Abstract
Data on current practices for management of renal replacement therapy in acute kidney injury (AKI) are limited, particularly with regard to the dosing of therapy. We conducted a survey of practitioners at the 27 study sites participating in the VA/NIH Acute Renal Trial Network (ATN) Study prior to initiation of subject enrollment to ascertain the local prevailing practices for management of renal replacement therapy in critically ill patients with AKI. Surveys were returned from 130 practitioners at 26 of 27 study sites; the remaining study site provided aggregate data. Intermittent hemodialysis and CRRT were the most commonly utilized modalities of renal replacement therapy, with SLED and other “hybrid” treatments used in fewer than 10% of patients. Intermittent hemodialysis was most commonly provided on a thrice weekly or every-other day schedule, with only infrequent assessment of the delivered dose of therapy. Most practitioners reported that they did not dose CRRT based on patient weight. The average prescribed dose of therapy corresponded to a weight-based dose of no more than 20 to 25 mL/kg/hr. These results provide insight into clinical management of renal replacement therapy and provide normative data for evaluation of the design of ongoing clinical trials.
Keywords: Acute kidney injury, Renal replacement therapy, Hemodialysis, Hemofiltration, CRRT, SLED
Data on current practices for management of renal replacement therapy in acute kidney injury (AKI) are limited, particularly with regard to the dosing of therapy. Surveys of nephrologists conducted in the United States in 1995 (1) and in Canada in 1999 (2) focused primarily on modality of renal replacement therapy utilized and did not address the prescribed or delivered dose of therapy. Similarly, observational studies such as the PICARD study provide data related to treatment modality, but not treatment dose (3). A survey, conducted in 2004 at an international course on critical care nephrology, evaluated dosing practices for renal replacement therapy, but only in patients with sepsis-associated AKI (4). Thus, although several recent clinical trials have suggested that more intensive regimens for the management of intermittent hemodialysis (IHD) (5) and continuous renal replacement therapy (CRRT) (6) are associated with improved survival, the impact of these studies on clinical practice is unclear.
The VA/NIH Acute Renal Failure Trial Network (ATN) Study is a multicenter randomized controlled trial comparing an intensive management strategy for renal replacement therapy in critically ill patients with AKI to a less intensive (“conventional”) dosing strategy (7). However, in the absence of robust data regarding clinical practice patterns, the relationship between the study’s protocol-driven treatment arms and usual care is uncertain. For this reason, a survey of practitioners at the 27 medical centers and university-affiliated VA hospitals participating as ATN Study sites was conducted prior to initiation of subject enrollment to ascertain the local prevailing practices for management of renal replacement therapy in critically ill patients with AKI.
Methods
Survey Methods
The survey was sent from the ATN Study Chairman’s Office to the site investigators at the 27 original sites participating in the ATN Study (Ann Arbor VA, Buffalo VA, Dallas VA, Houston VA, Indianapolis VA, Little Rock VA, West Los Angeles VA, Miami VA, Nashville VA, New Orleans VA, Pittsburgh VA, Portland VA, Richmond VA, San Diego VA, San Francisco VA, San Juan VA, Seattle VA, West Haven VA, Cleveland Clinic Foundation, Johns Hopkins University, Massachusetts General Hospital, University of Miami, University of California at San Francisco, University of Pittsburgh, University of Texas at Houston, Wake Forest University, Washington University at St. Louis) in October 2003, prior to initiation of study enrollment in November 2003. The site investigators at the 27 study sites were asked to distribute the survey to all practitioners responsible for prescribing renal replacement therapy at their site. Site investigators returned surveys to the ATN Study Chairman’s office at the VA Pittsburgh Healthcare System where the data was analyzed.
The survey was approved by the IRB at the VA Pittsburgh Healthcare System as a review preparatory to research. As data was collected with no information regarding the identity of individual practitioners other than specialty (nephrologist or intensivist) and study site, the analysis and reporting of data was approved by the IRB at the VA Pittsburgh Healthcare System as exempt research.
Survey Design
The survey consisted of 26 questions. (A copy of the survey is available as supplemental material at cjasn.asnjournals.org). Demographic questions asked the practitioner to identify his/her study site, specialty (nephrologists or intensivist) and estimated number of critically ill patients with AKI treated with renal replacement therapy per month. Seven questions related to the prescription of IHD, including estimation of his/her relative frequency of use of IHD as the modality of renal replacement therapy in critically ill patients with AKI and the frequency of his/her use of treatment schedules ranging from twice weekly to daily; specification of his/her usual prescribed blood flow rate, duration of treatment, and target dose of therapy (URR or Kt/Vurea); and whether and how frequently he/she assessed delivery of hemodialysis dose. A similar set of questions was asked about the use of sustained low-efficiency dialysis (SLED) and other forms of extended duration hemodialysis. Nine questions focused on the prescription of CRRT, including estimation of his/her relative frequency of use of CRRT as the modality of renal replacement therapy in critically ill patients with AKI; specification of the modalities of CRRT that he/she used (vascular access: arteriovenous or venovenous; modality: hemofiltration, hemodialysis, or hemodiafiltration); specification of his/her usual prescribed blood flow rate; characterization of whether he/she prescribed CRRT on the basis of patient weight and what his/her usual prescribed effluent flow rate (sum of dialysate flow rate and ultrafiltration rate) was (as mL/hr or mL/kg/hr); and specification of the composition of fluids he/she used as replacement fluid and dialysate.
Statistical Analysis
Aggregated data is summarized using descriptive statistics. All data was analyzed at the practitioner level unless otherwise noted. The relative frequency of utilization of each modality of RRT at the patient level and the frequency with which different treatment schedules were used in the management of IHD and SLED was calculated from the total number of patients treated per month and the percentage utilization of each modality of RRT as reported by each practitioner. Data is presented as mean ± standard deviation (SD), median and interquartile range (IQR), or as the percentage of patients treated, as appropriate.
Results
Survey Response Rate (Table 1)
Table 1.
