Abstract
Calsyntenins are members of the cadherin superfamily of cell adhesion molecules. They are present in postsynaptic membranes of excitatory neurons and in vesicles in transit to neuronal growth cones. In the current study, calsyntenin-1 (CST-1) and calsyntenin-3 (CST-3) were identified by mass spectrometric analysis (LC-MS/MS) of integral membrane proteins from highly enriched secretory granule preparations from bovine anterior pituitary gland. Immunofluorescence microscopy on thin frozen sections of rat pituitary revealed that CST-1 was present only in gonadotropes where it colocalized with follicle-stimulating hormone in secretory granules. In contrast, CST-3 was present not only in gonadotrope secretory granules but also in those of somatotropes and thyrotropes. Neither protein was detected in mammatropes. In addition, CST-1 was also localized to the glucagon-containing secretory granules of α cells in the pancreatic islets of Langerhans. Results indicate that calsyntenins function outside the nervous system and potentially are modulators of endocrine function. (J Histochem Cytochem 56:381–388, 2008)
Keywords: pituitary gland, pancreas, calsyntenin, secretory granules, follicle-stimulating hormone, glucagon, insulin, growth hormone, prolactin
Calsyntenins (CST) are three related type I transmembrane proteins that are members of the cadherin superfamily (Vogt et al. 2001; Hintsch et al. 2002). They are Ca2+-binding proteins that localize to postsynaptic membranes in excitatory neurons where they are postulated to modulate calcium-mediated postsynaptic signaling and cell adhesion. Their cytoplasmic domains also interact directly with kinesin-1 (KFC1) to regulate anterograde transport of a subset of cellular vesicles along microtubule tracks from the cell body toward neuronal growth cones (Konecna et al. 2006) and with the adaptor ×11/Mint2 to potentially stabilize the molecules at the cell surface (Araki et al. 2003). The ectodomain of CST-1 is released from motor neurons and accumulates in cerebrospinal fluid, whereas the transmembrane stump is internalized and accumulates within the postsynaptic spine (Vogt et al. 2001). When expressed by transfection in cultured cells, ectodomains of all the calsyntenins are proteolytically cleaved from the intact molecule and rapidly shed in a process analogous to that of Alzheimers' amyloid precursor protein APP (Vogt et al. 2001; Araki et al. 2004). It is therefore possible that the extracellular domain has functions independent of its synaptic activity.
In this study we report that calsyntenins are present in endocrine glands. In a preliminary analysis of secretory granule membrane proteins from anterior pituitary by mass spectrometry (LC/MS-MS), tryptic peptides derived from CST-1 and -3 were identified. Immunolocalization using specific antibodies to CST-1 and -3 confirmed that they were present in pituitary granules. In addition, CST-1 was localized to secretory granules in α cells in the pancreatic islets of Langerhans.
Materials and Methods
Isolation of Secretory Granules
Bovine pituitary granules were isolated using a modified version of our previously published procedure (Colomer et al. 1996). Pituitary glands obtained from a local slaughterhouse were kept on ice while being transported to the laboratory (2–3 hr). After excess tissue and the outer capsule layer were dissected away along with the infundibular stalk, anterior (together with intermediate) segments were washed in homogenization buffer [HB, 0.27 M sucrose, 10 mM Na HEPES, pH 7.2, 1 mM PMSF, 1 mM dithiothreitol (dTT); Sigma–Aldrich, St Louis, MO]. The tissue was finely minced and homogenized using a Teflon/glass homogenizer (Wheaton; Milville, NJ) and rotary drill (three strokes). The homogenate was centrifuged at 600 × g for 10 min to remove unbroken cells and nuclei. The pellet was rehomogenized and centrifuged again. The combined postnuclear supernatants were loaded onto a 1.75-M sucrose cushion and centrifuged at 3000 × g for 30 min, a speed that allows sedimentation of all granule subtypes (Misro and Conn 1988; unpublished data). The interface was collected, diluted in HB, and homogenized with one stroke of a loose-fitting Dounce homogenizer (Kontes; Vineland, NJ). This material was mixed with Percoll (Amersham Biosciences; Piscataway, NJ) to give a final concentration of 40% Percoll in 0.35 M sucrose, 6 mM HEPES, and 1 mM dTT. After centrifugation at 100,000 × g for 40 min, the white granule band near the bottom of the gradient was collected. After dilution with HB, this material was loaded on a sucrose gradient (0.8–2 M sucrose) and centrifuged at 150,000 × g for 4 hr. The broad granule layer was collected and diluted slowly with HB to 0.6 M sucrose. The granules were sedimented at 3000 × g for 40 min. Overall purification of the granules was 4- to 5-fold, based on the specific enrichment of the Prl and GH bands in the granule fraction as compared with the homogenate as measured by densitometry after SDS/PAGE and Coomassie Blue gel staining. Our preparations contained granules of different sizes as determined by electron microscopy and minimal contamination by other organelles (not shown).
