Abstract
The homeodomain transcription factor Nkx6-1 is essential for proper motor neuron development and development of insulin-producing pancreatic β-cells. Nkx6-1 is closely related to Nkx6-2 and Nkx6-3, and all three are expressed in the developing central nervous system and in the developing foregut. Immunohistochemical detection of protein expression is an important tool for description of the temporal differences in expression patterns. When several gene family members like the Nkx6 factors have overlapping or juxtaposed expression domains, there is an elevated risk of unrecognized cross-reactivity, and it is therefore crucial to determine the specificities of antibodies against such targets. In this study we have determined the epitope consensus sequences of four monoclonal antibodies against Nkx6-1 using SPOT membranes, and we refined the results by combined peptide recognition and blocking assays. We show that two of the monoclonal anti-Nkx6-1 antibodies specifically recognize Nkx6-1 and do not cross-react to Nkx6-2 and Nkx6-3. The other two monoclonal anti-Nkx6-1 antibodies are specific to Nkx6-1 in mice but do not recognize Nkx6-1 in chicken and human.(J Histochem Cytochem 56:415–424, 2008)
Keywords: Nkx6-1, pancreas, neural tube, development, antibody specificity
Since Nkx6-1 was first discovered (Rudnick et al. 1994), it has been shown to be instrumental in both neural and pancreatic development (Sander et al. 2000a,b) (Henseleit et al. 2005). In mice, Nkx6-1 is expressed along with its paralogs Nkx6-2 (also called Gtx) (Komuro et al. 1993) and Nkx6-3 (Nelson et al. 2005) in the developing foregut and in the central nervous system (Nelson et al. 2005; Pedersen et al. 2005; Alanentalo et al. 2006).
In the developing foregut, Nkx6-1 can be transiently detected in the prospective ventral pancreas of embryonic day 8.75 (E8.75) mouse embryos (Jørgensen et al. 2007). From E9.0 it marks the dorsal pancreas epithelium and reappears in the ventral pancreas epithelium at E10.5 (Jørgensen et al. 2007). At E13.5, Nkx6-1 is still widely expressed in the pancreatic epithelium but hereafter commences its restriction to the pancreatic β-cells (Sander et al. 2000b) to finally become exclusively expressed in the β-cells of the fully developed pancreas (Jensen et al. 1996; Sander et al. 2000b). Nkx6-2 and Nkx6-3 are also expressed in the developing gut tube area at the pancreas level (Nelson et al. 2005; Pedersen et al. 2005; Alanentalo et al. 2006). At E10.5, Nkx6-2 is coexpressed with Nkx6-1 in the pancreas buds but is also expressed in the duodenum and the posterior stomach epithelium, whereas Nkx6-3 is absent in the pancreas and to a large extent coexpressed with Nkx6-2 in the duodenum and posterior stomach (Pedersen et al. 2005; Alanentalo et al. 2006). This expression pattern is relatively well conserved in chicken, although Nkx6-1 appears to be more broadly expressed in the gut tube endoderm at the earliest stages of pancreas formation (Pedersen et al. 2005).
In the developing nervous system, Nkx6-1 and Nkx6-2 are both expressed in the ventral spinal cord, hindbrain, and midbrain (Qiu et al. 1998; Vallstedt et al. 2001; Pattyn et al. 2003), whereas Nkx6-3 expression is restricted to the caudal hindbrain (Alanentalo et al. 2006). As in the gut tube, there are also species differences between Nkx6 expression patterns in the developing spinal cord. In chicken, Nkx6-1 and Nkx6-2 overlap largely in the ventral neural tube, whereas they each mark distinct domains of neuronal progenitors in mice (Vallstedt et al. 2001).
Loss of Nkx6-1 function results in compromised β-cell development (Sander et al. 2000b) and failure in ventral interneuron and somatic motor neuron formation (Sander et al. 2000a), whereas Nkx6-2 mutant mice develop normally without any overt defects (Cai et al. 2001; Vallstedt et al. 2001; Henseleit et al. 2005). However, Nkx6-1/6-2 double homozygous mutants display an even more severe phenotype than the Nkx6-1 mutants (Vallstedt et al. 2001; Pattyn et al. 2003; Henseleit et al. 2005). The neuronal phenotype of Nkx6-1-deficient embryos is partly rescued by the action of Nkx6-2, which becomes derepressed and to some extent compensates for the loss of Nkx6-1 (Vallstedt et al. 2001; Henseleit et al. 2005). Also, Nkx6-1 and Nkx6-2 possess equal functional capabilities in pancreatic β-cell specification (Nelson et al. 2007). It has also been speculated that Nkx6-3 may compensate for Nkx6-2 in medullary reticular formation, explaining the lack of phenotype in the Nkx6-2 mutants (Nelson et al. 2005). However, the phylogenetic linkage between Nkx6-1 and Nkx6-2 is higher than with Nkx6-3 (Pedersen et al. 2005; Alanentalo et al. 2006), and Nkx6-1 and Nkx6-2 have been shown to have comparable functional activities (Nelson et al. 2007).