Modality of Renal Replacement Therapy
| Site | Practitioners responding N | Patients per Month with AKI Median (IQR) | Modality of RRT |
|||||
|---|---|---|---|---|---|---|---|---|
| Percentage of Practitioners who Use | Percentage of Patients Treated | |||||||
| IHD | CRRT | SLED | IHD | CRRT | SLED | |||
| VA Sites | ||||||||
| A | 5 | 3.5 | 100% | 100% | - | 31.8% | 68.2% | - |
| B | 2 | 2.8 | 100% | 100% | - | 25$ | 75% | - |
| C | 3 | 10 | 100% | 100% | - | 48.2% | 51.8% | - |
| D | 1 | 5 | 100% | - | - | 100% | - | - |
| E | 4 | 5 | 100% | 100% | - | 67.2% | 37.8% | - |
| F | 7 | 17.5 | 100% | 43% | 100% | 73% | 2.4% | 24.6% |
| G | 3 | 3.5 | 100% | - | - | 100% | - | - |
| H | 8 | 4.5 | 87.5% | 100% | 37.5% | 39.3% | 56.2 | 4.5% |
| I | 4 | 4 | 100% | 100% | - | 65.4% | 34.6% | - |
| J | 1 | 2 | 100% | - | 100% | 70% | - | 30% |
| K | 6 | 5.5 | 100% | 100% | 16.7% | 56.8% | 38.3% | 4.9% |
| L | 3 | 7.5 | 100% | 100% | - | 80.2% | 19.7% | - |
| M | 4 | 4.5 | 100% | 75% | - | 79.5% | 20.5% | - |
| N | 5 | 1 | 100% | 20% | 60% | 85.5% | 1.7% | 12.8% |
| O | 3 | 4 | 100% | - | - | 100% | - | - |
| P | 5 | 2 | 100% | 100% | - | 93.9% | 6.1% | - |
| All VA | 64 | 5(2–9) | 98.4% | 73.4% | 23.4% | 63.7% | 28.5% | 7.8% |
| Non-VA Sites | ||||||||
| Q | * | 30 | 100% | 100% | - | 40% | 60% | - |
| R | 4 | 35 | 100% | 100% | - | 36.4% | 63.6% | - |
| S | 6 | 2.8 | 100% | 83.3% | - | 18.3% | 81.7% | - |
| T | 7 | 10 | 100% | 100% | 28.6% | 32.9% | 65.8% | 1.4% |
| U | 13 | 25 | 100% | 100% | 100% | 60% | 20% | 20% |
| V | 5 | 5 | 100% | 100% | - | 46.5% | 53.5% | - |
| W | 11 | 12 | 100% | 100% | - | 74.1% | 25.8% | - |
| All Non-VA | 46 | 15 (5–25) | 100% | 97.8% | 15.2% | 54% | 36.8% | 9.1% |
| Combined VA/Non-VA Sites | ||||||||
| X | 8 | 10 | 87.5% | 100% | 12.5% | 40.7% | 59.2% | 0.1% |
| Y | 12 | 8.8 | 100% | 100% | 8.3% | 65% | 34% | 1% |
| All Combined | 20 | 10 (4.2–20) | 95% | 100% | 10% | 56% | 43.3% | 0.6% |
| All Sites | 130 | 7.5 (3.5–20) | 98.5% | 86.2% | 24.6% | 57% | 35.7% | 7.3% |
Site provided aggregate data for all practitioners
AKI: acute kidney injury; IQR: interquartile range; IHD: intermittent Hemodialysis; CRRT: continuous renal replacement therapy; SLED: sustained low-efficiency dialysis and other forms of hybrid therapy; VA Sites: Department of Veterans Affairs medical centers; Non-VA Sites: University-affiliated non-VA medical centers
Completed surveys were returned from all 27 study sites. One site provided aggregate data for all providers at that site; 130 surveys were returned from the remaining 26 sites. Eighty-four surveys were completed by providers at the 18 participating Department of Veterans Affairs (VA) medical centers and 66 surveys by providers at the 9 non-VA university study sites. Twenty practitioners provided care at both VA and university sites. There were a median of 5 respondents per site (IQR: 3–7) with a median of 4 respondents (IQR: 3–5.8) at the VA sites and 7.5 respondents (IQR: 5.8–11.2) at the university sites. Respondents reported treating a median of 7.5 critically ill patients with AKI each month (IQR: 3.5–20) with a median of 5 patients per month (IQR: 2–9) at the VA sites and 15 patients per month (IQR: 5–25) at the university sites. The 20 practitioners providing care at both VA and university sites reported treating a median of 10 patients per month (IQR: 4.2–20). Only one respondent identified themselves as an intensivist, two did not specify a specialty while all the remaining respondents identified themselves as nephrologists
Modality of Renal Replacement Therapy Used (Table 1)
All but two providers responded that they utilized IHD in the management of renal replacement therapy for critically ill patients with AKI. 112 providers (86.2%) responded that they used some form of CRRT in the management of critically ill patients with AKI while 32 providers (24.6%) reported using SLED. Eleven providers (8.5%) reported using only IHD; one (0.8%) reported using only CRRT; and 25 (19.2%) reported using all three modalities of renal support. While only one respondent at a non-VA site did not utilize CRRT, 17 respondents at 7 VA sites indicated they did not use these modalities. Adjusting for the number of patients that each provider reported treating, the relative frequency of utilization of each of the modalities of renal replacement therapy was 57% intermittent hemodialysis, 35.7% CRRT and 7.3% SLED.
Intermittent Hemodialysis (Table 2)
Table 2.
