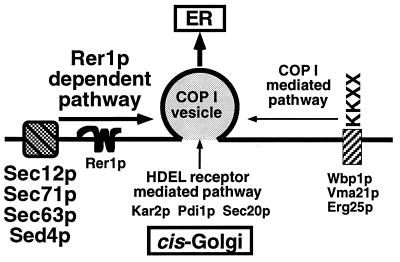
To compare with other retrieval signal systems, we constructed an Mfα1-Wbp1 fusion protein that harbors a typical dilysine signal. This protein was mislocalized in ret1–1 but not at all in the Δrer1 cells (unpublished observation). We have already shown that BiP, a soluble ER protein containing the HDEL signal, is not significantly missecreted in Δrer1 (13, 14). Probably these dilysine and HDEL signals are recognized by their specific receptors and directly packaged in the COP I vesicles. Our current model for ER protein sorting in the cis-Golgi is illustrated in Fig. 6. As far as we examined, the TMD of Sed4p also seems to act as an Rer1p-dependent signal (16). How Rer1p recognizes various types of membrane proteins would be the most important next question and awaits further biochemical experiments.
Figure 6.
A model for sorting ER proteins in the Golgi apparatus. Three pathways are shown for retrieval of proteins from the cis-Golgi to the ER. Membrane proteins that harbor KKXX signals bind to COP I subunits and are directly integrated into COP I vesicles. Proteins with the HDEL signal are recognized by its receptor, Erd2p, and are probably also packaged in COP I vesicles. Rer1p distinguishes another group of ER membrane proteins that contain neither of these signals and escorts them to COP I vesicles.
Acknowledgments
We are grateful to David Feldheim, Randy Schekman, and François Letourneur for strains and plasmids, and to the members of our laboratory for helpful comments on the manuscript. This study was supported by Grants-in-Aid for Scientific Research from the Ministry of Education, Science, and Culture of Japan, by a fund (Biodesign Project) from the Institute of Physical and Chemical Research of Japan (RIKEN), and by a Research Fellowship for Young Scientists (to K.S. and M.S.) from the Japan Society for the Promotion of Science.
ABBREVIATIONS
- ER
endoplasmic reticulum
- TMD
transmembrane domain
Note
While this manuscript was in a revise process, Boehm et al. (35) reported that another mutant allele of RET1 also mislocalized Sec12-invertase fusion proteins and that Rer1p recycled between the early Golgi and the ER in a COP I-dependent manner. These observations are consistent with our conclusions.
References
- 1.Munro S, Pelham H R B. Cell. 1987;48:899–907. doi: 10.1016/0092-8674(87)90086-9. [DOI] [PubMed] [Google Scholar]
- 2.Pelham H R B. EMBO J. 1988;7:913–918. doi: 10.1002/j.1460-2075.1988.tb02896.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Semenza J C, Hardwick K G, Dean N, Pelham H R B. Cell. 1990;61:1349–1358. doi: 10.1016/0092-8674(90)90698-e. [DOI] [PubMed] [Google Scholar]
- 4.Lewis M J, Sweet D J, Pelham H R B. Cell. 1990;61:1359–1363. doi: 10.1016/0092-8674(90)90699-f. [DOI] [PubMed] [Google Scholar]
- 5.Lewis M J, Pelham H R B. Nature (London) 1990;348:162–163. doi: 10.1038/348162a0. [DOI] [PubMed] [Google Scholar]
- 6.Jackson M R, Nillson T, Peterson P A. EMBO J. 1990;9:3153–3162. doi: 10.1002/j.1460-2075.1990.tb07513.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schutze M P, Peterson P A, Jackson M R. EMBO J. 1994;13:1696–1705. doi: 10.1002/j.1460-2075.1994.tb06434.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cosson P, Letourneur F. Science. 1994;263:1629–1631. doi: 10.1126/science.8128252. [DOI] [PubMed] [Google Scholar]
- 9.Letourneur F, Gaynor E C, Hennecke S, Démollière C, Duden R, Emr S D, Riezman H, Cosson P. Cell. 1994;79:1199–1207. doi: 10.1016/0092-8674(94)90011-6. [DOI] [PubMed] [Google Scholar]
