Abstract
Female fireflies of the genus Photuris, the so-called firefly “femmes fatales,” prey on male fireflies of the genus Photinus. The females are able to entrap the males by faking the flash signal characteristics of the Photinus female. We found that by feeding on Photinus males, Photuris females gain more than nutrients. They also acquire defensive steroidal pyrones called lucibufagins, which are contained in Photinus but which Photuris fireflies are unable to produce on their own. Photuris females that eat Photinus males or lucibufagin are rejected by Phidippus jumping spiders. Lucibufagin itself proved to be a deterrent to such spiders. Field-collected Photuris females contain lucibufagin in varying amounts. The more lucibufagin they contain the more unacceptable they are to Phidippus.
Keywords: steroidal pyrones, Coleoptera: Lampyridae, Photuris, Photinus, Phidippus
Fireflies, the familiar luminescent beetles of the family
Lampyridae, are protected against predation. They are known to be
unacceptable to a number of vertebrates (1–3), and we showed that
species of the genus Photinus are unpalatable to thrushes
(4). Novel steroidal pyrones are the basis of this unpalatability, and
we found these pyrones systemically in all species of
Photinus that we investigated (P. ignitus,
P. marginellus, and P. pyralis; refs. 4–7).
Because of their close structural relationship to the cardiotonic
steroids (e.g., bufalin) found in the venom of Chinese toads
(Bufo spp.) (8, 9), we called the compounds lucibufagins
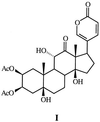
(LBG; for instance, I) (4).
Because LBG are of intrinsic chemical interest, we broadened our study to include other fireflies to see whether they might produce variants of these steroids. We found fireflies of another genus, Photuris, also to contain LBG. However, we learned that these species do not produce LBG themselves, acquiring them instead by preying on Photinus. Herein we detail the particulars of this chemical usurpation strategy.
We knew that Photuris females hunt species of Photinus. Female Photuris, appropriately called firefly “femmes fatales,” lure male Photinus by masquerading as females of the latter (10, 11). In firefly courtship, males fly about at night, flashing their light organs in a characteristic, species-specific pattern. Females answer their respective males from their perches, usually with single flashes, timed to follow the male “calls” with a species-specific delay. Photuris females lure Photinus males by flashing with a delay imitative of that of the Photinus female. The Photinus males are thereby drawn to the femmes fatales, only to be caught and eaten (12–15). The femmes fatales, in turn, are enriched with LBG.
The data we present here demonstrate that (i) female Photuris, in the field, contain LBG, as do Photinus; (ii) female Photuris, at adult emergence, are LBG-free, but become LBG-laden if fed on Photinus males or on pure LBG; (iii) female Photuris, fed on male Photinus or LBG, are less vulnerable to predation by Phidippus spiders than unfed controls; and (iv) the degree of protection of field-collected Photuris females against Phidippus is a function of the female’s LBG content.
MATERIALS AND METHODS
Statistics and Conventions.
Statistical analyses (except of frequency data) were performed by using systat (16). All t tests compared means of independent samples. Following ANOVAs that indicated significant difference (P < 0.05), multiple comparison tests were conducted (Tukey–Kramer, experiment wide α = 0.05). Contingency tests of frequency data employed the G-statistic (with Williams correction) (17). All values are given as mean ± SE.
The one LBG available to us for testing in pure form, 12-oxo-2β,3β-di-O-acetyl-2β,5β-11α-trihydroxybufalin (I), the principal LBG from P. pyralis (5), is abbreviated as LBG-I throughout the paper.
Fireflies (Photinus and Photuris).
The Photinus were of two species, P. ignitus and P. marginellus (Fig. 1 B and C). They were collected as adults at night in meadows in the vicinity of Ithaca, Tompkins County, New York. Most were taken in flight, detected by their flashes.