Due to the overlapping expression domains and the high degree of amino acid sequence similarity between members of the Nkx6 family, there is a significant risk that antibodies raised against, e.g., Nkx6-1 do not specifically recognize Nkx6-1 as intended but also Nkx6-2 or Nkx6-3. Here we identify the epitopes recognized by four recently generated monoclonal antibodies against Nkx6-1 (Pedersen et al. 2006) and use the information gained to analyze their species as well as paralog specificity.
Materials and Methods
Multiple Peptide SPOT Membrane Assay
The C-terminal 66-amino acid sequence used for immunization to generate the monoclonal anti-Nkx6-1 antibodies (Pedersen et al. 2006) is represented by a series of 19 13-mer peptides overlapping by 10 residues and synthesized on a cellulose membrane (Sigma–Genosys; Suffolk, UK) using the SPOT technique (Frank 2002). In accordance with the manufacturer's instructions, the membrane with bound peptides was probed using the same procedure as for Western blots. The membrane was stripped in stripping buffer (Pierce Biotechnology; Rockford, IL) between each assay with the four monoclonal anti-Nkx6-1 antibodies. Goat anti-mouse horseradish peroxidase (HRP)-conjugated secondary antibody (Santa Cruz Biotechnology; Santa Cruz, CA) was used as secondary antibody and tested for nonspecific binding before carrying out the assays.
Immunohistochemistry
Purified monoclonal anti-Nkx6-1 antibodies (F55A10, F55A12, F64A6B4, and F65A2; Antibody Core, Beta Cell Biology Consortium, Vanderbilt University, Nashville, TN) (Pedersen et al. 2006) were analyzed by immunohistochemical staining of frozen pancreas sections from adult wild-type (WT) NMRI mice, E4 White Leghorn chicken embryos, or adult human pancreas. Tissue samples were fixed overnight at 4C in Lilly's formalin buffer, pH 7.4 (Bie and Berntsen; Rodovre, Denmark), cryoprotected in 30% sucrose in PBS, embedded in Tissue-Tek (Sakura; Vaerlose, Denmark), and cut into 8-μm sections on a Leica cryostat (Leica Microsystems; Herlev, Denmark).
Antigen retrieval was performed by microwave treatment of mouse and human tissue sections in 200 ml 0.01 M citrate buffer for 4 min at 600 W followed by 15 min at 250 W and left to cool for 20 min at room temperature. Tissue sections were rinsed in PBS, quenched in 3% H2O2 for 5 min, and rinsed in PBS again. Sections were then blocked in 0.1 M Tris–HCl, pH 7.5, 0.15 M NaCl, and 0.5% blocking reagent (TNB; PerkinElmer, Hvidovre, Denmark) for 30 min before overnight incubation with the monoclonal anti-Nkx6-1 antibodies diluted 1:200 in 0.5% TNB. Sections were washed three times for 5 min each in PBS followed by incubation with Cy3-conjugated donkey anti-mouse secondary antibody (Jackson ImmunoResearch Europe Ltd.; Soham, UK) for 45 min. Sections were finally washed three times for 5 min each in PBS. Immunostainings for insulin were performed subsequently with guinea pig anti-insulin (Abcam; Cambridge, UK) primary antibody diluted 1:500 in TNB and applied overnight. The sections were washed three times for 5 min in PBS and incubated for 45 min with Cy2-conjugated donkey anti-guinea pig (Jackson ImmunoResearch Europe Ltd.) secondary antibody diluted 1:500 in TNB. Finally, the sections were washed three times for 5 min each in PBS and mounted with 20% glycerol in 40 mM Tris base, pH 8.4. All steps in the staining procedure were performed at room temperature.
Chicken tissue sections were rinsed in PBS, blocked for 30 min in TNB, and incubated overnight with the monoclonal anti-Nkx6-1 antibodies diluted 1:200 and the polyclonal rabbit anti-Nkx6-1 antiserum diluted 1:700 in TNB. Sections were washed three times for 5 min each in PBS followed by 30-min incubation with Cy3-conjugated donkey anti-mouse and Cy2-conjugated donkey anti-rabbit (Jackson ImmunoResearch Europe Ltd.) secondary antibodies diluted 1:500 in TNB. Sections were washed three times for 5 min in PBS and mounted with 20% glycerol in 40 mM Tris base, pH 8.4. All steps in the staining procedure were performed at room temperature.
For blocking experiments, peptides DDDYNKPLDP, DDEYNKPLDP, DDEYNRPLDP (Sigma–Genosys) and peptides KITQLLKKHKSSG, KIAQLLKKHKPGA, KITRLLKKHKPSN, KITRLLKKHKSTN, KIRLLLRKHRAAF (GL Biochem; Shanghai, China) were added to the indicated antibody solutions at a final concentration of 300 ng/μl prior to application.
Images were obtained by confocal microscopy using a Zeiss LSM510 META Axio Imager (Zeiss; Birkerod, Denmark).