Management of Intermittent Hemodialysis
| Site | Respondents Using IHD | Treatment Frequency |
Median Treatment Duration (hours) | Median BFR (ml/min) | Monitoring of Delivered Dose (# practitioner) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 2/week | 3/week | QOD | 4/week | 5/week | 6/week | 7/week | |||||
| VA Sites | |||||||||||
| A | 5 | - | 47.8% | - | 38.1% | 14.1% | - | - | 4 | 400 | 5/5 |
| B | 2 | - | 15.9% | 25.5% | 31.8% | 26.8% | - | - | 4 | 350 | - |
| C | 3 | - | 75.7% | 0.5% | 23.8% | - | - | - | 4 | 300 | - |
| D | 1 | - | 75% | - | 13% | 12% | - | - | 3.5 | 300 | - |
| E | 4 | - | 69.1% | - | 12.7% | 18.2% | - | - | 4 | 310 | 1/4 |
| F | 7 | 1.9% | 65.4% | 0.3% | 29.9% | 1.6% | 0.9% | - | 4 | 400 | 1/7 |
| G | 3 | 3.1% | 60.6% | - | 24.4% | 11.9% | - | - | 4 | 300 | - |
| H | 7 | - | 45.9% | 5.4% | 22.5% | 16.2% | 6.9% | 3.0% | 4 | 300 | 1/7 |
| I | 4 | - | 40.6% | - | 29.5% | 18.9% | 6.0% | 5.0% | 4 | 350 | - |
| J | 1 | - | 69% | - | 15% | 10% | 5% | 1% | 4 | 350 | - |
| K | 6 | - | 13.3% | 14.8% | 26.7% | 29.2% | 16.1% | - | 3.25 | 300 | 2/6 |
| L | 3 | - | 49.5% | 7.8% | 24.5% | 7.3% | 5.5% | 5.4% | 3 | 350 | - |
| M | 4 | - | 39.4% | 38% | 16.9% | - | - | 10.6% | 3.5 | 360 | - |
| N | 5 | - | 18.2% | 18.1% | 36.8% | 13.3% | 6.6% | 7.1% | 4 | 300 | - |
| O | 3 | - | 37.5% | - | 22.5% | 35% | 38% | 1.2% | 3.5 | 325 | - |
| P | 5 | - | 90% | - | - | - | 10% | - | 4 | 350 | - |
| All VA | 63 | 0.7% | 51.4% | 5.7% | 25.7% | 10.9% | 3.7% | 2.0% | 4 | 350 | 10/63 |
| Non-VA Sites | |||||||||||
| Q | * | - | 88.9% | 11.1% | - | - | - | - | 4 | 350 | * |
| R | 4 | - | 56.9% | 15.7% | 13.7% | 6.7% | 1.2% | 5.9% | 3 | 325 | - |
| S | 6 | - | 73.8% | 2.9% | 23.1% | 18.4% | - | - | 4 | 350 | 5/6 |
| T | 7 | - | 23.1% | 2.5% | 54.4% | 15% | 2.5% | 2.5% | 3.5 | 350 | - |
| U | 13 | - | 16.5% | 11.6% | 55.2% | 8.0% | 0.7% | 8.0% | 4 | 350 | - |
| V | 5 | - | 81.5% | - | 14.8% | 3.7% | - | - | 4 | 300 | 1/5 |
| W | 11 | - | 77.2% | 1.8% | 13.5% | 3.9% | 2.2% | 1.4% | 3.5 | 375 | 10/11 |
| All Non-VA | 46 | - | 41.8% | 8.3% | 36.7% | 6.8% | 1.2% | 5.2% | 4 | 350 | 16/46 |
| Combined VA/Non-VA Sites | |||||||||||
| X | 7 | - | 12.9% | 2.2% | 17.9% | 41.2% | 16.6% | 9.2% | 3.5 | 400 | - |
| Y | 12 | 1.2% | 57.8% | 1.8% | 29.9% | 4.2% | 5.1% | - | 4 | 350 | 1/12 |
| All Combined | 19 | 0.9% | 45.5% | 1.9% | 26.6% | 14.3% | 8.2% | 2.5% | 4 | 350 | 1/19 |
| All Sites | 128 | 0.4% | 45.4% | 6.4% | 31.6% | 9.3% | 3.2% | 3.8% | 4 | 350 | 27/128 |
Site provided aggregate data for all practitioners; IHD: intermittent hemodialysis; QOD: every other day; BFR: blood flow rate; VA Sites: Department of Veterans Affairs medical centers; Non-VA Sites: University-affiliated non-VA medical centers;
The frequency with which different treatment schedules are used in the management of IHD was calculated based on the reported number of patients treated per month, frequency of use of IHD and reported frequency of use of dialysis treatment schedules ranging from twice weekly to daily dialysis (Figure 1). Less than 1% of patients were prescribed hemodialysis less frequently than three times per week. Overall, approximately 52% of patients were treated on a three times per week or every other day schedule, 32% of patients received IHD four times per week and approximately 7% of patients received IHD six or more times per week. There was substantial variation in the frequency of use of different treatment schedules between sites (Figure 1), however practitioners at only one site reported prescription of IHD six or more times per week for more than 20% of patients. There was no significant difference in the frequency of IHD treatments at VA as compared to non-VA sites.
Figure 1.