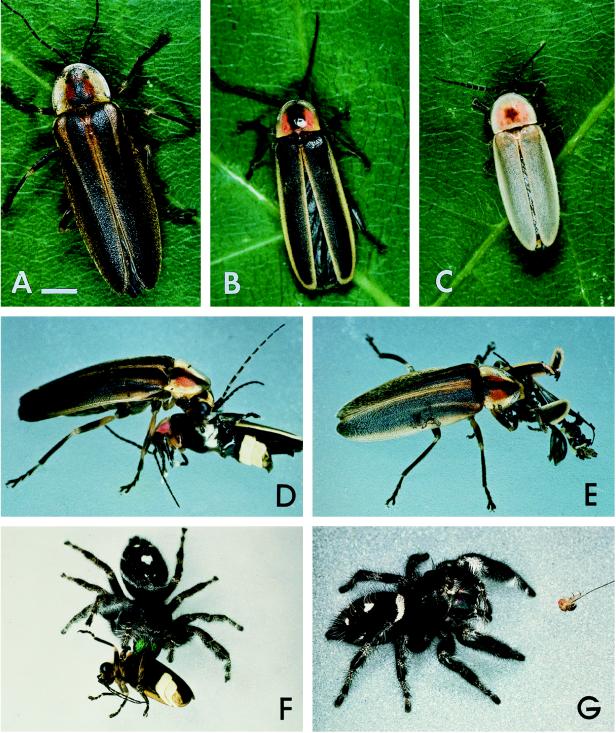
Figure 1.
(A) Photuris (versicolor complex). (B) P. ignitus. (C) P. marginellus. (D) Photuris female feeding on a P. ignitus male. (E) Comparable to preceding, later stage of meal. (F) P. audax eating a field-collected Photuris female. (G) P. regius being offered a freshly killed D. melanogaster. (A–C, Bar = 1 mm.)
Most of the Photuris also came from Ithaca. Field-collected adults (Fig. 1A) were taken at night at the same sites and times as the Photinus. Individuals designated as “reared” were collected as full-grown larvae on the ground in meadows in late summer and fall. Larvae emit a faint sporadic glow, by which they were spotted. During winter, larvae were kept in the dark at 10°C, except when taken to room temperature for a day, at 1- to 2-week intervals, to feed on freshly cut-up freshwater snails and/or earthworms. In midspring they were transferred to a 16-hr light/8-hr dark photocycle at 24°C, which caused them to pupate and to emerge in early summer, when they would ordinarily have been active, and when Photinus were available for capture.
A few Photuris were reared from larvae taken in Maryland (golf course near Bethesda, Montgomery County) and in Florida (shores of Lake Alice, Gainesville, Alachua County). On the advice of J. E. Lloyd (University of Florida, Gainesville), we are tentatively designating the Photuris from our three sources as P. versicolor, with the understanding that they may represent a complex of sibling species indistinguishable by current taxonomic criteria. We are therefore referring to Photuris throughout this paper by their generic designation. All experiments with Photuris, except one (specified in text), were done with Ithaca specimens.
Adult Photuris raised from larvae were used in experiments at varying ages (few days to over 2 weeks following adult emergence). They were maintained as adults individually in small Petri dishes (4.8-cm diameter) and given only water (soaked cotton wad).
Spiders (Phidippus).
Two congeneric species of jumping spider (family Salticidae) were used, Phidippus audax, collected in Ithaca and surrounding area, and Phidippus regius, taken at various sites in Florida. All were maintained individually in cylindrical plastic containers (8.0-cm diameter and height) and fed mostly houseflies. The tests with spiders were carried out in these containers.
Firefly Reflex Bleeding.
When disturbed, fireflies commonly emit droplets of blood, mostly from the elytra and pronotum (Fig. 2; ref. 18). Emission may occur from sites traumatized (for example, ruptured elytral veins) but may occur even from regions not apparently injured. The amount of blood emitted by such “reflex bleeding” may be considerable, and the behavior is generally regarded to be defensive (1, 19). We found both Photinus and Photuris to reflex-bleed readily, and to withstand such blood loss without ill effect (individuals checked days after being induced to reflex-bleed seemed normal). In conjunction with some of our experiments, Photuris females were induced to reflex-bleed to yield blood samples for LBG analysis.