To lyse the granules, granule pellets were resuspended in 25 mM Tris–HCl, pH 9, 10 mM reduced glutathione (Sigma–Aldrich), freeze/thawed, and homogenized with a Dounce homogenizer. Membranes were separated from content by centrifugation at 150,000 × g for 3 hr on a sucrose step gradient (0.75 M/1.0 M). The granule lysate (content) above the gradient was saved. Membranes at the interface were collected and briefly sonicated. They were recovered by centrifugation at 200,000 × g. To collect total membranes, the postnuclear supernatant was diluted in lysis buffer, freeze-thawed, and sonicated. Membranes were recovered by centrifugation at 200,000 × g. Total rat pituitary membranes were recovered from frozen pituitaries using the same methodology. To remove peripheral membrane proteins, a carbonate extraction procedure was used (Rindler and Hoops 1990). Membranes were resuspended (in 0.1 M Na2CO3, 10 mM GSH, pH 11), sonicated, and incubated for 1 hr at 4C. Membranes were collected by centrifugation on a sucrose step gradient (0.6/1.0) in the same buffer (200,000 × g for 40 min). The band at the interface was collected and diluted in carbonate buffer. Supernatants were also recovered. After sonication, membranes were sedimented.
LC-MS/MS Analysis
Integral membrane proteins from carbonate-washed membranes were separated by SDS/PAGE on 10% gels. Fifty μg of protein was loaded on each of two adjacent lanes. Gels were fixed in 46% methanol, 7% acetic acid, and 0.1% Coomassie Blue R-250, destained in this solution without Coomassie, and stored in 1% acetic acid until use. For this preliminary study, two separate slices (3–4 mm) corresponding approximately to 55 and 100 kDa were excised from the gel and digested using mass spectrometry grade trypsin (Promega; Madison, WI) at 12.5 to 25.0 ng/μl in 25 mM NH4HCO3 buffer. Acetonitrile (ACN; 10%) was added to the digestion buffer. The resulting peptides were extracted and dried under vacuum and then resuspended in 10 μl of 0.1% formic acid. Four to 6 μl of peptide mixtures were analyzed using nanoflow LC/ESI-MS/MS, with the configuration of a CapLC HPLC coupled directly to a QTOF Micro MS (Waters; Milford, MA).
A C18 precolumn was used to load the sample to a 75-μm × 15-cm fused silica C18 PepMap100 analytical column (LC Packings; Dionex, Sunnyvale, CA). A gradient of 2–40% ACN in 0.1% formic acid was delivered over 120 min at a flow rate of 200 nL/min through a fused silica distal end-coated tip nano-electrospray needle (New Objective; Woburn, MA). Data acquisition involved MS survey scans and automatic data-dependent MS/MS acquisitions, which were invoked after selected ions met preset parameters of minimum signal intensity of 8 counts per second, ion charge state 2+, 3+, or 4+, and appropriate retention time. Survey scans of 1 sec were followed by MS/MS of the three most intense ions for up to 8.8 sec each or until 6000 total MS/MS ion counts per precursor peptide were obtained. Raw MS data were subsequently processed using ProteinLynx software (Waters), which generated DTA files from each MS/MS spectrum and were merged into a single file containing all spectra from the gel bands.