Western Blots
Nuclear extracts were made from SV40 large T-antigen-transformed βTC-3 insulinoma cells (Efrat et al. 1988) treated with lysis buffer (10 mM Hepes, pH 7.9, 10 mM KCl, 0.1 mM EDTA, 0.1 mM EGTA) for 15 min. Nonidet P-40 was added to a final concentration of 0.6% and vortexed for 10 sec. Nuclei were spun down (12,000 × g, 1 min at 4C), and nuclear proteins were released by incubation for 15 min on a shaker in a high-salt buffer (20 mM Hepes, pH 7.9, 25% glycerol, 0.4 M NaCl, 1 mM EDTA, 1 mM EGTA). Samples were centrifuged (12,000 × g, 5 min at 4C), and the supernatants containing the nuclear proteins were kept. All reactions were done on ice, and all buffers contained a protease inhibitor cocktail (Roche Diagnostics; Hvidovre, Denmark) and 1 mM 4-(2-aminoethyl)benzenesulfonyl fluoride.
Nuclear extract samples containing a total of 30 μg protein were denatured for 5 min at 80C in NuPAGE 4X lithium dodecyl sulfate sample buffer (Invitrogen; Taastrup, Denmark) and 100 mM dithiothreitol and loaded on a NuPAGE 10% Bis–Tris Gel (Invitrogen). The gel was electroblotted at 400 mA for 75 min at 4C onto a nitrocellulose membrane in transfer buffer (20 mM Tris base, 150 mM glycine, 20% EtOH). The membrane was washed 5 min in TBS (50 mM Tris, 150 mM NaCl), blocked for 1 hr in TBST–milk (TBS, 0.1% Tween 20, 5% skimmed milk powder), washed three times for 5 min each in TBST, and incubated overnight at 4C with one of the four monoclonal anti-Nkx6-1 antibodies diluted 1:1000 in TBST–milk. For the blocking experiments, 10 μg/ml peptide was added to the primary anti-Nkx6-1 antibody solutions. The membrane was washed three times for 5 min in TBST and incubated for 30 min with goat anti-mouse HRP-conjugated secondary antibody (Santa Cruz Biotechnology) diluted 1:10,000 in TBST–milk. Chemiluminescence was detected with Lumigen TSA-6 (Amersham Biosciences; Piscataway, NJ) using BioSpectrum AC Imaging system (Ultra-Violet Products Ltd.; Cambridge, UK) and visualized by VisionWorks LS (Ultra-Violet Products Ltd.).
Dot Blots
Two-μl aliquots containing amounts between 0 and 20 μg of peptide dissolved in H2O were spotted manually on nitrocellulose membranes and allowed to dry at room temperature. Membranes were then processed exactly as for Western blots as described above.
Results
Identification of Epitopes Recognized by the Monoclonal Anti-Nkx6-1 Antibodies
The four monoclonal antibodies (F55A10, F55A12, F64A6B4, and F65A2) against the Nkx6-1 homeodomain transcription factor have previously been shown to recognize two different epitopes (Pedersen et al. 2006). To accurately define these epitopes we have used the SPOT method to perform a detailed epitope mapping (Frank 2002). Hence, 19 overlapping peptides spanning the C-terminal 66 amino acids of the rat Nkx6-1 sequence used for generation of the antibodies (Pedersen et al. 2006) were synthesized and spotted onto a cellulose membrane support (Figure 1). Three peptides were recognized for each of the four antibodies when applied to the SPOT membrane (Figures 1A–1C). F55A10 and F55A12 recognize the same three peptides (Figures 1A and 1C), which have the seven amino acid stretch DDDYNKP in common (Figure 1D). Likewise, the antibodies F64A6B4 and F65A2 both recognize a second set of three peptides (Figures 1B and 1C), which share the seven amino acid stretch QLLKKHK (Figure 1D).
Figure 1.
The four monoclonal anti-Nkx6-1 antibodies (F55A10, F55A12, F64A6B4, and F65A2) recognize two different epitopes. (A–C) The SPOT membrane with 19 different peptides covering the C-terminal part of the rat/mouse Nkx6-1 sequence used for immunization when the antibodies were developed. (A) F55A10 recognizes peptide numbers 7–9. (B) F64A6B4 recognizes peptide numbers 13–15. (C) Combined immunoblot showing that F55A12 recognizes peptide numbers 7–9, whereas F65A2 recognizes peptide number 13–15. White dotted circles illustrate the positioning of the peptides on the membrane. (D) Line-up of the 19 peptide sequences that are spotted on the membrane. With 10 amino acid overlaps, the 19 peptides cover the 66 amino acid C-terminal part of rat/mouse Nkx6-1. The epitope consensus sequence DDDYNKP recognized by F55A10 and F55A12 is highlighted in green, and the epitope consensus sequence QLLKKHK recognized by F64A6B4 and F65A2 is highlighted in red.