Figure 2.
Photuris female, reflex-bleeding. The beetle had been picked up in forceps, causing it to emit a droplet of blood from the right elytron. Other fireflies, including species of Photinus, reflex-bleed similarly when disturbed.
Chemistry.
The assays for LBG, whether of firefly whole bodies or of Photuris blood samples, were based on our previously published procedures (4–6). In essence, following a preliminary hexane extraction of the samples to remove lipids, dichloromethane extracts were prepared, dried, and evaporated. The residue was subjected to HPLC analysis. LBG peaks, characterized by their ultraviolet absorption in the 300 nm region, were integrated to give the total LBG content per sample.
Photuris: Experimental Feedings.
Offerings of Photinus males to Photuris females were effected simply by introducing the former singly into Petri dishes housing the latter.
Feedings of LBG-I involved offering, to individual Photuris females, measured volumes of aqueous suspension of the chemical (6 μg/μl). The fluid was presented to the Photuris in glass microcapillary tubes, one end of which was held directly against the mouth of the firefly. The latter usually imbibed the fluid promptly, draining the full measure of liquid from the tube.
Tests with Phidippus.
These tests were of two types. One involved introducing single Photuris females or P. ignitus into the containers with the individual spiders and monitoring the results. The spiders were deprived of food for several days beforehand.
The second test was used to assess the feeding deterrency of LBG-I. In preliminary experimentation we had found that Phidippus can be enticed readily to take freshly killed fruit flies (Drosophila melanogaster killed by freezing), presented to them at close range, suspended from a human hair affixed to the end of a rod (Fig. 1G). Such flies, if untreated, are grasped by the spiders, ground up in the chelicers, sucked dry, and reduced to a small pellet of solid remains. Experimental items, in contrast, consisting of fruit flies treated topically by addition of a chemical deterrent, are rejected outright or partly consumed. We had used this assay previously for assessment of deterrency of a defensive acetylenic acid from cantharid beetles (20). For assessment of LBG-I, we applied the chemical to the fruit flies at different dosages in 1 μl vol of dichloromethane solution and after allowing for some seconds for solvent evaporation, offered the flies to the spiders. Fruit flies that were offered as controls were treated by addition of 1 μl dichloromethane.
RESULTS
LBG Content of Field-Collected Photuris and Photinus.
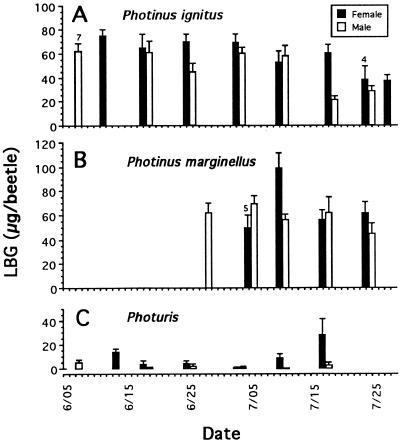
A total of 349 fireflies of both sexes (144 P. ignitus, 85 P. marginellus, 120 Photuris) was collected at intervals of several days over the course of 2 summer months in the Ithaca area and individually analyzed for total LBG content. The fireflies were frozen on the day of capture and stored frozen until analyzed. P. marginellus appears later in the season than the other two fireflies, accounting for the absence of samples of this species for most of June.
As is evident from the results (Fig. 3), the Photinus contained substantially more LBG than the Photuris. Both species of Photinus contained about equal amounts of the components (in the order of 60 μg per beetle), and there were no gender differences. Photuris contained on average less than 20 μg per beetle, and the females tended to contain substantially more than the males.
Figure 3.