Protein Identification Criteria
Criteria for determining whether protein matches were genuine were similar to what we have previously used for neuronal postsynaptic density and pancreatic secretory granule proteins (Jordan et al. 2004; Rindler et al. 2007). DTA files were used to search the International Protein Index non-redundant bovine protein database using the Mascot search engine (Version 2.1.0; Matrix Science, Boston, MA). Search parameters included peptide mass tolerance of up to 1.5 Da, MS/MS mass tolerance of up to 0.5 Da, and variable oxidation of methionines with up to one missed tryptic cleavage allowed. The Mascot algorithm was used to determine peptide and protein expectation values. Each protein in the peptide summary report that met the probability-based Mowse threshold [p(x)<0.05] determined by Mascot was analyzed by a stricter set of criteria to determine the number of unique peptide matches. To pass our threshold, peptides had to have a minimum of six residues, a Mascot probability-based score >15, an expectation value of <1, a precursor ion mass error of <0.5 Da, no missed cleavages, and a rank of 1. In addition, the peptide could not appear in other proteins of higher score and, for each protein, only the highest scoring peptide (lowest expectation value) with a given amino acid sequence was considered. Spectra of peptides from proteins scoring 50 or below were visually inspected to assure that the peptide assignments were reliable.
Immunofluorescence Microscopy
Male Sprague Dawley rats were obtained from Taconic (Rensselaer, NY). They were maintained and sacrificed according to protocols approved by the Institutional Animal Care and Use Committee of New York University School of Medicine. Rat pituitary or pancreas was cut into 2-mm blocks and fixed for 3 hr in 8% paraformaldehyde in 60 mM PIPES, 25 mM HEPES, 2 mM MgCl2, 10 mM EGTA, pH 6.9 (PHEM). Blocks were washed in PHEM buffer and PBS with 20 mM glycine. Blocks were infused sequentially in Hank's PBS containing 2% and 10% gelatin and then equilibrated with 2.3 M sucrose in PBS overnight at 4C. After incubation at −80C, thin (0.5 μm) frozen sections were prepared on a Leica cryomicrotome (Bannockburn, IL) and bound to Superfrost Plus Gold slides (Electron Microscopic Sciences; Hatfield, PA) with silicone isolators. Sections were stored at 4C in 2.3 M sucrose in PBS until use. After washing five times in PBS and once in PBS with 1% non-fat milk, antibodies were applied overnight (1:250–1:1000). Antibodies against the N-terminal (luminal) moiety of calsyntenin-1 (R85) were raised in rabbits by immunization with a recombinant fragment comprising the two cadherin domains. Recombinant immunogen was produced the same way as reported previously for the corresponding segment of chicken calsyntenin-1 (Vogt et al. 2001). To raise antibodies against the C-terminal (cytoplasmic) segments of calsyntenin-1 (R82) and calsyntenin-3 (R90), peptides comprising the entire cytoplasmic parts of 101 and 88 amino acids, respectively, were used as immunogens. A detailed description of the production of antibody R90 has previously been given (Hintsch et al. 2002). Guinea pig antibodies to prolactin (Prl), growth hormone (GH), thyroid-stimulating hormone (TSH), and follicle-stimulating hormone (FSH) were obtained from the National Institute of Diabetes and Digestive and Kidney Diseases National Hormone and Peptide Program. Guinea pig antibodies to insulin and glucagon were purchased from Linco Research (St Charles, MO). After washing in PBS, Alexa 488 goat anti-rabbit IgG and Alexa 594 anti-guinea pig IgG secondary antibodies (Invitrogen; Carlsbad, CA) were applied. After washing, gelvatol (Rodriguez and Deinhardt 1960) was placed on the slide and covered with a coverslip. Digital images were collected on a Zeiss LSM510 confocal microscope (Carl Zeiss; Thornwood, NY).
Expression of Calsyntenins in Transfected Cells
CST-1 and -3 were expressed in HEK-293T cells using lipofectamine 2000 (Invitrogen) and pcDNA3.1 plasmids encoding murine CST-1 or -3 (Vogt et al. 2001). At 42 hr post-transfection, cells were washed and incubated for 2–3 hr in serum-free DMEM. Cells were then scraped from the dishes in PBS, pelleted, and sonicated in 10 mM Tris, 1 mM EDTA, pH 7.5, with protease inhibitors [0.5 mM PMSF, aprotinin (Sigma–Aldrich), pepstatin, and leupeptin (Roche Applied Science; Indianapolis, IN)]. Membranes were recovered by sedimentation at 200,000 × g.
