Abstract
Natural ecosystems contain many individuals and species interacting with each other and with their abiotic environment. Such systems can be expected to exhibit complex dynamics in which small perturbations can be amplified to cause large changes. Here, we document the reorganization of an arid ecosystem that has occurred since the late 1970s. The density of woody shrubs increased 3-fold. Several previously common animal species went locally extinct, while other previously rare species increased. While these changes are symptomatic of desertification, they were not caused by livestock grazing or drought, the principal causes of historical desertification. The changes apparently were caused by a shift in regional climate: since 1977 winter precipitation throughout the region was substantially higher than average for this century. These changes illustrate the kinds of large, unexpected responses of complex natural ecosystems that can occur in response to both natural perturbations and human activities.
Ecosystems are composed of many individuals of multiple species of organisms which interact with each other and their abiotic environment to produce complex structures and dynamics. Some seemingly modest perturbations may be amplified to have large effects, whereas other equally large changes can be buffered so as to have little apparent impact. The highly variable effects on different ecosystems of certain native keystone and introduced exotic species are examples of such complex behavior (1–7). Similar amplified or damped changes in ecosystems should be expected to occur in response to small shifts in abiotic conditions, such as climate changes due to either natural causes or human activities. Here, we show how a coordinated syndrome of changes, symptomatic of desertification, has been triggered in a Chihuahuan Desert ecosystem by recent climate change.
Major changes have occurred in arid ecosystems throughout the world during recorded history (8). These have included diminished vegetative cover, reduced productivity, increased soil erosion, and invasion of exotics and loss of native species. Such desertification has usually been attributed primarily to human activities, especially to livestock grazing, and secondarily to changes in climate, especially prolonged and recurrent drought (9–14). The extensive degradation of arid grasslands to desert shrublands in southwestern North America within the last 125 years is consistent with this global pattern (9–17). Because water is the most limiting resource in arid ecosystems, changes in global and regional precipitation regimes, even of relatively small magnitude and short duration, may be expected to have substantial effects.
Here, we use data from our long-term research in southeastern Arizona (18–20) to document large changes which have occurred since the late 1970s in three components in a Chihuahuan Desert ecosystem: precipitation, vegetation, and animal populations. Our 20-hectare study site, at 1330 m elevation in the San Simon Valley approximately 7 km east of Portal, Arizona (Fig. 1), is near the transition between desert shrubland and arid grassland habitat. Historical records indicate that grassland vegetation covered the site prior to the 1880s, but large increases in woody vegetation and extensive soil erosion had occurred by the 1930s (21–23). When we initiated our study in 1977, the vegetation of the site consisted of widely scattered large shrubs of the genera Acacia, Ephedra, Flourensia, Prosopis, Mimosa, and Chilopsis, an admixture of small shrubs of the genera Gutierrezia, Haplopappus, and Zinnia, and large open areas covered primarily with the grass Tridens puchella. At that time we fenced the site to exclude domestic livestock, but cattle have continued to graze the immediately adjacent area. The climate of the region is characterized by bimodal precipitation, with nearly all of the rainfall concentrated in winter (November to March) and summer (July and August).
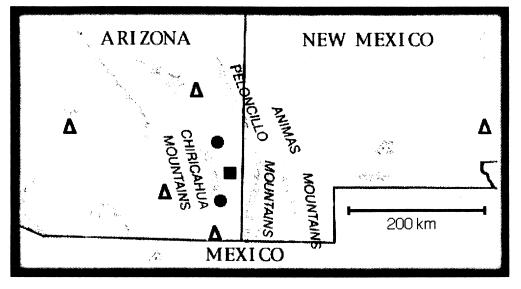
Figure 1.
Map of southeastern Arizona and southwestern New Mexico, showing the location of the long-term study site (▪), the two other sites where vegetation changes were measured (•), and the five weather stations from which the precipitation data were obtained (▵).