Body LBG content of field-collected male and female fireflies, plotted as a function of date of collection (Ithaca, NY); n = 10 per column, except where otherwise indicated.
LBG Content of Photuris That Ate Photinus.
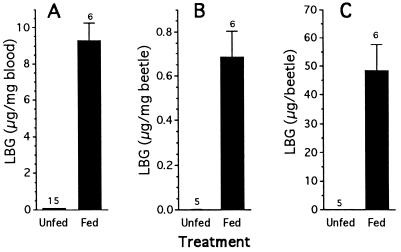
Six reared Photuris females were offered two P. ignitus males each, which they ate. The attacks proceeded quickly and resulted in most soft parts of the P. ignitus being consumed, so that only a tangle of cuticular fragments remained of the prey (Fig. 1 D and E). The interval between the two P. ignitus offerings was a few days at most.
Within the day after consumption of the second P. ignitus the Photuris were “bled.” They were individually seized in forceps until they gave off one or two blood droplets by reflex bleeding, and these droplets were picked up with glass microcapillary tubes, transferred to vials, weighed, and analyzed. The bled bodies of the Photuris, weighed before the bleeding, were also analyzed. The data obtained permitted calculation of the LBG concentration in the blood, the total LBG per beetle (= LBG in bled beetle, plus LBG in blood sample), and from the latter figure, the calculation of LBG concentration in the body.
As controls, 15 reared Photuris females (a mixed lot from our three geographic locations) that were kept unfed were also bled and the blood samples analyzed for LBG content. Five of these Photuris were treated like the experimentals and analyzed whole for LBG content after bleeding.
It is clear from the data (Fig. 4) that upon emergence from the pupa Photuris females are essentially LBG-free, and that by feeding on P. ignitus they acquire LBG. Moreover, they allocate the acquired chemical in high measure to the blood.
Figure 4.
LBG content of Photuris females that ate two P. ignitus males (n = 6) or were kept unfed (controls; n = 15). LBG values are expressed as concentration (in blood and body) and as whole body content. Only 5 of the 15 controls were analyzed for whole body content. In all three cases, differences were significant (t tests, P < 0.01).
LBG Content of Photuris That Ate LBG-I.
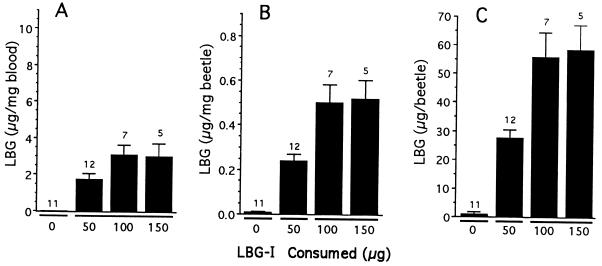
An experiment identical to the preceding was carried out with reared Photuris females that were fed LBG-I solution. Three sets of females (n = 12, 7, and 5) were given volumes of solution containing 50, 100, and 150 μg, respectively, of the chemical. Eleven control females were kept unfed. All females were bled, and both the blood and their bled bodies were analyzed for LBG content.
The results (Fig. 5) show that ingestion of LBG itself leads to LBG sequestration in the Photuris female, and that the female stores the chemical at high levels in the blood. They show further that there may be a limit to the amount of LBG that can be retained by Photuris: when fed 150 μg of LBG-I the female sequestered no more of the chemical than when it consumed 100 μg.
Figure 5.
LBG content of Photuris females, plotted as a function of LBG-I consumed. Data are presented as concentration and whole body content, as in Fig. 4. In all three cases, significant differences were detected [ANOVAs of log (x + 1) transformed data, P < 0.0001]. Within a plot, columns not sharing underlining are significantly different (experiment-wide α = 0.05).
Phidippus vs. Field-Collected P. ignitus.
A total of 41 P. ignitus (36 males and 5 females) were individually offered to P. audax and all were rejected. The spiders invariably attacked, but all dropped the prey, usually promptly after contact. Twenty-nine of the P. ignitus survived the attack and were alive when checked after 24 hr. P. ignitus is evidently protected against Phidippus.