Western Blotting
Immunoblotting was performed after SDS/PAGE of samples from postnuclear supernatants, granules, and cultured cells on 10% polyacrylamide gels. After transfer to nitrocellulose and blocking in 5% nonfat milk and 0.5% Tween-20 in PBS, antibodies were applied overnight (1:500–1:2000). Secondary antibodies directed against rabbit IgG and coupled to horseradish peroxidase (Jackson Immunoresearch; West Grove, PA) were used with a chemiluminescence substrate (Western Lightning Plus; Perkin Elmer, Boston. MA) to detect immunoreactivity. The film was digitally scanned and processed using Adobe Photoshop (San Jose, CA).
Results
Identification of Calsyntenins in Pituitary Gland Secretory Granules by Mass Spectrometry
A highly enriched fraction of secretory granules from bovine anterior pituitary gland was prepared as described in Materials and Methods. Membrane proteins from this preparation were separated by SDS/PAGE, and two gel slices were excised. Peptides in each slice were analyzed by LC-MS/MS after tryptic digestion of the proteins. Calsyntenins CST-1 and -3 were identified in one of the gel slices (Figure 1A; Table 1). Other proteins detected included islet cell autoantigen ICA512/IA-2A and carboxypeptidase E/H (data not shown), known granule membrane proteins found in all endocrine cells and expressed in pituitary gland (Fricker 1988; Lan et al. 1994; Dirkx et al. 1998).
Figure 1.
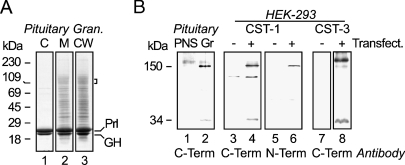
Preparation of pituitary membranes and immunoblotting of CST-1 and -3. Bovine secretory granule (Gr) and postnuclear supernatant fractions (PNS) were prepared as described in Materials and Methods. (A) Equal amounts (10 μg) of proteins from granule content (C), membrane (M), and carbonate-washed membranes (CW) were separated by SDS/PAGE on a 12% acrylamide gel and stained with Coomassie Blue. Molecular mass markers are indicated in the left margin. On the right margin (bracket) is marked the region excised from the preparative gel and used for LC-MS/MS. Prolactin (Prl) and growth hormone (GH) were the major proteins of the content (Lane 1) as well as the membrane fractions (Lane 2) even after carbonate washing (Lane 3). (B) PNS and granule membranes were prepared and separated by SDS/PAGE on 10% gels before Western blotting was performed. Using antibodies to the cytoplasmic domain (C-term) of CST-1, specific bands representing full-length 150-kDa CST and 34-kDa membrane anchor (the stump after shedding of the 115-kDa fragment) were detected in granule membranes (∼5-fold enriched over total membranes; Lanes 1 and 2) and bands of similar sizes in membranes prepared from HEK-293 transfected with a plasmid encoding murine CST-1 (+, Lane 4) but not in membranes from mock transfected cells (−, Lane 3). As expected, antibodies to the CST-1 luminal domain (N-term) recognized only the ∼150-kDa band in HEK-293 cells expressing CST-1 (Lanes 5 and 6). It did not give a significant signal on bovine pituitary tissue (not shown). Although the C-terminal CST-1 antibody did react weakly with an additional band, this was apparently due to a reaction with the secondary antibody used and was observed in all lanes of the gel, including those with nonspecific first antibodies (data not shown). Similarly, antibodies against the cytoplasmic domain of CST-3 reacted with ∼150 and ∼30 kDa proteins in membranes from cells transfected with a plasmid encoding murine CST-3 but not in membranes from mock-transfected cells (Lanes 7 and 8).
Table 1.