METHODS
Regional precipitation data were obtained from five weather stations surrounding the study area and within 250 km (Fig. 1; ref. 24). For each weather station, we compiled the total precipitation in winter (October to April) and summer (May to September) for each year since 1977, and we compared these values with the long-term averages. Vegetation changes were measured using chronosequences of aerial photographs of our experimental site and two other locations in the San Simon Valley. The experimental site was photographed in 1979 and 1995 by a private contractor, and in 1958 by the U.S. Forest Service (Coronado National Forest Archives, Albuquerque, NM). The two other grazed sites, 20 km northwest and 15 km southwest of the experimental site and at 1350 m and 1550 m elevation, respectively, were photographed by the U.S. Forest Service. On all photos for each location, permanent landmarks and a stratified random design were used to identify 50 × 50 m plots; we used six plots on each side of the livestock exclusion fence at the experimental site and eight plots on each of the two additional sites. Plots were located on relatively flat terrain, away from dry watercourses. We counted all shrubs >1.0 m in diameter (primarily Acacia, Ephedra, Flourensia, and Prosopis). Populations of rodents and ants have been monitored on the experimental site since 1977, with rodents censused monthly and ant colonies counted annually (18, 19).
RESULTS
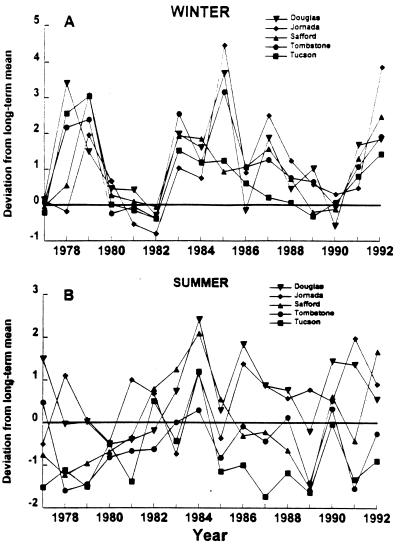
The recent climate of southeastern Arizona and southwestern New Mexico has changed substantially from earlier in the century (24). Since 1977 winter precipitation at all five weather stations was significantly greater than the long-term averages (Fig. 2A). Much higher than average winter rainfall was recorded at most stations in 1978–79, 1983–1988, and 1991–92. Several of these peaks correspond to the El Niño events of 1977–1978, 1982–1983, 1986–1987, and 1991–1992 (25, 26). Summer rainfall did not deviate consistently from the long-term averages (Fig. 2B).
Figure 2.
Yearly differences (measured in standard deviations) in precipitation from long-term averages (set equal to zero) for winter (A) and summer (B) seasons. Data were analyzed from 1976 back to the following years: 1903 (Douglas, AZ), 1914 (Jornada, NM), 1898 (Safford, AZ), 1889 (Tombstone, AZ), and 1867 (Tucson, AZ). At all stations, 1977–1992 winter precipitation was significantly higher than prior years (Mann–Whitney U tests): Douglas, P = 0.008; Jornada, P = 0.003; Safford, P = 0.001; Tombstone, P = 0.001; Tucson, P = 0.005. In the years 1977–1992 summer precipitation was: significantly higher than prior years at Douglas, P = 0.014, and Jornada, P = 0.020; significantly lower than prior years at Tucson, P = 0.001; and not significantly different from prior years at Safford, P = 0.980, and Tombstone, P = 0.610.
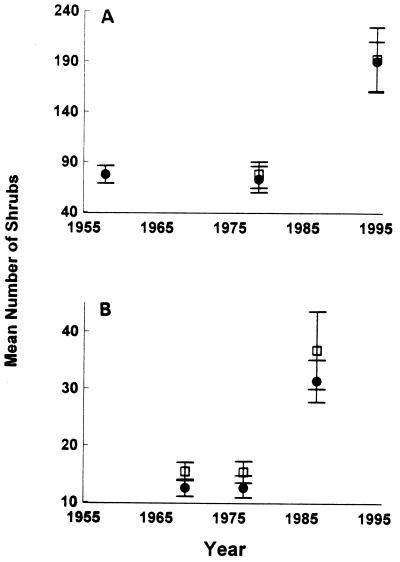
Large increases in shrub cover occurred during this period of increased winter precipitation. At all three sites, aerial photographs revealed no significant change in shrub density for several decades prior to the late 1970s, but a 3-fold increase after 1980 (Fig. 3). Earlier aerial photos and landscape paintings show no detectable change in woody vegetation from the 1930s through the mid 1970s. Increases since the 1970s include not only greater shrub numbers (Fig. 3) but also comparable increases in total cover of large woody shrubs at all sites (P < 0.001).
Figure 3.