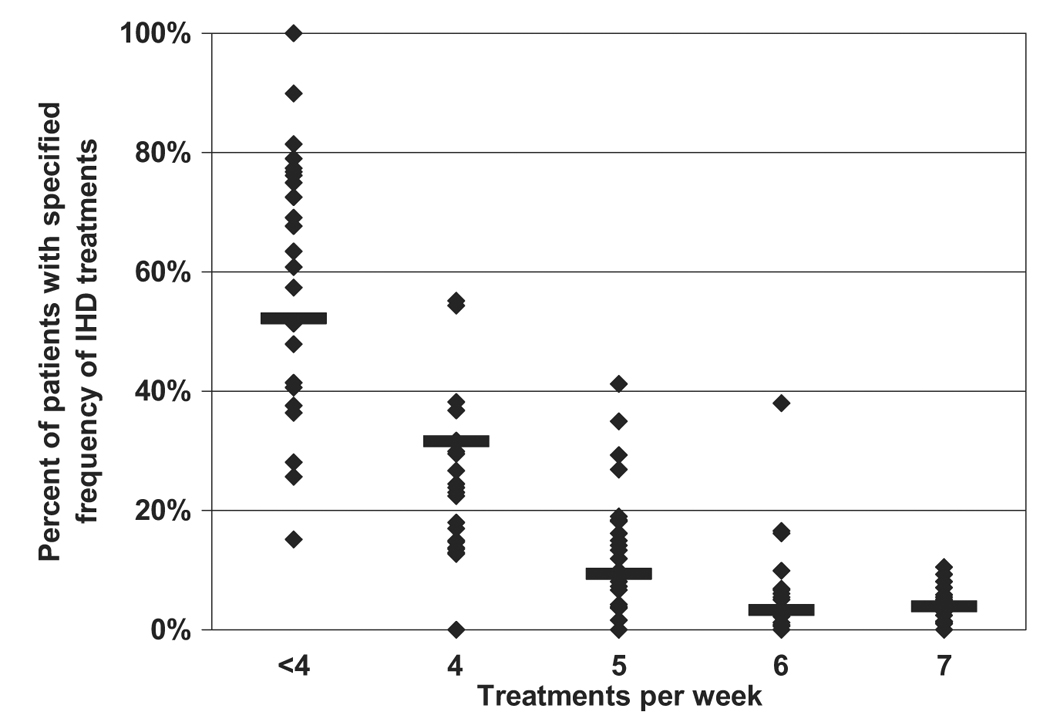
Percent of patients with specified frequency of intermittent hemodialysis (IHD) treatment. Horizontal lines represent pooled data from all sites while the symbols represent data for individual sites. Data for treatment schedules less frequent than four-times per week (two-times per week, three-times per week and alternate-day treatment schedules) are pooled.
The median reported blood flow rate used for IHD was 350 mL/min (IQR: 300–360 mL/min) and the median reported duration for the IHD treatments was 4.0 hours (IQR: 3.5–4.0 hours). Practitioners were asked to identify their target dose of prescribed hemodialysis. Seven percent indicated that they prescribed IHD with a goal of delivering a Kt/Vurea of at least 1.2 per treatment and 31% reported a target urea reduction ratio (URR) of at least 0.65. 56% of practitioners responded that they had no particular target dose of hemodialysis for critically ill patients with AKI. 78.9% of practitioners reported that they did not routinely assess the delivered dose of hemodialysis; 21.1% of practitioners indicated that they measured the delivered dose of therapy at least once per week with 11.7% reporting that they assessed the delivered dose of IHD more than once per week. There was clustering of responses by institution, with more than 70% of the respondents who indicated that they routinely assessed the delivered dose of IHD practicing at only 3 of the 26 institutions providing individual responses.
SLED (Table 3)
Table 3.
Management of SLED
| Site | Respondents Using SLED | Treatment Frequency |
Median Treatment Duration (hours) | Median BFR (ml/min) | Median BFR (ml/min) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| 3/week | QOD | 4/week | 5/week | 6/week | 7/week | |||||
| VA Sites | ||||||||||
| F | 7 | 19.7% | 4% | 42.6% | 19% | 14.3% | 0.4% | 12 | 200 | 200 |
| H | 3 | 1.4% | 2.9% | 7.1% | 50% | 14.3% | 24.3% | 24 | 150 | 100 |
| J | 1 | - | - | - | 10% | 70% | 20% | 10 | 200 | 200 |
| K | 1 | - | - | - | - | - | 100% | NR | NR | NR |
| N | 3 | 15.8% | - | - | 14.2% | 30.5% | 39.5% | 8 | 150 | 100 |
| All VA | 15 | 16.9% | 3.3% | 34.3% | 180.6% | 15.6% | 11.2% | 12 | 200 | 100 |
| Non-VA Sites | ||||||||||
| T | 2 | - | - | - | 50% | 50% | - | 6 | 200 | 500 |
| U | 13 | - | - | - | - | - | 100% | 24 | 150 | 100 |
| All Non-VA | 15 | - | - | - | 0.7% | 0.7% | 98.6% | 24 | 150 | 100 |
| Combined VA/Non-VA Sites | ||||||||||
| X | 1 | 60% | - | 20% | 20% | - | - | 8 | 250 | 300 |
| Y | 1 | - | - | - | 20% | 75% | 5% | 5.5 | 200 | 500 |
| All Combined | 2 | 3.8% | - | 1.2% | 20% | 70.3% | 4.7% | 6.75 | 225 | 400 |
| All Sites | 32 | 5% | 1% | 10% | 6.2% | 6.1% | 71.7% | 19 | 150 | 100 |
Site provided aggregate data for all practitioners; IHD: intermittent hemodialysis; QOD: every other day; BFR: blood flow rate; VA Sites: Department of Veterans Affairs medical centers; Non-VA Sites: University-affiliated non-VA medical centers; NR: no response
Only 24.6% of respondents, at 9 institutions, reported use of SLED in the management of critically ill patients with AKI. 72.6% of patients receiving SLED received treatments on a daily basis. The median reported blood flow rate was 150 mL/min (IQR: 150–200 mL/min), the median dialysate flow rate was 100 mL/min (IQR 100–200 mL/min) and the median duration of therapy was 19 hours (IQR 10–24 hours).
Continuous Renal Replacement Therapy (Table 4)
Table 4.