Calsyntenins identified by LC-MS/MS in bovine secretory granules
| Observed | Mol mass (Da) (expt) | Mol mass (Da) (calc) | δ | Score | Expectation value | Peptide sequence | |
|---|---|---|---|---|---|---|---|
| Calsyntenin 1 | 1008.0676 | 2014.1206 | 2013.9684 | 0.1522 | 58 | 2.0e–4 | R.AASEFESSEGVFLFPELR.I |
| (NCBI Access #119908402) | |||||||
| Mass = 109724 | |||||||
| Score: 58 | |||||||
| Matched: 1 | |||||||
| Calsyntenin 3 | 752.4241 | 1502.8336 | 1502.7762 | 0.0573 | 38 | 0.033 | R.ESLLLDMASLQQR.G |
| (NCBI Access #115495495) | 817.9556 | 1633.8967 | 1633.8100 | 0.0866 | 26 | 0.6 | R.VNDVNEFAPVFVER.L |
| Mass = 106132 | 866.5150 | 1731.0155 | 1730.9355 | 0.0799 | 60 | 1.9e–4 | R.IEYAPGAGSLALFPGIR.L |
| Score: 80 | |||||||
| Matched: 3 |
Peripheral membrane proteins from bovine pituitary secretory granules were separated by SDS/PAGE and the peptides from individual gel slices determined by LC-MS/MS as described in Materials and Methods. For each protein, the mass (Mol mass), Mascot score, and number of identified peptides are given. Individual calsyntenin peptide sequences are listed along with the observed, experimental (expt), and calculated (calc) masses of the separated peptides and the precursor ion mass error (δ), the Mascot score, and expectation value for each. The Mascot score 95% confidence threshold was 36.
Localization of CST-1 and -3 in Anterior Pituitary Gland
To confirm that these proteins were in secretory granules in pituitary gland, Western blotting was performed. Using membranes from HEK-293 cells expressing murine CST-1, both antibodies directed against the luminal N- and the cytoplasmic C-termini specifically recognized a protein of ∼150 kDa, the expected size for CST-1. In addition, the C-terminal antibody reacted with a 34-kDa fragment that represents the membrane-associated stump of the protein after shedding of its ectodomain containing the N-terminus (Vogt et al. 2001). The C-terminal antibody also recognized proteins of 150 kDa and 34 kDa in bovine pituitary membranes (Figure 1B). Moreover, there was enrichment of CST-1 in granule membranes vs total cellular membranes, supporting the notion that it is a granule membrane protein. Antibody directed against the cytoplasmic tail of murine CST-3 also recognized proteins of similar sizes in transfected HEK-293 cells expressing murine CST-3 (Figure 1B). This antibody did not cross-react with bovine membranes (data not shown).
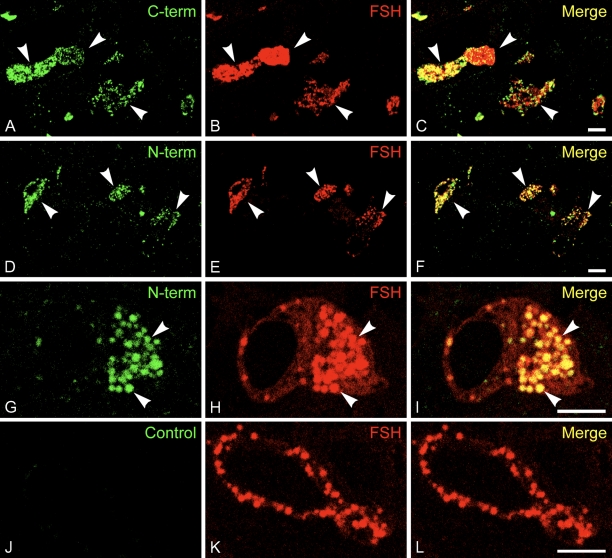
The same antibodies were used to localize CST-1 and -3 on frozen thin sections of rat anterior pituitary gland. As shown in Figure 2, both antibodies to CST-1 labeled a subpopulation of the cells, which were identified as gonadotropes containing FSH. Moreover, the predominant localization was to the secretory granules labeled with anti-FSH. Similar localization was observed with the N- and C-terminal antibodies. No significant localization of CST-1 over the plasma membrane was observed. Some vesicle staining for CST-1 did not correspond to secretory granules containing FSH, especially when the C-terminal antibodies were used. This would indicate that at least a small fraction of the CST-1 is in a different subcellular compartment, perhaps endosomes as was previously observed in neurons (Vogt et al. 2001).