Vegetation changes at three sites over time. (A) Mean shrub number (±1 SD) on 0.25-hectare plots inside (□) and outside (•) the livestock fence at the experimental site. Ungrazed and grazed plots did not differ significantly in shrub density (repeated measures ANOVA, F1, 10 = 0.13, P = 0.73), but both had significantly more shrubs in 1995 compared with 1977 (repeated measures ANOVA, F1, 10 = 172.9, P < 0.001). (B) Mean shrub number (±1 SD) on 0.25-hectare plots at two additional grazed sites. Each site had significantly more shrubs in 1987 compared with earlier years (repeated measures ANOVA, NW site (•): F2, 21 = 151.3, P < 0.001; SW site (□): F2, 21 = 71.5, P < 0.001).
In this and other regions of grassland–shrubland transition, increases in shrub cover are generally thought to be symptomatic of desertification caused by a combination of livestock grazing and drought (9–14). These factors likely contributed to the degradation of the study area and surrounding habitat from grassland to shrubland between the 1870s and 1930s. The changes in vegetation since the 1970s cannot be attributed to grazing, however; the increases in shrubs were virtually identical on our experimental site, fenced to exclude livestock since 1977, and on immediately adjacent grazed areas (Fig. 3). And the vegetation changes were associated with an altered climatic regime that consisted not of drought, but of increased winter precipitation. This supports Neilson’s (27) observation that episodes of shrub increases in the Chihuahuan Desert region earlier in this century occurred during periods of unusually high winter precipitation, which favored the establishment of cool-season-active C3 woody shrubs at the expense of warm-season-active C4 grasses (28–30).
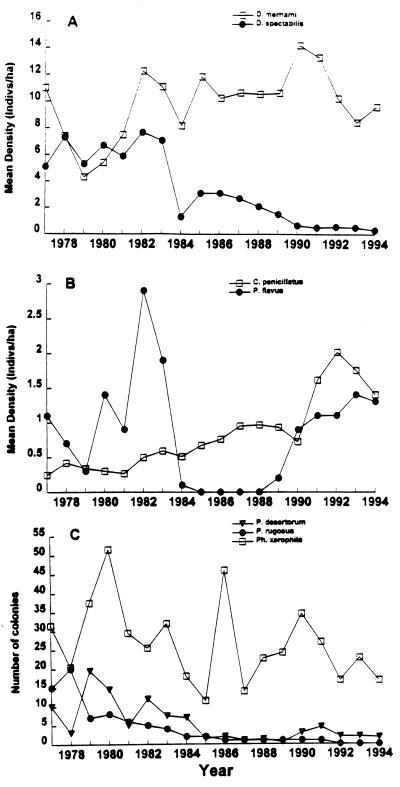
Coinciding with these recent shifts in climate and vegetation, major changes have occurred in population dynamics and community composition of animals on the experimental study site (Fig. 4). Several previously abundant species went locally extinct, at least one previously absent species colonized, some rare species increased, and other species persisted with little change. The species that decreased included the keystone kangaroo rat, Dipodomys spectabilis, another seed-eating rodent, Perognathus flavus, and two seed-harvesting ants, Pogonomyrmex rugosus and Pogonomyrmex desertorum. D. spectabilis and Perognathus flavus were the second and third most abundant rodent species, respectively, prior to 1984. Pogonomyrmex desertorum was the second most abundant harvester ant prior to 1983. Pogonomyrmex rugosus could have been considered the dominant harvester ant, because its colonies contained extremely large numbers of workers which were highly aggressive toward other ant species. These rodent and ant species are characteristic of open arid grassland or desert habitat, and they store seeds in large subterranean larders (23). Their precipitous declines can be attributed to some combination of the vegetation and climatic changes. That the declining species are most abundant in habitats with sparse shrub cover suggests that the three-fold increases in woody vegetation represented a decline in habitat quality. That all four species are larder hoarders suggests that wetting of seed stores may have been a contributing factor (23, 31). Populations of some seed-eating animals characteristic of shrubbier habitats, such as the rodent Chaetodipus penicillatus (32), increased substantially (Fig. 4). Another rodent, Chaetodipus bayleii, also typical of desert shrub habitats but never previously recorded on the site, colonized in 1995 and has been consistently present ever since. These increases can probably be attributed to some combination of greater shrub cover facilitating predator avoidance and higher food availability due to declines in competing species. Populations of other animal species typical of desert shrub habitats, including the rodent Dipodomys merriami and the ant Pheidole xerophila, remained essentially unchanged (Fig. 4).