F55A10 and F55A12 Recognize Specifically Nkx6-1
To assess the possibility that the four antibodies could cross-react to Nkx6-2 or Nkx6-3, we aligned the C termini of the Nkx6 proteins in rat, mouse, human, chicken, and zebrafish and compared the sequence stretches containing the two epitopes (Figure 2). Overall, there is a high degree of sequence similarity between species and between the three Nkx6 proteins. The DDDYNKP epitope recognized by F55A10 and F55A12 is completely conserved in Nkx6-1 of all five species, whereas the corresponding motifs in Nkx6-2 and Nkx6-3 differ by one or two amino acid residues (Figure 2). The QLLKKHK epitope recognized by F64A6B4 and F65A2 is also conserved in Nkx6-1 of all five species, whereas the corresponding motifs in Nkx6-2 and Nkx6-3 differ by one or three amino acid residues, respectively (Figure 2).
Figure 2.
Motifs recognized by the antibodies display a high degree of sequence similarity among Nkx6 proteins in different species. ClustalW alignment of the Nkx6-1, Nkx6-2, and Nkx6-3C termini of rat (Rr), mouse (Mm), human (Hs), chicken (Gg), and zebrafish (Dr) demonstrating the high degree of similarity among Nkx6 proteins. Positions of the two motifs DDDYNKP and QLLKKHK are boxed in gray. Peptide sequences used in the blocking experiments of F55A10 and F55A12 are highlighted in green, and those used in the blocking experiments of F64A6B4 and F65A2 are highlighted in red.
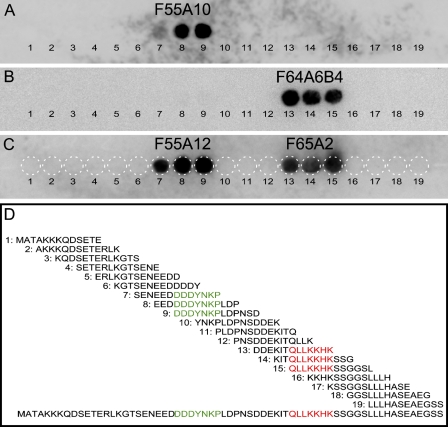
To test the specificity of the monoclonal anti-Nkx6-1 antibodies against different Nkx6-1 homo- and paralogs, we performed blocking experiments with Nkx6-1-, Nkx6-2-, and Nkx6-3-specific peptides. As previously demonstrated (Pedersen et al. 2006), all four antibodies recognize specifically Nkx6-1 at 44–46 kDa in βTC-3 nuclear extracts in Western blot assays. The expected size of both Nkx6-2 and Nkx6-3 is 29 kDa, and these would easily have been distinguishable from Nkx6-1 had they been recognized. As expected, F55A10 and F55A12 were both completely blocked by the Nkx6-1-specific peptide DDDYNKPLDP in Western blot assays (Figures 3A and 3B) and in immunohistochemical stainings (Figures 3C–3F). In contrast, the peptide DDEYNKPLDP corresponding to the matching amino acid stretches in Nkx6-2 found in chicken and zebrafish, as well as in Nkx6-3 in rat, mouse, human, and chicken (Figure 2), did not block immunoreactivity of F55A10 and F55A12 in Western blot assays (Figures 3A and 3B) or in stainings on tissue sections (Figures 3G–3J). Similarly, the DDEYNRPLDP peptide corresponding to the equivalent sequences found in rat, mouse, and human Nkx6-2 (Figure 2) did not block the activity of F55A10 and F55A12 in these assays (Figures 3A,B,K–N). This demonstrates that the two monoclonal anti-Nkx6-1 antibodies F55A10 and F55A12 specifically recognize Nkx6-1 and do not cross-react to Nkx6-2 and Nkx6-3, and that the aspartic acid at position 3 of the epitope is essential for binding.
Figure 3.
F55A10 and F55A12 recognize specifically Nkx6-1. Blocking experiments with the monoclonal anti-Nkx6-1 antibodies F55A10 and F55A12 (A,B) by Western blot assays and (C–N) by immunohistochemical stainings on adult mouse pancreas sections. (A) Western blot showing F55A10 immunoreactivity to Nkx6-1 at the expected size in nuclear extracts from βTC-3 cells with the indicated peptides added. (B) Western blot showing F55A12 immunoreactivity to Nkx6-1 at the expected size in nuclear extracts from βTC-3 cells with the indicated peptides added. (C,D,G,H,K,L) F55A10 immunostainings of Nkx6-1 (red) in cell nuclei of pancreatic β-cells are shown. Insulin immunostainings are shown in green. D,H,L are adjacent sections to C,G,K, respectively, and with the indicated peptides added. (E,F,I,J,M,N) F55A12 immunostainings of Nkx6-1 (red) in cell nuclei of pancreatic β-cells are shown. Insulin immunostainings are shown in green. F,J,N are adjacent sections to E,I,M, respectively, and with the indicated peptides added. Bar = 20 μm.