Figure 2.
CST-1 is a gonadotrope secretory granule protein. Frozen thin sections (0.5 μm) of rat pituitary gland were stained for immunofluorescence using rabbit antibodies to the C-terminal (A–C) or N-terminal (D–I) segments of mouse CST-1 and Alexa 488-conjugated anti-rabbit IgG (green). Guinea pig antibodies to follicle-stimulating hormone (FSH) and Alexa 594-conjugated anti-guinea pig IgG (red) were used to identify gonadotropes and FSH-enriched granules within them. Antibodies to CST-1 labeled ∼5% of the cells, which corresponded to those labeled by antibodies to FSH (C,F). Higher magnification views (G,H) confirmed the nearly perfect correspondence between anti-CST-1 and anti-FSH, indicating that CST is in secretory granules (arrowheads). C-terminal antibody also labeled some vesicles in gonadotropes that did not contain FSH. As controls, CST-1 was used alone in single-labeling experiments with the same localization (not shown). In addition, a nonspecific rabbit antiserum was used as a control, and photographs were taken using the same settings (J–L). Bar = 5 μm.
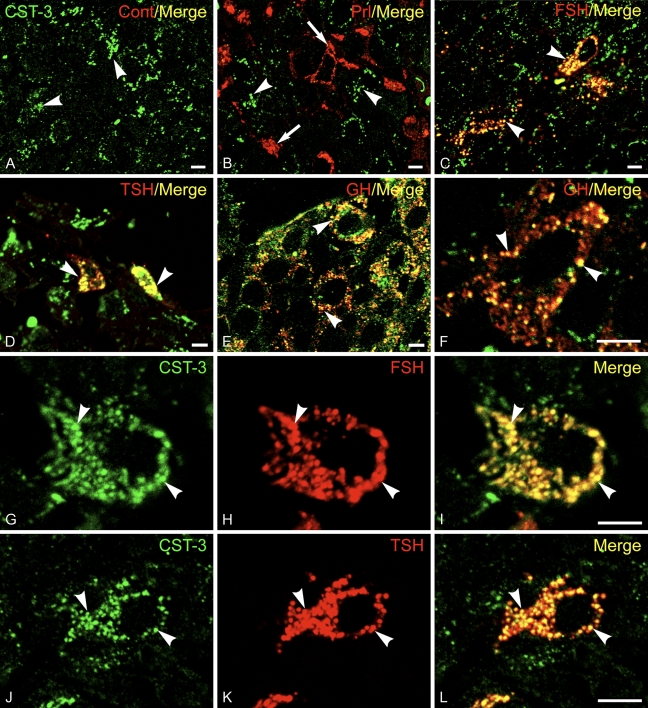
Antibodies directed against CST-3 were also used in immunofluorescence labeling experiments using sections of rat anterior pituitary gland. CST-3 was found to be in secretory granules of gonadotropes, somatotropes, and thyrotropes, which were identified using antibodies to FSH, GH, and TSH (Figure 3). No labeling of Prl-containing mammatropes was observed. As was the case for CST-1, there was little CST-3 localized to the plasma membrane. Results indicate that CST-1 and -3 are secretory granule proteins in anterior pituitary cells.
Figure 3.
CST-3 is a secretory granule protein of anterior pituitary cells. Frozen thin sections of rat pituitary gland were stained for immunofluorescence using rabbit antibodies to CST-3 and Alexa 488-conjugated anti-rabbit IgG (green). Guinea pig antibodies to (A) insulin (as a nonspecific control), (B) Prl, (C) FSH, (D) thyroid-stimulating hormone (TSH), and (E,F) GH as well as Alexa 594-conjugated anti-guinea pig IgG (red) were used to identify the various pituitary cell types and label the granules within them (arrowheads). Antibodies to CST-3 labeled the majority of the cells (arrows), which overlapped (yellow) with those also labeled by antibodies to FSH, TSH, and GH but not Prl. High magnification matched images from sections incubated with anti-CST-3 (G,J) together with anti-FSH (H) or anti-TSH (K) show good correspondence between the staining for CST and the granule proteins (I,L), indicating that CST-3 is in secretory granules. Bar = 5 μm.