Figure 4.
Population trends of seed-eating rodents and ants since 1977. (A) Density (individuals per hectare) of the kangaroo rats Dipodomys spectabilis and Dipodomys merriami. (B) Density of the pocket mice Perognathus flavus and Chaetodipus penicillatus. (C) Number of colonies of the seed-harvester ants Pogonomyrmex desertorum and Pheidole xerophila (colonies per 0.25-hectare plot) and Pogonomyrmex rugosus (total colonies on the study site).
DISCUSSION
Additional evidence indicates that pervasive changes have occurred in the entire regional ecosystem since the late 1970s. During the 1980s, Aphaenogaster cockerellii, another large harvester ant that stores seeds in subterranean larders, went locally extinct at another site in the San Simon Valley where it had previously been abundant (23). Horned lizards (Phrynosoma cornutum and Phrynosoma modestum), abundant on our experimental site in the late 1970s (33), have become extremely rare. Horned lizards are specialized predators on the large harvester ants that disappeared. Populations of the burrowing owl, Athene cunicularia, in this region “collapsed in the 1980’s” (34). Mojave rattlesnakes (Crotalus scutulatus) decreased to an estimated 15–25% of their original numbers between the late 1960s and late 1980s (35). The near extinction of the keystone rodent, D. spectabilis, probably accounts for declines of the owls and rattlesnakes (36): both live in kangaroo rat burrows, and the rattlesnakes feed on these rodents. Thus, changes in the entire ecosystem and in populations of many plants and animals can be attributed to the new climatic regime affecting the organisms either directly, through increased winter moisture, or indirectly, through altered biotic interactions. Despite dramatic changes in species composition, however, overall species diversity has remained virtually unchanged; declines and extinctions in some species have been offset by increases and colonizations of others (37).
We do not claim that the recent increases in cool-season precipitation caused a reorganization symptomatic of desertification in all arid ecosystems in the region. Although the increases in winter precipitation since 1977 occurred over much of southwestern North America, the dramatic increases in shrub cover documented above were more localized. No detectable shrub increase occurred in large areas of arid grassland habitat, some within 20 km of our study site. Many factors, including climate, soil, fire, herbivory, and existing vegetation, influence the boundary between arid grassland and desert shrubland (9–14, 38–41). Causes of shrub increases may include counterintuitive mechanisms, such as increases in precipitation during critical seasons, as well as commonly accepted factors, such as livestock grazing and drought. These are the kinds of responses to perturbations that are likely to occur in a complex dynamic system. While in some cases the ecological interactions may amplify the effects of a perturbation, causing wholesale reorganization, in other cases, the complex relationships may lead to negative feedbacks, resulting in little change.
The changes in this arid ecosystem may be related to recent ecological changes documented elsewhere in the world. Shifts in ocean and intertidal ecosystems off the California coast have been linked to changes in oceanographic circulation and El Niño events (42–44), which were also associated with increased winter precipitation in southwestern North America (26). Changes in species composition of tropical forests in several locations around the world have been tentatively attributed to increased atmospheric CO2 concentrations and temperature (44). All of these changes are consistent with the hypothesis that global climate has been altered due to human influences (46, 47).
Possible effects of global climate change on populations and ecosystems have thus far been addressed largely by laboratory experiments or computer simulation models (refs. 48–52, but see refs. 53 and 54). These simplified systems cannot be expected to contain the diversity of interactions and hence to exhibit the range of complex responses that are inherent in entire ecosystems. Further, because of their complicated networks of interactions, it will be difficult to predict how ecosystems will respond to such perturbations. In the present case, a modest shift in the seasonal pattern of precipitation since the late 1970s led to multiple and unexpected changes in an arid ecosystem. An increase in winter rainfall triggered the kinds of changes in vegetation and animal populations that would usually be expected to occur as a result of increased aridity or anthropogenic desertification. Due largely to the effects on keystone species and other biotic relationships, the changes in climate were amplified by the network of interactions to alter the structure and dynamics of the entire ecosystem. These results suggest that comparably large and unexpected changes may occur in other ecosystems throughout the world in response to both natural fluctuations and human activities.