Phidippus vs. Photinus-Fed Photuris.
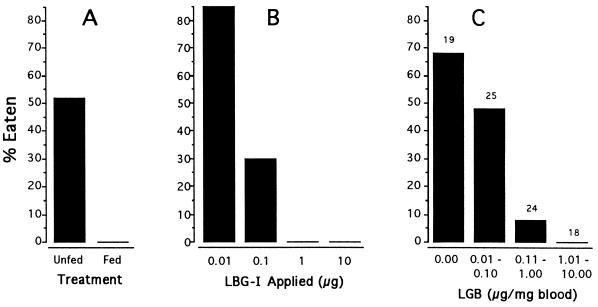
A total of 58 Photuris females were offered individually to P. audax. Half of these females had been fed two P. ignitus males each during previous days, whereas the other half had been kept unfed. While the spiders rejected each of the fed Photuris (28 of 29 were alive after 24 hr), they killed and partially consumed 15 of the controls (Fig. 6A).
Figure 6.
Predation tests with Phidippus spiders (A and C, P. audax; B, P. regius). (A) Acceptability of Photuris females, plotted as a function of whether or not they had eaten 2 P. ignitus males (n = 29 per category). (B) Acceptability of D. melanogaster, plotted as a function of quantity of LBG-I added topically (n = 20 per category). (C) Acceptability of field-collected female Photuris, plotted as a function of LBG concentration in blood. Acceptability was dependent upon treatment (A and B) or blood LBG content (C) (G-tests, in each case, P < 0.00001).
Because of limitation in numbers, we were unable to undertake this experiment with raised Photuris, and used instead field-collected individuals. Because field-collected Photuris females could be expected to contain some LBG even before they were experimentally fed on Photinus, we anticipated that the controls would not be fully acceptable, as turned out to be the case.
Phidippus vs. Fruit Flies Treated with LBG-I.
These tests were carried out with P. regius rather than P. audax. The spiders were offered 80 individual freshly killed D. melanogaster, in four categories of 20 each, treated by topical addition of 0.01, 0.1, 1, and 10 μg LBG-I, respectively. As is evident from the results (Fig. 6B), the chemical proved to be a deterrent at the higher two dosages: all flies in these categories were rejected uneaten. At the dosage of 0.1 μg, the chemical was partly effective and it proved essentially ineffective at the lowest dosage tested.
Phidippus vs. Field-Collected Photuris.
A total of 86 Photuris females were field-collected and individually induced to reflex bleed 1–4 days after capture, by being held in forceps. The blood they emitted was collected in microcapillary tubes, weighed, and analyzed for LBG content. For each individual female, a value was therefore obtained reflective of its systemic LBG load. The females were then individually offered to P. audax.
The results (Fig. 6C) confirmed that Photuris females in the field contain variable quantities of LBG (in Fig. 6, the females are grouped into four categories, according to their blood LBG level). More importantly, they showed that there is a correlation between the vulnerability of Photuris to predation and their LBG content. Photuris that contained 1–10 μg LBG per mg blood were all rejected live by the spiders, while a proportion of those containing lesser amounts were eaten (that is, killed and at least partially consumed). Of the total of 59 Photuris rejected in this experiment, 58 were alive when checked after 24 hr.
DISCUSSION
It seems established that the Photuris femme fatale obtains more than a nutritional boost by feeding on male Photinus. By luring and consuming such prey she is able to procure free-of-charge chemicals that she appears unable to produce on her own and that protect her against predation. While LBG have been shown to be a deterrent only to Phidippus and thrushes (4), there is no reason why the chemicals should not be effective against other predators as well. We have tentative data, for instance, that LBG deter ants, and that spiders besides Phidippus, including certain lycosids and orb-weavers, reject both Photinus and field-collected Photuris females. It would be interesting to test for the effect of LBG on predatory bats (21).