Management of Continuous Renal Replacement Therapy
| Site | Respondents Using CRRT | CRRT Modalities Used N (% of respondents) | Weight Based Dosing | Non-Weight Based Dosing | |||||
|---|---|---|---|---|---|---|---|---|---|
| AV Modalities | CVVH | CVVHD | CVVHDF | N (%) | Median Dose (mL/kg/hr) | N (%) | Median Dose (mL/hr) | ||
| VA Sites | |||||||||
| A | 5 | - | 1 (20%) | 5 (100%) | - | - | - | 5 (100%) | 2000 |
| B | 2 | - | 2 (100%) | - | - | - | - | 2 (100%) | NR |
| C | 3 | - | - | 1 (33.3%) | 3 (100%) | 2 (66.7%) | 35 | 1 (3.33%) | 1850 |
| E | 4 | - | 4 (100%) | 2 (50%) | 2 (50%) | 1 (25%) | 35 | 3 (75%) | 1000 |
| F | 3 | 2 (66.7%) | 1 (33.3%) | 1 (33.3%) | 1 (33.3%) | - | - | 3 (100%) | 1000 |
| H | 8 | 1 (12.5%) | 2 (25%) | 8 (100%) | - | - | - | 8 (100%) | 1000 |
| I | 4 | - | 3 (75%) | 3 (75%) | 4 (100%) | - | - | 4 (100%) | 2500 |
| K | 6 | - | - | 2 (33.3%) | 4 (66.7%) | - | - | 6 (100%) | 2000 |
| L | 3 | - | 1 (33.3%) | 1 (33.3%) | 2 (66.7%) | 1 (33.3%) | 25 | 2 (66.7%) | 1800 |
| M | 3 | - | 2 (66.7%) | 3 (100%) | 3 (100%) | - | - | 3 (100%) | 1100 |
| N | 1 | - | 1 (100%) | - | - | - | - | 1 (100%) | 1500 |
| P | 5 | - | 5 (100%) | 5 (100%) | 4 (80%) | - | - | 4 (100%) | NR |
| All VA | 47 | 3 (6.4%) | 22 (46.8%) | 31 (66%) | 23 (48.9%) | 4 (8.5%) | 35 | 42 (89.4%) | 1800 |
| Non-VA Sites | |||||||||
| Q | * | * | * | * | * | * | - | * | 2000 |
| R | 4 | - | - | 4 (100%) | - | - | - | 4 (100%) | 1500 |
| S | 5 | - | 4 (80%) | 1 (20%) | - | - | - | 5 (100%) | 1600 |
| T | 7 | 2 (28.6%) | 7 (100%) | 2 (28.6%) | 7 (100%) | 5 (71.4%) | 35 | 2 (28.6%) | 1000 |
| U | 13 | - | - | 13 (100%) | 13 (100%) | - | - | 13 (100%) | 2400 |
| V | 5 | - | - | 3 (60%) | 3 (60%) | - | - | 5 (100%) | 1000 |
| W | 11 | 2 (18.2%) | 2 (18.2%) | 9 (81.8%) | 4 (36.4%) | 1 (9.1%) | 35 | 10 (90.9%) | 1500 |
| All Non-VA | 45 | 4 (8.9%) | 13(28.9%) | 32 (71.1%) | 27 (60%) | 6 (13.3%) | 35 | 39 (86.7%) | 1600 |
| Combined VA/Non-VA Sites | |||||||||
| X | 8 | 2 (25%) | 3 (37.5%) | 4 (50%) | 6 (75%) | 5 (62.5%) | 35 | 3 (37.5%) | 2250 |
| Y | 12 | - | 5 (41.7%) | 11 (91.7%) | 11 (91.7%) | 5 (41.7%) | 35 | 7 (58.3%) | 2300 |
| All Combined | 20 | 2 (10%) | 8 (40%) | 15 (75%) | 17 (85%) | 10 (50%) | 35 | 10 (50%) | 2400 |
| All Sites | 112 | 9 (8%) | 43 (38.4%) | 78 (69.6%) | 67 (59.7%) | 20 (17.9%) | 35 (IQR: 35-35) | 91 (81.2%) | 1825 (IQR: 1200–2400) |
Site provided aggregate data for all practitioners; IHD: intermittent hemodialysis; QOD: every other day; BFR: blood flow rate; VA Sites: Department of Veterans Affairs medical centers; Non-VA Sites: University-affiliated non-VA medical centers; IQR: interquartile range
One hundred seven of the 112 providers (95.5%) who reported use of CRRT indicated that they used venovenous modalities of therapy; five providers (4.5%) reported using both venovenous and arteriovenous modalities and four providers (3.6%) reported use of only arteriovenous CRRT. Forty-three of the 112 providers (38.4%) reported use of continuous hemofiltration; 78 (69.6%) reported use of continuous hemodialysis; and 67 (59.8%) reported use of hemodiafiltration. The median reported blood flow during venovenous therapy was 150 mL/min (IQR: 125–170 mL/min). Only 20 practitioners (17.9%) reported dosing CRRT based on patient weight with 16 of the 20 (80%) prescribing an effluent flow rate of at least 35 mL/kg/hr. Of the practitioners who did not report weight-based prescription, the median reported effluent flow rate was 1825 mL/hr (IQR: 1200–2400 mL/hr).
There was wide variation in the composition of dialysate and replacement fluids. Of the 99 practitioners describing dialysate composition, 30.3% indicated that they used bicarbonate-buffered dialysate, 26.2% that they used lactate-buffered dialysate; 14.1% that they used both bicarbonate- and lactate-buffered dialysate, 14.1% that they used citrate-buffered dialysate, 2% that they used acetate-buffered dialysate and 13.1% did not specify a buffer. Of the 98 respondents describing the composition of replacement fluids, 35.7% indicated that they used bicarbonate-buffered fluids, 17.3% that they used normal saline, 5.1% that they used citrate-buffered fluids, 4.1% that they used lactate-buffered fluids and 37.8% used selected buffer-composition on a case-by-case basis or did not specify buffer composition.
Discussion
Clinical practice patterns for the management of renal replacement therapy in patients with acute kidney injury are poorly characterized. Our survey was conducted at 27 academic university-affiliated and Department of Veterans Affairs medical centers prior to the initiation of a multicenter randomized controlled trial comparing two strategies of intensity of renal replacement therapy in critically ill patients with acute kidney injury at those sites (7). The survey demonstrated that there was wide variability in the management of RRT. There was less utilization of CRRT at VA sites, but otherwise there were no differences in prescribing practices by facility type.