Acknowledgments
We thank all who have contributed to the Portal Project over the years. The research was supported by the National Science Foundation (most recent Grant DEB-9221238) and by a grant from California State University, Northridge.
References
- 1.Paine R T. Am Nat. 1966;100:65–75. [Google Scholar]
- 2.Paine R T. Oecologia. 1974;15:93–120. doi: 10.1007/BF00345739. [DOI] [PubMed] [Google Scholar]
- 3.Drake J A, Mooney H A, di Castri F, Groves R H, Kruger F J, Rejmanek M, Williamson M, editors. Biological Invasions. New York: Wiley; 1989. [Google Scholar]
- 4.Hengeveld R. Dynamics of Biological Invasions. Cambridge, U.K.: Cambridge Univ. Press; 1989. [Google Scholar]
- 5.Brown J H, Heske E J. Science. 1990;250:1705–1707. doi: 10.1126/science.250.4988.1705. [DOI] [PubMed] [Google Scholar]
- 6.Jones C G, Lawton J H. Linking Species and Ecosystems. London: Chapman and Hall; 1994. [Google Scholar]
- 7.Menge B A. Ecol Monogr. 1995;65:21–74. [Google Scholar]
- 8.Mabbutt J A, Floret C, editors. Case Studies on Desertification. Scientific and Cultural Organization, Paris: United Nations Educational; 1980. [Google Scholar]
- 9.Glendening G E. Ecology. 1952;33:319–328. [Google Scholar]
- 10.Buffington L D, Herbel C H. Ecol Monogr. 1965;35:139–164. [Google Scholar]
- 11.Madany M H, West N E. Ecology. 1983;64:661–667. [Google Scholar]
- 12.Schlesinger W H, Reynolds J F, Cunningham G L, Huenneke F A, Jarrell W M, Virginia R A, Whitford W G. Science. 1990;247:1043–1048. doi: 10.1126/science.247.4946.1043. [DOI] [PubMed] [Google Scholar]
- 13.Dick-Peddie W A. New Mexico, Vegetation Past, Present, and Future. Albuquerque: Univ. of New Mexico Press; 1993. [Google Scholar]
- 14.Archer S. In: Ecological Implications of Livestock Herbivory in the West. Vavre M, Laycock W, Pieper R, editors. Denver: Society of Range Management; 1994. pp. 13–68. [Google Scholar]
- 15.Hastings J R, Turner R M. The Changing Mile. Tucson: Univ. of Arizona Press; 1965. [Google Scholar]
- 16.Humphrey R R. 90 Years and 535 Miles: Vegetation Changes along the Mexican Border. Albuquerque: Univ. of New Mexico Press; 1987. [Google Scholar]
- 17.Bahre C J. A Legacy of Change: Historic Human Impact on Vegetation of the Arizona Borderlands. Tucson: Univ. of Arizona Press; 1991. [Google Scholar]
- 18.Brown J H, Munger J C. Ecology. 1985;66:1545–1563. [Google Scholar]
- 19.Brown J H, Davidson D W, Munger J C, Inouye R S. In: Community Ecology. Diamond J A, Case T J, editors. New York: Harper and Row; 1986. pp. 41–61. [Google Scholar]
- 20.Valone T J, Brown J H. In: Long-term Studies of Vertebrate Communities. Cody M L, Smallwood J A, editors. Orlando, FL: Academic; 1996. pp. 555–583. [Google Scholar]
- 21.Chew R M, Chew A E. Ecol Monogr. 1965;35:355–375. [Google Scholar]
- 22.Kelt D A, Valone T J. Oecologia. 1995;103:191–195. doi: 10.1007/BF00329079. [DOI] [PubMed] [Google Scholar]
- 23.Chew R M. Am Midl Nat. 1995;134:75–83. [Google Scholar]
- 24.Karl T R, Williams C N, Jr, Quinlan F T, Boden T A. HCN Serial Temperature and Precipitation Data. Carbon Dioxide Information Analysis Center, Oak Ridge, TN: HCN Environmental Sciences Division; 1990. , Publication 3404. [Google Scholar]