- 10.Cosson P, Démollière C, Hennecke S, Duden R, Letourneur F. EMBO J. 1996;15:1792–1798. [PMC free article] [PubMed] [Google Scholar]
- 11.Nakano A, Brada D, Schekman R. J Cell Biol. 1988;107:851–863. doi: 10.1083/jcb.107.3.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.d’Enfert C, Barlowe C, Nishikawa S, Nakano A, Schekman R. Mol Cell Biol. 1991;11:5727–5734. doi: 10.1128/mcb.11.11.5727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nishikawa S, Nakano A. Proc Natl Acad Sci USA. 1993;90:8179–8183. doi: 10.1073/pnas.90.17.8179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sato K, Nishikawa S, Nakano A. Mol Biol Cell. 1995;6:1459–1477. doi: 10.1091/mbc.6.11.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boehm J, Ulrich H D, Ossig R, Schmitt H D. EMBO J. 1994;13:3696–3710. doi: 10.1002/j.1460-2075.1994.tb06679.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sato M, Sato K, Nakano A. J Cell Biol. 1996;134:279–293. doi: 10.1083/jcb.134.2.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.von Heijne G. J Membr Biol. 1990;115:195–201. doi: 10.1007/BF01868635. [DOI] [PubMed] [Google Scholar]
- 18.Kurihara T, Silver P. Mol Biol Cell. 1993;4:919–930. doi: 10.1091/mbc.4.9.919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Feldheim D, Yoshimura K, Admon A, Schekman R. Mol Biol Cell. 1993;4:931–939. doi: 10.1091/mbc.4.9.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Feldheim D, Rothblatt J A, Schekman R. Mol Cell Biol. 1992;12:3288–3296. doi: 10.1128/mcb.12.7.3288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Deshaies R J, Schekman R. J Cell Biol. 1989;109:2653–2664. doi: 10.1083/jcb.109.6.2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rothblatt J A, Deshaies R J, Sanders S L, Daum G, Schekman R. J Cell Biol. 1989;109:2641–2652. doi: 10.1083/jcb.109.6.2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sadler I, Chiang A, Kurihara T, Rothblatt J A, Way J, Silver P. J Cell Biol. 1989;109:2665–2675. doi: 10.1083/jcb.109.6.2665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Green N, Fang H, Walter P. J Cell Biol. 1992;116:597–604. doi: 10.1083/jcb.116.3.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brodsky J L, Schekman R. J Cell Biol. 1993;123:1355–1363. doi: 10.1083/jcb.123.6.1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Feldheim D, Schekman R. J Cell Biol. 1994;126:935–943. doi: 10.1083/jcb.126.4.935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Panzner S, Dreier L, Hartmann E, Kostka S, Rapoport T A. Cell. 1995;81:561–570. doi: 10.1016/0092-8674(95)90077-2. [DOI] [PubMed] [Google Scholar]
- 28.Ng D T W, Brown J D, Walter P. J Cell Biol. 1996;132:499–509. doi: 10.1083/jcb.132.4.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sikorski R S, Hieter P. Genetics. 1989;122:19–27. doi: 10.1093/genetics/122.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ohya Y, Goebl M, Goodman L E, Petersen-Bjφrn S, Anraku Y. J Biol Chem. 1991;266:12356–12360. [PubMed] [Google Scholar]
- 31.Keszenman-Pereyra D, Hieda K. Curr Genet. 1988;13:21–23. doi: 10.1007/BF00365751. [DOI] [PubMed] [Google Scholar]
- 32.Sanger F, Nicklen S, Coulson A R. Proc Natl Acad Sci USA. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nishikawa S, Nakano A. Biochim Biophys Acta. 1991;103:135–143. doi: 10.1016/0167-4889(91)90114-d. [DOI] [PubMed] [Google Scholar]
- 34.Nishikawa S, Umemoto N, Ohsumi Y, Nakano A, Anraku Y. J Biol Chem. 1990;265:7440–7448. [PubMed] [Google Scholar]
- 35.Boehm J, Letourneur F, Ballensiefen W, Ossipov D, Démolliére C, Schmitt H D. J Cell Sci. 1997;110:991–1003. doi: 10.1242/jcs.110.8.991. [DOI] [PubMed] [Google Scholar]