Intermittent hemodialysis was the most widely utilized modality of renal support, utilized by virtually all practitioners and accounting for more than half of all patient treatments. The majority of patients treated with IHD received treatments on a thrice weekly or every-other day schedule with only 7% of patients receiving treatments 6 or more times per week. More than half of practitioners did not specify a target delivered dose of IHD in this population and more than three-quarters of practitioners indicated that they did not routinely monitor the delivered dose of therapy. These results are notable in light of the fact that the survey was conducted more than 18 months after publication of a study demonstrating improved survival with daily intermittent hemodialysis as compared to alternate-day therapy in patients with AKI (5). In addition, although studies evaluating the optimal delivered dose per treatment in AKI have not been conducted, expert consensus has recommended a minimum Kt/Vurea of at least 1.2 delivered three times per week in this population (8). The absence of assessment of the actual delivered dose of therapy is also notable in light of studies documenting large discrepancies between the prescribed and delivered dose of dialysis in this population (9, 10, 5).
The survey provides only limited data on the use of SLED and other forms “hybrid” therapy. These modalities of treatment were reported as used at only one-third of the institutions. Although approximately one-quarter of respondents reported using these modalities, the majority also used CRRT in hemodynamically unstable patients. Based on the survey data, fewer than 10% of patients were treated with SLED. When SLED is utilized, the vast majority of patients are treated on a daily basis. No providers reported routine monitoring of the delivered dose of therapy during SLED.
More than 95% of providers reported use of CRRT. Nine percent of reported use of arteriovenous therapies, either as their exclusive modality of CRRT or in addition to use of venovenous therapy, despite the widespread availability of equipment for venovenous CRRT and the markedly lower rate of vascular complications with venovenous therapy (11). There was substantial variation in the reported modalities of CRRT used with greater use of diffusive as compared to convective therapies. Fewer than 20% of practitioners reported using weight based dosing of CRRT, with the majority of these prescribing an effluent flow at least equal to the intermediate dose arm (35 mL/kg/hr) in the study by Ronco, et al (6). The median effluent flow rate of 1800 mL/hr reported prescribed by practitioners who did not use weight-based dosing represents a flow rate of 20–25 mL/kg/hr, assuming an average patient weight of 80 kg. This is a dose that corresponds to the low-dose arm in the study by Ronco et al., published three years prior to the survey, that demonstrated improved survival with higher doses of CVVH (6).
The results of this survey suggest an absence of a consistent standard for the prescription and monitoring of renal replacement therapy in the setting of AKI. This is in stark contrast to the management of dialysis in the chronic setting, where there are well established clinical practice guidelines and performance measures. While much of the variability in treatment in the acute setting can be attributed to the absence of a robust evidence base to support treatment standards, the absence of monitoring of delivered treatment dose is surprising.
The results of this survey are also of relevance to the design and ethical conduct of clinical trials of renal replacement therapy in AKI in general, and specifically to the conduct of the ATN Study (7). The relationship between clinical trial interventions and concurrent clinical practice has been the subject of intense controversy (12–17). Two years after publication of the results of the Acute Respiratory Distress Syndrome Network’s (ARDSNet) ARMA Trial, which evaluated the efficacy of lower tidal volume ventilation in the acute respiratory distress syndrome (18), the design of the study was challenged as ethically unsound, based on the contention that it compared groups that reflected extremes of practice rather than intermediate tidal volumes that were putatively more commonly utilized (19, 17). The critics argued that both protocol-driven treatment arms constituted experimental interventions and that a non-protocol driven arm (“wild-type therapy”) was ethically required as a control group for assessment of both safety and efficacy. Although the Office for Human Research Protections (OHRP) of the United States Department of Health and Human Services ultimately did not find fault with the ARDSNet study design, OHRP faulted the institutional review boards (IRBs) responsible for oversight of the ARDSNet studies for failing to obtain sufficient information required to assess the risks to subjects (20). Specifically, OHRP opined that the IRBs should have been provided “a clear detailed description of concurrent routine clinical practice at the ARDS Network trial sites with respect to management of tidal volume in patients with ALI and ARDS” and “a detailed comparison of the tidal volume management strategies that were to be used in the two experimental groups relative to concurrent routine clinical practice” (20).
This OHRP opinion impacts the design and conduct of trials of renal replacement therapy in acute kidney injury (AKI). As was the case with the ARDS Network studies, the ATN Study compares two protocol driven strategies of titrated therapy and does not include a concurrent, non-protocol directed control arm. We therefore conducted this survey after the release of the OHRP determination in July 2003 to assess the relationship between the treatment arms of ATN Study and concurrent clinical practice. In the less intensive “conventional” treatment arm of the ATN Study, intermittent hemodialysis and SLED are provided on a thrice-weekly schedule, with a target delivered Kt/Vurea of 1.2/treatment, and CVVHDF is provided at a prescribed effluent flow rate of 20 mL/kg/hr. In the intensive therapy arm, intermittent hemodialysis and SLED are provided on a six-times per week schedule, with a target delivered Kt/Vurea of 1.2/treatment, and CVVHDF is provided at a prescribed effluent flow rate of 35 mL/kg/hr. We believe that the survey results suggest that “wild-type” therapy at the time of initiation of the study was most similar to the ATN Study’s less intensive “conventional” treatment arm. Intermittent hemodialysis was provided with a treatment frequency of between 3 and 4 times per week, in more than 80% of patients, with the actual delivered dose of therapy not being monitored. Similarly, although there was wider variation in the dosing of CRRT, the practitioner responses suggest that the majority of patients are prescribed doses that are similar to or less than the dose of therapy specified in the “conventional” treatment arm of the ATN Study. In contrast, the survey indicates that the majority of “wild-type” patients treated with SLED received treatment on a daily treatment schedule rather than the thrice-weekly schedule of the conventional treatment-arm. Overall, however, there was minimal use of SLED, and this more intensive dosing used for this modality has only little impact on the overall intensity of “wild-type” therapy.