By reflex-bleeding when disturbed, Photuris females essentially externalize the very fluid that acts as a major, if not the major, carrier of the acquired LBG. The amount of blood that females emit reflexively is substantial. The quantities that we obtained experimentally for analysis from the individual females ranged from 0.22 to 4.40 mg (2.24 ± 0.12; n = 46 females), amounting on average to approximately 2% of female body mass. In females that ate two Photinus, or ingested 100 μg of LBG-I (the approximate equivalent of LBG in two Photinus), such masses of blood contain on average over 8 μg LBG, well in excess of the 1 μg proved to be absolutely deterrent to Phidippus.
Vulnerability of field-collected Photuris females to Phidippus proved to be a remarkably precise correlate of their LBG content. Females containing in the order of 1 to 10 μg LBG per mg blood, a concentration they could attain from ingestion of a single Photinus, were without exception rejected by the spiders. Females containing lesser amounts, as expected, proved less protected.
One wonders how females in the field come to possess LBG in quantities lower than expected from ingesting a single Photinus. One possibility is that they do not always thoroughly consume their prey, but this is contraindicated by what we observed in the laboratory (on the other hand, in the field, females might be subject to interruption while feeding). Another possibility is that they are often induced to reflex bleed. Most likely perhaps is that females void the acquired chemical over time, not by excretion, but with the eggs. As we will report elsewhere, Photuris females endow their eggs with LBG, presumably protecting them (and possibly the emergent larvae) as a result. It is therefore impossible to estimate from our data on LBG content of field-collected females, how many male Photinus female Photuris consume in the wild. In the laboratory we have observed individual females to eat as many as six Photinus over a period of days.
Unexplained so far is the finding that male Photuris in the field also contain LBG, albeit in lesser amounts than the female. We know from chemical analyses that males lack LBG upon emergence from the pupa, suggesting that they too acquire the compound from an exogenous source. Is that source Photinus? Although there is no direct evidence, Photuris males could possibly prey on Photinus males in flight. Predation by aerial “hawking” may be more widespread among fireflies than suspected, and may be practiced by Photuris females as well (14, 22, 23). Whatever the means by which male Photuris acquire LBG in nature, we know that they attack and ingest Photinus in the laboratory, and that they sequester LBG as a result. One could even imagine male Photuris transferring acquired LBG to the female at mating, for possible bestowal upon the eggs. Biparental endowment of eggs with defensive chemical is not without precedent in insects (24).
Photuris exemplifies a common strategy in nature, the procurement of ready-made defensive chemicals from an exogenous source. Insects, for instance, acquire defenses from plants or other insects (25), plants benefit from chemicals produced by microorganisms (26, 27), and we humans put countless natural products to medicinal use. But Photuris is nonetheless anomalous in that it procures its defenses by aggressive mimicry, from what are essentially close, chemically more gifted, phyletic relatives.
Acknowledgments
This paper is 148 in the series Defense Mechanisms of Arthropods; no. 147 is ref. 28. James E. Lloyd provided inspiration, guidance in the field, taxonomic help, much useful information, and comments on the manuscript. John B. Buck was most helpful with information on firefly rearing and collection. Karen Hicks, Kenneth R. Dodge, and Maria Eisner provided technical assistance. Several individuals helped collect fireflies and Phidippus, including Stephen Nowicki, the late Randall P. Grant, and W. Mitchell Masters. The study was supported by National Institutes of Health Grants AI02908, AI12020, and GM53830.