F64A6B4 and F65A2 Are Specific to Nkx6-1 in Mice but May Cross-react to Nkx6-2 in Chicken
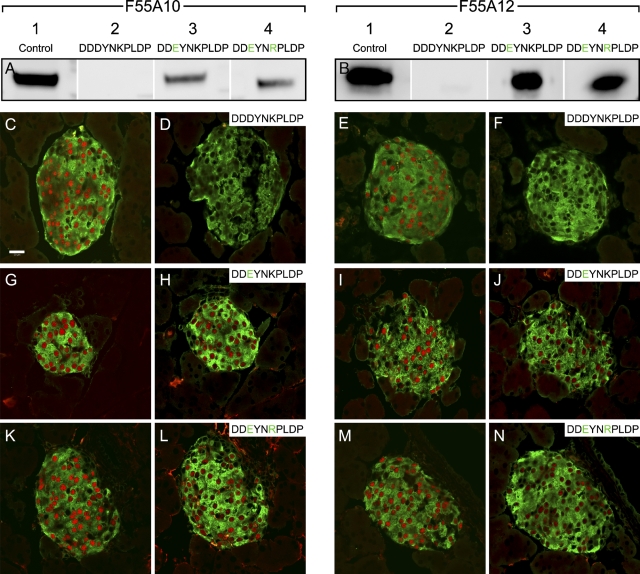
Blocking experiments were also performed with F64A6B4 and F65A2 (Figure 4). Here the peptide sequences were designed as 13-mers because 10-mers were not functional in these assays. As expected, preabsorption with the peptide KITQLLKKHKSSG, which contains the identified epitope and corresponds to the Nkx6-1 sequences in rat and mouse (Figure 2), could efficiently block both F64A6B4 and F65A2 in Western blot assays (Figures 4A and 4B) and in immunohistochemical stainings (Figures 4C–4F). The equivalent Nkx6-1 peptide sequence from chicken, KIAQLLKKHKPGA (Figure 2), was not able to block F64A6B4 and F65A2 in the Western blot assays (Figures 4A and 4B), and immunoreactivity of F64A6B4 but not F65A2 seemed to be only partially blocked by the chicken-specific Nkx6-1 peptide on tissue sections (Figures 4G–4J). This indicates that F64A6B4 and F65A2 may fail to detect Nkx6-1 in assays using chicken tissue, even though the core motif of the epitope QLLKKHK is identical to that found in mouse and rat.
Figure 4.
F64A6B4 and F65A2 recognize specifically Nkx6-1 in rat and mouse but not in chicken. Blocking experiments with the monoclonal anti-Nkx6-1 antibodies F64A6B4 and F65A2 (A,B) by Western blot assays and (C–V) by immunohistochemical stainings on adult mouse pancreas sections. (A) Western blot showing F64A6B4 immunoreactivity to Nkx6-1 at the expected size in nuclear extracts from βTC-3 cells with the indicated peptides added. (B) Western blot showing F65A2 immunoreactivity to Nkx6-1 at the expected size in nuclear extracts from βTC-3 cells with the indicated peptides added. (C,D,G,H,K,L,O,P,S,T) F64A6B4 immunostainings of Nkx6-1 (red) in cell nuclei of pancreatic β-cells are shown. Insulin immunostainings are shown in green. D,H,L,P,T are adjacent sections to C,G,K,O,S, respectively, and with the indicated peptides added. Inset in H shows a close-up of a few β-cells. (E,F,I,J,M,N,Q,R,U,V) F65A2 immunostainings of Nkx6-1 (red) in cell nuclei of pancreatic β-cells are shown. Insulin immunostainings are shown in green. F,J,N,R,V are adjacent sections to E,I,M,Q,U, respectively, and with the indicated peptides added. Bar = 20 μm.
The peptide KITRLLKKHKPSN corresponding to the matching amino acid stretches in Nkx6-2 from rat, mouse, and human (Figure 2) did not block immunoreactivity of F64A6B4 and F65A2 in Western blot assays (Figures 4A and 4B) or in stainings on tissue sections (Figures 4K–4N). Surprisingly, preabsorption with the chicken Nkx6-2-specific peptide KITRLLKKHKSTN (Figure 2) turned out to block F64A6B4 and F65A2. F65A2 was efficiently blocked, whereas F64A6B4 was blocked to a lesser degree in the Western blot assays (Figures 4A and 4B). Both were completely blocked in the immunohistochemical stainings (Figures 4O–4R), indicating that F64A6B4 and F65A2 may cross-react to chicken Nkx6-2.
Finally, the peptide KIRLLLRKHRAAF corresponding to the matching amino acid stretches in Nkx6-3 from rat, mouse, human, and chicken (Figure 2) did not block immunoreactivity of F64A6B4 and F65A2 in Western blot assays (Figures 4A and 4B) or in stainings on tissue sections (Figures 4S–4V).