There are significant limitations to this survey. The results of this survey may not be generalizable beyond the 27 participating sites. In addition, the reliability of practitioner reported prescribing practices was not validated by comparison to actual treatment data. Prescribing practices may also change over time, in part influenced by the ongoing clinical trial. For these reasons, observational data on the prescription and delivery of renal replacement therapy is being collected on patients at participating study sites who meet the eligibility criteria for the interventional study but for whom informed consent could not be obtained. This data will provide a more objective assessment of the relationship between study therapy and concurrent treatment outside of the study and will allow assessment of changes in “wild-type” therapy over the duration of the study.
In summary, we conducted a survey of practitioners at the 27 sites participating in the ATN Study prior to subject enrollment to assess patterns of clinical practice for the management of renal replacement therapy in patients with AKI. Intermittent hemodialysis and CRRT were the most commonly utilized modalities of therapy, with SLED and other “hybrid” treatments used in fewer than 10% of patients. Intermittent hemodialysis was most commonly provided on a thrice weekly or every-other day schedule, with only infrequent assessment of the delivered dose of therapy. Most practitioners reported that they did not dose CRRT based on patient weight and their average prescribed dose of therapy corresponded to a weight based dose of no more than 20 to 25 mL/kg/hr. These dosing patterns correspond to the non-intensive treatment arm of the VA/NIH Acute Renal Failure Trial Network (ATN) Study. While some practitioners reported using dosing strategies similar to the intensive treatment arm of the ATN Study, very few reported the use of intermediate dosing strategies.
Supplementary Material
Acknowledgements
The ATN Study is supported by the Cooperative Studies Program of the Department of Veterans Affairs Office of Research and Development and by the National Institute of Diabetes, Digestive and Kidney Diseases by interagency agreement Y1-DK-3508-01.
This research was presented at the 37th Annual Meeting and Scientific Exposition of the American Society of Nephrology in St. Louis, MO, October 27-November 1, 2004.
Appendix
VA/NIH Acute Renal Failure Trial Network (ATN) Study
Executive Committee
Paul M. Palevsky, MD, Chairman, VA Pittsburgh Healthcare System, Pittsburgh, PA
Theresa Z. O’Connor, PhD, CSP Coordinating Center, VA Connecticut Healthcare System, West Haven, CT
Jane H. Zhang, PhD, CSP Coordinating Center, VA Connecticut Healthcare System, West Haven, CT
Glenn M. Chertow, MD, MPH, University of California, San Francisco, San Francisco, CA
Susan Crowley, MD, VA Connecticut Healthcare System, West Haven, CT
Devasmita Dev, MD, VA North Texas Healthcare System, Dallas, TX
John Kellum, MD, University of Pittsburgh, Pittsburgh, PA
Emil Paganini, MD, Cleveland Clinic Foundation, Cleveland, OH
Roland M. H. Schein, MD, Miami VA Medical Center, Miami, FL
B. Taylor Thompson, MD, Massachusetts General Hospital, Boston, MA
Mark W. Smith, HERC, VA Palo Alto Healthcare System, Menlo Park, CA
Kathy Swanson, RPh, CSP Research Pharmacy Coordinating Center, New Mexico VA Healthcare System, Albuquerque, NM
Peter Peduzzi, PhD, (Ex Officio), Director CSP Coordinating Center, VA Connecticut Healthcare System, West Haven, CT
Robert Star, MD (Ex Officio), Senior Advisor, NIDDK, Bethesda, MD
Participating Sites (Primary Site Investigator)
VA Ann Arbor Healthcare System, Ann Arbor, MI (Eric Young, MD)
VA Western NY Healthcare System, Buffalo, NY (James Lohr, MD)
VA North Texas Healthcare System, Dallas, TX (Devasmita Dev, MD)
Houston VA Medical Center, Houston, TX (George Dolson, MD)
Richard L Roudebush VA Medical Center, Indianapolis, IN (Robert Bacallao, MD)
Central Arkansas Veterans Healthcare Center, Little Rock, AK (May Jo Shaver, MD)
West Los Angeles VA Healthcare Center, Los Angeles, CA (Jeffrey Kraut, MD)
Miami VA Medical Center, Miami, FL (Roland M. H. Schein, MD)
VA Tennessee Valley Healthcare System, Nashville, TN (T. Alp Ikizler, MD)
New Orleans VA Medical Center, New Orleans, LA (Vecihi Batuman, MD)
VA Pittsburgh Healthcare System, Pittsburgh, PA (Mohan Ramkumar, MD)
Portland VA Medical Center, Portland, OR (Suzanne Watnick, MD)
Hunter Holmes McGuire VA Medical Center, Richmond, VA (George Feldman, MD)
VA San Diego Healthcare System, San Diego, CA (Francis Gabbai, MD)
San Francisco VA Medical Center, San Francisco, CA (Kirsten Johansen, MD)
San Juan VA Medical Center, San Juan, PR (Carlos Rosado-Rodriguez, MD)
VA Puget Sound Healthcare System, Seattle, WA (Dennis Andress, MD)
VA Connecticut Healthcare System, West Haven, CT (Susan Crowley, MD)
Cleveland Clinic Foundation, Cleveland, OH (Emil Paganini, MD)
Johns Hopkins University (Hamid Rabb, MD)
Massachusetts General Hospital, Boston, MA (John Niles, MD)
University of California, San Francisco, San Francisco, CA (Glenn Chertow, MD)
University of Miami, Miami, FL (Gabriel Contreras, MD, MPH)
University of Pittsburgh, Pittsburgh, PA (Nabeel Aslam, MD)
University of Texas at Houston, Houston, TX (Kevin Finkel, MD and Andrew Shaw, MD)
Wake Forest University, Winston-Salem, NC (Michael Rocco, MD)
Washington University at St. Louis, St. Louis, MO (Anitha Vijayan, MD)