- 25.Betancourt J L. In: Shrubland Ecosystem Dynamics in a Changing Environment. Barrow J R, McArthur E D, Sosebee R E, Tausch R J, editors. Forest Service, U.S. Dept. of Agriculture, Ogden, UT: Intermountain Research Station; 1996. pp. 5–9. [Google Scholar]
- 26.Molles M C, Dahm C N. J North Am Benth Soc. 1990;9:68–76. [Google Scholar]
- 27.Neilson R P. Science. 1986;232:27–34. doi: 10.1126/science.232.4746.27. [DOI] [PubMed] [Google Scholar]
- 28.Cable D R. Ecology. 1975;56:981–986. [Google Scholar]
- 29.Kemp P R. J Ecol. 1983;71:427–436. [Google Scholar]
- 30.Bahre C J, Shelton M L. J Biogeog. 1993;20:489–504. [Google Scholar]
- 31.Valone T J, Brown J H, Jacobi C. J Mammal. 1995;76:428–436. [Google Scholar]
- 32.Hoffmeister D F. Mammals of Arizona. Tucson: Univ. of Arizona Press and the Arizona Game and Fish Department; 1986. [Google Scholar]
- 33.Munger J C. Oecologia. 1984;62:251–260. doi: 10.1007/BF00384267. [DOI] [PubMed] [Google Scholar]
- 34.Taylor R C. Location Checklist to the Birds of the Chiricauhua Mountains. Tucson, AZ: Borderlands Productions; 1993. [Google Scholar]
- 35.Mendelson J R, III, Jennings W B. J Herpetol. 1992;26:38–45. [Google Scholar]
- 36.Hawkins L K, Nicoletto P F. J Arid Envir. 1992;23:199–208. [Google Scholar]
- 37.Valone T J, Brown J H. Science. 1995;267:880–883. doi: 10.1126/science.7846530. [DOI] [PubMed] [Google Scholar]
- 38.Goldberg D E, Turner R M. Ecology. 1986;67:695–712. [Google Scholar]
- 39.Turner R M. Ecology. 1990;71:464–477. [Google Scholar]
- 40.Bush J K, Van Auken O W. Ecology. 1995;76:1603–1609. [Google Scholar]
- 41.McAuliffe J R. In: The Desert Grassland. McClaran M, editor. Tucson: Univ. of Arizona Press; 1995. [Google Scholar]
- 42.Roemmich D, McGowan J. Science. 1995;267:1324–1326. doi: 10.1126/science.267.5202.1324. [DOI] [PubMed] [Google Scholar]
- 43.Barry J P, Baxter C H, Sagarin R D, Gilman S E. Science. 1995;267:672–675. doi: 10.1126/science.267.5198.672. [DOI] [PubMed] [Google Scholar]
- 44.Holbrook, S. J., Schmitt, R. J. & Stephens, J. S. (199?) Ecological Applications, in press.
- 45.Phillips O L, Gentry A H. Science. 1994;263:954–958. doi: 10.1126/science.263.5149.954. [DOI] [PubMed] [Google Scholar]
- 46.Vitousek P M. Ecology. 1994;75:1861–1876. [Google Scholar]
- 47.Houghton H T, Meira Filho L G, Bruce J, Lee H, Callander B A, Haites E, Harris N, Mashell K, editors. Climate Change 1994. Cambridge, U.K.: Cambridge Univ. Press; 1995. [Google Scholar]
- 48.Kareiva P M, Kingsolver J G, Huey R, editors. Biotic Interactions and Global Change. Sunderland, MA: Sinauer; 1993. [Google Scholar]
- 49.Naeem S, Thompson L J, Lawler J L, Lawton J H, Woodfin R M. Nature (London) 1994;368:734–737. [Google Scholar]
- 50.Mayeux H S, Johnson H B, Polley H W. In: Noxious Range Weeds. James L F, Evans J O, Ralphs M H, Childs R D, editors. Boulder, CO: Westview; 1991. pp. 62–74. [Google Scholar]
- 51.Polley H W, Johnson H B, Mayeux H S. Ecology. 1994;75:976–988. [Google Scholar]
- 52.Archer S, Schimel D S, Holland E A. Climate Change. 1995;29:91–99. [Google Scholar]
- 53.Parmesan C. Nature (London) 1996;382:765–766. [Google Scholar]
- 54.Mitchell, W. A., Curtin, C. G. & Porter, W. P. (1997) Oikos, in press.