Together this demonstrates that the F64A6B4 and F65A2 monoclonal anti-Nkx6-1 antibodies specifically recognize Nkx6-1 in rat and mouse, where they do not cross-react to Nkx6-2 and Nkx6-3. In contrast, based on the absorption results, F64A6B4 and F65A2 may not recognize chicken Nkx6-1 very well and will possibly cross-react to chicken Nkx6-2.
F55A10 and F55A12, but Not F64A6B4 and F65A2, Recognize Nkx6-1 in Chicken and Human
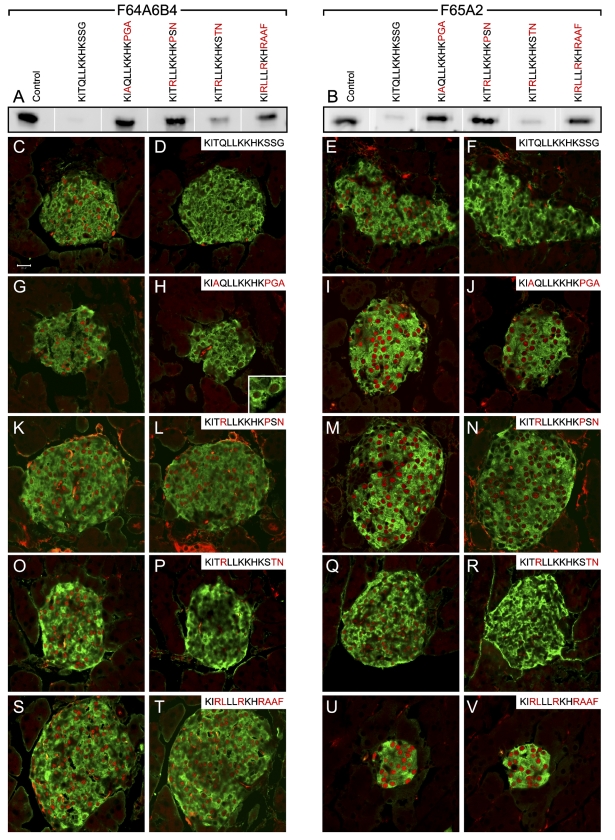
As different species specificities were predicted from the absorption results, we spotted the different peptides on a nitrocellulose membrane and assayed for binding of the four monoclonal antibodies (Figure 5). F55A10 and F55A12 recognized only the Nkx6-1-specific peptide DDDYNKPLDP but not the Nkx6-2- and Nkx6-3-specific peptides (Figures 5A and 5B). F64A6B4 and F65A2 recognized only the mouse Nkx6-1-specific peptide KITQLLKKHKSSG but not the chicken Nkx6-1-specific peptide KIAQLLKKHKPGA or any of the Nkx6-2- and Nkx6-3-specific peptides (Figures 5C and 5D), indicating that F64A6B4 and F65A2 will not recognize chicken Nkx6-2. However, these two antibodies can be preabsorbed with the chicken Nkx6-2-specific peptide (Figure 4).
Figure 5.
Recognition of specific peptides by the four monoclonal anti-Nkx6-1 antibodies. Dot blots with 0–20 μg of the indicated peptides spotted on nitrocellulose membranes and assayed for immunoreactivity to (A) F55A10, (B) F55A12, (C) F64A6B4, and (D) F65A2. White dashed circles illustrate the positioning of the peptide spots on the membranes.
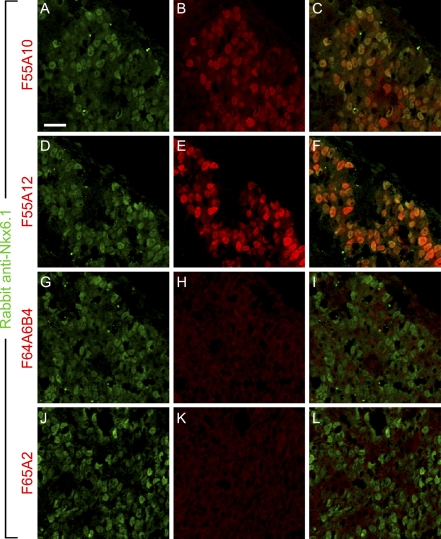
We next tested immunoreactivity of the monoclonal anti-Nkx6-1 antibodies on tissue sections from developing chicken pancreas where Nkx6-1 as well as Nkx6-2 are expressed (Pedersen et al. 2005). As expected, F55A10 and F55A12 both showed a strong staining of the nuclei of pancreatic epithelial cells (Figures 6B and 6E) that colocalize with the staining obtained with a polyclonal anti-Nkx6-1 antiserum (Jensen et al. 1996) (Figures 6A–6F). F64A6B4 and F65A2 did not show any immunoreactivity on chicken pancreas sections (Figures 6H and 6K), verifying the lack of chicken Nkx6-1 reactivity of these antibodies and demonstrating that in spite of the absorption data these antibodies display no detectable cross-reactivity to Nkx6-2, at least under the staining conditions used here.
Figure 6.