References
- 1.Mehta RL, Letteri JM. Current status of renal replacement therapy for acute renal failure. A survey of US nephrologists. The National Kidney Foundation Council on Dialysis. Am J Nephrol. 1999;19:377–382. doi: 10.1159/000013481. [DOI] [PubMed] [Google Scholar]
- 2.Hyman A, Mendelssohn DC. Current Canadian approaches to dialysis for acute renal failure in the ICU. Am J Nephrol. 2002;22:29–34. doi: 10.1159/000046671. [DOI] [PubMed] [Google Scholar]
- 3.Mehta RL, Pascual MT, Soroko S, Savage BR, Himmelfarb J, Ikizler TA, Paganini EP, Chertow GM. Spectrum of acute renal failure in the intensive care unit: the PICARD experience. Kidney Int. 2004;66:1613–1621. doi: 10.1111/j.1523-1755.2004.00927.x. [DOI] [PubMed] [Google Scholar]
- 4.Ricci Z, Ronco C, D'Amico G, De Felice R, Rossi S, Bolgan I, Bonello M, Zamperetti N, Petras D, Salvatori G, Dan M, Piccinni P. Practice patterns in the management of acute renal failure in the critically ill patient: an international survey. Nephrol Dial Transplant. 2006;21:690–696. doi: 10.1093/ndt/gfi296. [DOI] [PubMed] [Google Scholar]
- 5.Schiffl H, Lang SM, Fischer R. Daily hemodialysis and the outcome of acute renal failure. N Engl J Med. 2002;346:305–310. doi: 10.1056/NEJMoa010877. [DOI] [PubMed] [Google Scholar]
- 6.Ronco C, Bellomo R, Homel P, Brendolan A, Dan M, Piccinni P, La Greca G. Effects of different doses in continuous veno-venous haemofiltration on outcomes of acute renal failure: a prospective randomised trial. Lancet. 2000;356:26–30. doi: 10.1016/S0140-6736(00)02430-2. [DOI] [PubMed] [Google Scholar]
- 7.Palevsky PM, O'Connor T, Zhang JH, Star RA, Smith MW. Design of the VA/NIH Acute Renal Failure Trial Network (ATN) Study: intensive versus conventional renal support in acute renal failure. Clin Trials. 2005;2:423–435. doi: 10.1191/1740774505cn116oa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kellum JA, Mehta RL, Angus DC, Palevsky P, Ronco C. The first international consensus conference on continuous renal replacement therapy. Kidney Int. 2002;62:1855–1863. doi: 10.1046/j.1523-1755.2002.00613.x. [DOI] [PubMed] [Google Scholar]
- 9.Evanson JA, Himmelfarb J, Wingard R, Knights S, Shyr Y, Schulman G, Ikizler TA, Hakim RM. Prescribed versus delivered dialysis in acute renal failure patients. Am J Kidney Dis. 1998;32:731–738. doi: 10.1016/s0272-6386(98)70127-1. [DOI] [PubMed] [Google Scholar]
- 10.Evanson JA, Ikizler TA, Wingard R, Knights S, Shyr Y, Schulman G, Himmelfarb J, Hakim RM. Measurement of the delivery of dialysis in acute renal failure. Kidney Int. 1999;55:1501–1508. doi: 10.1046/j.1523-1755.1999.00355.x. [DOI] [PubMed] [Google Scholar]
- 11.Bellomo R, Parkin G, Love J, Boyce N. A prospective comparative study of continuous arteriovenous hemodiafiltration and continuous venovenous hemodiafiltration in critically ill patients. Am J Kidney Dis. 1993;21:400–404. doi: 10.1016/s0272-6386(12)80268-x. [DOI] [PubMed] [Google Scholar]
- 12.Steinbrook R. How best to ventilate? Trial design and patient safety in studies of the acute respiratory distress syndrome. N Engl J Med. 2003;348:1393–1401. doi: 10.1056/NEJMhpr030349. [DOI] [PubMed] [Google Scholar]
- 13.Steinbrook R. Trial design and patient safety--the debate continues. N Engl J Med. 2003;349:629–630. doi: 10.1056/NEJMp038133. [DOI] [PubMed] [Google Scholar]
- 14.Miller FG, Silverman HJ. The ethical relevance of the standard of care in the design of clinical trials. Am J Respir Crit Care Med. 2004;169:562–564. doi: 10.1164/rccm.200311-1577CP. [DOI] [PubMed] [Google Scholar]
- 15.Silverman HJ, Miller FG. Control group selection in critical care randomized controlled trials evaluating interventional strategies: An ethical assessment. Crit Care Med. 2004;32:852–857. doi: 10.1097/01.ccm.0000114814.62759.06. [DOI] [PubMed] [Google Scholar]
- 16.Silverman HJ. The acute respiratory distress syndrome network controversy: lessons and legacy. Curr Opin Crit Care. 2004;10:560–564. doi: 10.1097/01.ccx.0000144766.73540.52. [DOI] [PubMed] [Google Scholar]
- 17.Deans KJ, Minneci PC, Eichacker PQ, Natanson C. Defining the standard of care in randomized controlled trials of titrated therapies. Curr Opin Crit Care. 2004;10:579–582. doi: 10.1097/01.ccx.0000145097.36069.44. [DOI] [PubMed] [Google Scholar]
- 18.The Acute Respiratory Distress Syndrome Network. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. 2000;342:1301–1308. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 19.Eichacker PQ, Gerstenberger EP, Banks SM, Cui X, Natanson C. Meta-analysis of acute lung injury and acute respiratory distress syndrome trials testing low tidal volumes. Am J Respir Crit Care Med. 2002;166:1510–1514. doi: 10.1164/rccm.200208-956OC. [DOI] [PubMed] [Google Scholar]
- 20.Borror K, Carome M. Letter to Ronald S. Newbower, PhD, Massachusetts General Hospital; Lee E. Limbird, PhD, Vsanderbilt University; and Robert Kay, MD, The Cleveland Clinic Foundation. Office for Huamn Research Protections, Department of Health and Human Services; 2003. Jul 3, [Accessed August 23, 2006]. Human research subject protections under Federalwide Assurance (FWA) 3136, Multiple Project Assurances (MPA) M-1331, M-1363, and M-1338 and the OHRP-approved assurances for all ARDS Network institutions. Available at http://www.hhs.gov/ohrp/detrm_letrs/YR03/jul03a.pdf. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