Localization of CST-1 in Pancreatic Islets
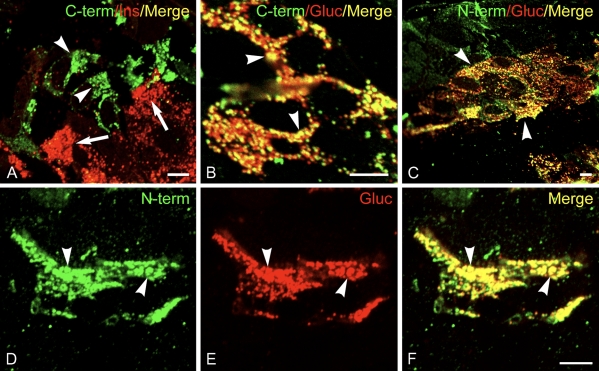
As previously noted, a number of secretory granule membrane proteins are widely expressed in endocrine tissues, including pancreatic islets. We therefore investigated whether calsyntenins may be present in secretory granules in endocrine pancreas. Antibodies to CST-1 also labeled a small number of cells in sections of pancreas (Figure 4). These cells turned out to be primarily α cells of pancreatic islets of Langerhans that also stain with anti-glucagon antibodies. CST-1 colocalized with glucagon in the secretory granules in these cells as well. However, no labeling of insulin-containing β cells was observed. An additional cell population had granular labeling when antibodies to the N- and C-termini of CST-1 were used but were not stained with antibodies to glucagon. These cells are most likely δ cells. No specific labeling was observed when antibodies to CST-3 were applied to the pancreatic sections.
Figure 4.
CST-1 localizes to α cells in islets of Langerhans. Frozen thin sections of rat pancreas were stained with rabbit anti-CST-1 C-terminal (A,B) or N-terminal (C–F) antibodies and guinea pig anti-insulin (A) or glucagon (B–F) as well as Alexa 488-conjugated anti-rabbit IgG (green) and Alexa 594-conjugated anti-guinea pig IgG (red). CST-1 localized to glucagon granules in α cells (arrowheads) and did not stain insulin-containing β cells (arrows). CST-1 also localized to a few cells that did not label with glucagon, taken to be δ cells. Bar = 5 μm.
Discussion
In this study we have shown that calsyntenins are present in endocrine granules of pituitary gland and pancreas. Pituitary data for both CST-1 and -3 were based on two experimental findings: LC-MS/MS analysis on highly enriched granule membrane fractions and immunofluorescence microscopy on frozen sections from anterior pituitary. In addition, by immunoblotting, CST-1 showed enrichment in secretory granule membranes. Moreover, labeling of granules by immunofluorescence microscopy was obtained with two different antibodies for CST-1. The same antibodies were also used to stain pancreatic islets, where CST-1 was found in glucagon granules in α cells and perhaps in granules in δ cells. Interestingly, CST-1 was not detected in β cells in the pancreas and was specific to gonadotropes in the pituitary. The reason for this cellular specificity and that of CST-3, which is lacking in pituitary lactotropes, is not understood. With regard to other endocrine tissues, by immunofluorescence microscopy we did observe weak staining of adrenal chromaffin cells with both anti-CST-1 and anti-CST-3 antibodies (data not shown), which may indicate that calsyntenins are secretory granule proteins in this tissue as well.
Calsyntenins are postulated to be involved in establishment and maintenance of the postsynaptic densities in neurons. However, their function in endocrine cells is very likely to be different. It is conceivable that they play a role at the cell surface of endocrine cells after granule exocytosis. Localization in pituitary cells was somewhat different for N- and C-terminal antibodies. Whereas N-terminal antibody labeling was confined primarily to secretory granules, the C-terminal antibody also labeled other vesicles in the cells. This would be consistent with a scenario whereby the transmembrane stump, after rapid release of ectodomain from the membrane, would be routed to the endocytic pathway as in other cell types (Vogt et al. 2001). Given the well-documented shedding of their ectodomains, it is also reasonable to hypothesize that soluble calsyntenins are released locally, where they could potentially serve as modulators of endocrine function.