ABBREVIATION
- LBG
lucibufagin
References
- 1.Lloyd J E. Coleopt Bull. 1973;27:91–106. [Google Scholar]
- 2.Blum M S, Sannasi A. J Insect Physiol. 1974;20:451–460. [Google Scholar]
- 3.Sydow S L, Lloyd J E. Fla Entomol. 1975;58:312. [Google Scholar]
- 4.Eisner T, Wiemer D F, Haynes L W, Meinwald J. Proc Natl Acad Sci USA. 1978;75:905–908. doi: 10.1073/pnas.75.2.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Meinwald J, Wiemer D F, Eisner T. J Am Chem Soc. 1979;101:3055–3060. [Google Scholar]
- 6.Goetz M A, Wiemer D F, Haynes L W, Meinwald J, Eisner T. Helv Chim Acta. 1979;62:1396–1400. [Google Scholar]
- 7.Goetz M A, Meinwald J, Eisner T. Experientia. 1981;37:679–680. [Google Scholar]
- 8.Fieser L F, Fieser M. Natural Products Related to Phenanthrene. New York: Reinhold; 1949. pp. 561–564. [Google Scholar]
- 9.Budavari S. In: The Merck Index. Budavari S, O’Neil M J, Smith A, Heckelman P E, editors. Rahway, NJ: Merck; 1989. [Google Scholar]
- 10.Lloyd J E. Science. 1965;149:653–654. doi: 10.1126/science.149.3684.653. [DOI] [PubMed] [Google Scholar]
- 11.Lloyd J E. Science. 1975;187:452–453. doi: 10.1126/science.187.4175.452. [DOI] [PubMed] [Google Scholar]
- 12.Lloyd J E. Sci Am. 1981;245:139–145. [Google Scholar]
- 13.Lloyd J E. Oxf Surv Evol Biol. 1984;1:48–84. [Google Scholar]
- 14.Lloyd J E. Fla Entomol. 1984;67:368–376. [Google Scholar]
- 15.Lloyd J E. In: The Evolution of Mating Systems in Insects and Arachnids. Choe J C, Crespi B J, editors. Cambridge, U.K.: Cambridge Univ. Press; 1997. pp. 184–192. [Google Scholar]
- 16.Wilkinson L. SYSTAT: The System for Statistics. Evanston, IL: SYSTAT; 1989. [Google Scholar]
- 17.Sokal R R, Rohlf F J. Biometry: The Principles and Practice of Statistics in Biological Research. New York: Freeman; 1995. [Google Scholar]
- 18.Kloft W J, Lloyd J E, Bhatkar A P. Experientia. 1975;31:450. doi: 10.1007/BF02026373. [DOI] [PubMed] [Google Scholar]
- 19.Williams F X. J NY Entomol Soc. 1917;25:11–33. [Google Scholar]
- 20.Eisner T, Hill D, Goetz M, Jain S, Alsop D, Camazine S, Meinwald J. J Chem Ecol. 1981;7:1149–1158. doi: 10.1007/BF00987634. [DOI] [PubMed] [Google Scholar]
- 21.Lloyd J E. Coleopt Bull. 1989;43:83–91. [Google Scholar]
- 22.Lloyd J E. Science. 1980;210:669–671. doi: 10.1126/science.210.4470.669. [DOI] [PubMed] [Google Scholar]
- 23.Lloyd J E, Wing S R. Science. 1983;222:634–635. doi: 10.1126/science.222.4624.634. [DOI] [PubMed] [Google Scholar]
- 24.Eisner T, Meinwald J. In: Chemical Ecology. Eisner T, Meinwald J, editors. Washington, DC: Natl. Acad. Press; 1995. pp. 119–132. [Google Scholar]
- 25.Rosenthal G A, Berenbaum M R. Herbivores: Their Interactions with Secondary Plant Metabolites. New York: Academic; 1992. [Google Scholar]
- 26.Cooke R. The Biology of Symbiotic Fungi. New York: Wiley; 1977. [Google Scholar]
- 27.Pirozynski K A, Hawksworth D L. Coevolution of Fungi with Plants and Animals. New York: Academic; 1988. [Google Scholar]
- 28.Eisner T, Morgan RC, Attygalle AB, Smedley S R, Herath K B, Meinwald J. J.Exp. Biol. in press; 1997. [DOI] [PubMed] [Google Scholar]