F55A10 and F55A12, but not F64A6B4 and F65A2, recognize chicken Nkx6-1. Double-immunohistochemical stainings of E4 chicken pancreas showing Nkx6-1 immunoreactivity by a polyclonal anti-Nkx6-1 rabbit serum in green and by the indicated monoclonal antibodies in red. (A) Staining with the polyclonal anti-Nkx6-1 antiserum overlaps with (B) F55A10 as (C) shown by overlay of the pictures. (D) Likewise, staining with the polyclonal anti-Nkx6-1 antiserum overlaps with (E) F55A12 as (F) shown by overlay of the pictures. (G–L) There is no detectable immunoreactivity of (H) F64A6B4 or (K) F65A2. Bar = 20 μm.
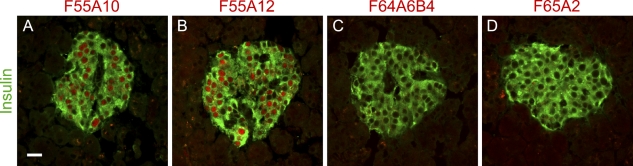
We finally tested whether the monoclonal anti-Nkx6-1 antibodies would recognize human Nkx6-1 on tissue sections from human pancreas. F55A10 and F55A12 both stained the nuclei of islet β-cells (Figures 7A and 7B), whereas F64A6B4 and F65A2 did not show any immunoreactivity (Figures 7C and 7D). This demonstrates that despite the fact that the primary amino acid sequences of mouse and human Nkx6-1 are identical for both epitopes, it is only the one recognized by F55A10 and F55A12 that is conserved, whereas the other epitope diverges, probably due to conformational differences.
Figure 7.
F55A10 and F55A12 but not F64A6B4 and F65A2 recognize human Nkx6-1. Double-immunohistochemical stainings of adult human pancreas showing the indicated monoclonal antibodies in red and insulin immunoreactivity in green. (A) F55A10 demonstrates immunoreactivity toward human Nkx6-1 expressed in β-cells of the islets of Langerhans. (B) Likewise, F55A12 recognizes human Nkx6-1. There is no detectable immunoreactivity of (C) F64A6B4 or (D) F65A2. Bar = 20 μm.
Discussion
Previous data from competitive ELISA experiments suggested that F55A10 and F55A12 recognize the same epitope, just as F64A6B4 and F65A2 share another epitope (Pedersen et al. 2006). In this study we confirm these data and fine map the amino acid sequences of the two epitopes recognized by the four monoclonal antibodies against the transcription factor Nkx6-1 as being DDDYNKP for F55A10 and F55A12 and QLLKKHK for F64A6B4 and F65A2. It is demonstrated that F55A10 and F55A12 both are uniquely specific for Nkx6-1, as peptides corresponding to the same region of other Nkx6 proteins were unable to block the function of the antibodies. The data set underlines the crucial importance of aspartic acid in position 3 as an essential component of the epitope. The F64A6B4 and F65A2 recognized three peptides on the SPOT membrane that have the QLLKKHK sequence in common, but the results obtained in the blocking experiments suggest that the following serine (as found in the mouse Nkx6-1 and chicken Nkx6-2) is also important for epitope recognition, which seems to be obstructed by the presence of a proline at that position (as found in chicken Nkx6-1 and mouse Nkx6-2). It is possible that F64A6B4 and F65A2 not only recognize a linear but also a conformational epitope, which is inhibited by the proline and may be facilitated by the serine. We conclude that F64A6B4 and F65A2 are specific for Nkx6-1 in rat and mouse but cannot be used in assays based on chicken tissue because of their inability to recognize Nkx6-1 and because of the potential risk of cross-reactivity to chicken Nkx6-2. However, dot blots and F64A6B4 and F65A2 immunostainings of chicken tissue presented here do not show any apparent cross-reactivity to Nkx6-2, but we cannot exclude that this may occur under different conditions. In fact, given optimal conditions, F64A6B4 or F65A2 may potentially provide unique tools to study Nkx6-2 expression in the chicken system.
It would be ideal to use tissue from the Nkx6.1 knockout mouse as a control for cross-reactivity to Nkx6-2 or Nkx6-3 because any positive immunohistochemical staining here would demonstrate cross-reactivity. However, because we do not have easy access to Nkx6-1 knockout tissue, peptides corresponding to Nkx6-2 and Nkx6-3 provide useful alternatives.
Finally, our results pave the way for the use of site-directed mutagenesis to engineer novel specificities into these monoclonals such that Nkx6-2- and Nkx6-3-specific monoclonal antibodies may be obtained in the future.
Acknowledgments
This work was supported by National Institutes of Health (Grant #1U01DK-072473) and the EU 6th framework programme.
We thank Karsten Marckstrøm for expert technical assistance and the Beta Cell Biology Consortium for the supply of anti-Nkx6-1 antibodies.
References
- Alanentalo T, Chatonnet F, Karlen M, Sulniute R, Ericson J, Andersson E, Ahlgren U (2006) Cloning and analysis of Nkx6.3 during CNS and gastrointestinal development. Gene Expr Patterns 6:162–170 [DOI] [PubMed] [Google Scholar]
- Cai J, Qi Y, Wu R, Modderman G, Fu H, Liu R, Qiu M (2001) Mice lacking the Nkx6.2 (Gtx) homeodomain transcription factor develop and reproduce normally. Mol Cell Biol 21:4399–4403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efrat S, Linde S, Kofod H, Spector D, Delannoy M, Grant S, Hanahan D, et al. (1988) Beta-cell lines derived from transgenic mice expressing a hybrid insulin gene-oncogene. Proc Natl Acad Sci USA 85:9037–9041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank R (2002) The SPOT-synthesis technique. Synthetic peptide arrays on membrane supports principles and applications. J Immunol Methods 267:13–26 [DOI] [PubMed] [Google Scholar]
- Henseleit KD, Nelson SB, Kuhlbrodt K, Hennings JC, Ericson J, Sander M (2005) NKX6 transcription factor activity is required for α- and β-cell development in the pancreas. Development 132:3139–3149 [DOI] [PubMed] [Google Scholar]
- Jensen J, Serup P, Karlsen C, Nielsen TF, Madsen OD (1996) mRNA profiling of rat islet tumors reveals nkx 6.1 as a β-cell-specific homeodomain transcription factor. J Biol Chem 271:18749–18758 [DOI] [PubMed] [Google Scholar]
- Jørgensen MC, Ahnfelt-Rønne J, Hald J, Madsen OD, Serup P, Hecksher-Sørensen J (2007) An illustrated review of early pancreas development in the mouse. Endocr Rev 28:685–705 [DOI] [PubMed] [Google Scholar]
- Komuro I, Schalling M, Jahn L, Bodmer R, Jenkins NA, Copeland NG, Izumo S (1993) Gtx: a novel murine homeobox-containing gene, expressed specifically in glial cells of the brain and germ cells of testis, has a transcriptional repressor activity in vitro for a serum-inducible promoter. EMBO J 12:1387–1401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson SB, Janiesch C, Sander M (2005) Expression of Nkx6 genes in the hindbrain and gut of the developing mouse. J Histochem Cytochem 53:787–790 [DOI] [PubMed] [Google Scholar]
- Nelson SB, Schaffer AE, Sander M (2007) The transcription factors Nkx6.1 and Nkx6.2 possess equivalent activities in promoting beta-cell fate specification in Pdx1+ pancreatic progenitor cells. Development 134:2491–2500 [DOI] [PubMed] [Google Scholar]
- Pattyn A, Vallstedt A, Dias JM, Sander M, Ericson J (2003) Complementary roles for Nkx6 and Nkx2 class proteins in the establishment of motoneuron identity in the hindbrain. Development 130:4149–4159 [DOI] [PubMed] [Google Scholar]
- Pedersen IL, Klinck R, Hecksher-Sorensen J, Zahn S, Madsen OD, Serup P, Jorgensen MC (2006) Generation and characterization of monoclonal antibodies against the transcription factor Nkx6.1. J Histochem Cytochem 54:567–574 [DOI] [PubMed] [Google Scholar]
- Pedersen JK, Nelson SB, Jorgensen MC, Henseleit KD, Fujitani Y, Wright CV, Sander M, et al. (2005) Endodermal expression of Nkx6 genes depends differentially on Pdx1. Dev Biol 288:487–501 [DOI] [PubMed] [Google Scholar]
- Qiu M, Shimamura K, Sussel L, Chen S, Rubenstein JL (1998) Control of anteroposterior and dorsoventral domains of Nkx-6.1 gene expression relative to other Nkx genes during vertebrate CNS development. Mech Dev 72:77–88 [DOI] [PubMed] [Google Scholar]
- Rudnick A, Ling TY, Odagiri H, Rutter WJ, German MS (1994) Pancreatic beta cells express a diverse set of homeobox genes. Proc Natl Acad Sci USA 91:12203–12207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sander M, Paydar S, Ericson J, Briscoe J, Berber E, German M, Jessell TM, et al. (2000a) Ventral neural patterning by Nkx homeobox genes: Nkx6.1 controls somatic motor neuron and ventral interneuron fates. Genes Dev 14:2134–2139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sander M, Sussel L, Conners J, Scheel D, Kalamaras J, Dela Cruz F, Schwitzgebel V, et al. (2000b) Homeobox gene Nkx6.1 lies downstream of Nkx2.2 in the major pathway of beta-cell formation in the pancreas. Development 127:5533–5540 [DOI] [PubMed] [Google Scholar]
- Vallstedt A, Muhr J, Pattyn A, Pierani A, Mendelsohn M, Sander M, Jessell TM, et al. (2001) Different levels of repressor activity assign redundant and specific roles to Nkx6 genes in motor neuron and interneuron specification. Neuron 31:743–755 [DOI] [PubMed] [Google Scholar]