Acknowledgments
This study was supported by National Institutes of Health (NIH) Grant DK-067283 (to MJR), NIH–National Institute of Neurological Disorders and Stroke Grant P30 NS-050276, and NIH Shared Instrumentation Grant S10 RR-017990 (to TAN), and the Swiss National Science Foundation, the National Center of Competence in Research (NCCR) Neural Plasticity and Repair, Olga Mayenfisch Foundation, the Jubilaumsstiftung of Rentenanstalt/Swisslife, and the EMDO-Foundation (to PS).
We thank David Sabatini for continuing support and advice and Gustav Hintsch and Katja Fink for assistance.
References
- Araki Y, Miyagi N, Kato N, Yoshida T, Wada S, Nishimura M, Komano H, et al. (2004) Coordinated metabolism of Alcadein and amyloid β-protein precursor regulates FE65-dependent gene transactivation. J Biol Chem 279:24343–24354 [DOI] [PubMed] [Google Scholar]
- Araki Y, Tomita S, Yamaguchi H, Miyagi N, Sumioka A, Kirino Y, Suzuki T (2003) Novel cadherin-related membrane proteins, Alcadeins, enhance the X11-like protein-mediated stabilization of amyloid β-protein precursor metabolism. J Biol Chem 278:49448–49458 [DOI] [PubMed] [Google Scholar]
- Colomer V, Kicska GA, Rindler MJ (1996) Secretory granule content proteins and the luminal domains of granule membrane proteins aggregate in vitro at mildly acidic pH. J Biol Chem 271:48–55 [DOI] [PubMed] [Google Scholar]
- Dirkx R Jr, Hermel JM, Rabin DU, Solimena M (1998) ICA 512, a receptor tyrosine phosphatase-like protein, is concentrated in neurosecretory granule membranes. Adv Pharmacol 42:243–246 [DOI] [PubMed] [Google Scholar]
- Fricker LD (1988) Carboxypeptidase E. Annu Rev Physiol 50:309–321 [DOI] [PubMed] [Google Scholar]
- Hintsch G, Zurlinden A, Meskenaite V, Steuble M, Fink-Widmer K, Kinter J, Sonderegger P (2002) The calsyntenins: a family of postsynaptic membrane proteins with distinct neuronal expression patterns. Mol Cell Neurosci 21:393–409 [DOI] [PubMed] [Google Scholar]
- Jordan BA, Fernholz BD, Boussac M, Xu C, Grigorean G, Ziff EB, Neubert TA (2004) Identification and verification of novel rodent postsynaptic density proteins. Mol Cell Proteomics 3:857–871 [DOI] [PubMed] [Google Scholar]
- Konecna A, Frischknecht R, Kinter J, Ludwig A, Steuble M, Meskenaite V, Indermuhle M, et al. (2006) Calsyntenin-1 docks vesicular cargo to kinesin-1. Mol Biol Cell 17:3651–3663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan MS, Lu J, Goto Y, Notkins AL (1994) Molecular cloning and identification of a receptor-type protein tyrosine phosphatase, IA-2, from human insulinoma. DNA Cell Biol 13:505–514 [DOI] [PubMed] [Google Scholar]
- Misro MM, Conn PM (1988) Isolation and characterization of gonadotropin-rich secretory granules from rat: depletion by GnRH and by a high affinity agonist. Mol Cell Endocrinol 55:131–140 [DOI] [PubMed] [Google Scholar]
- Rindler MJ, Hoops TC (1990) The pancreatic membrane protein GP-2 localizes specifically to secretory granules and is shed into the pancreatic juice as a protein aggregate. Eur J Cell Biol 53:154–163 [PubMed] [Google Scholar]
- Rindler MJ, Xu CF, Gumper I, Smith NN, Neubert TA (2007) Proteomic analysis of pancreatic zymogen granules: identification of new granule proteins. J Proteome Res 6:2978–2992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez J, Deinhardt F (1960) Preparation of a semipermanent mounting medium for fluorescent antibody studies. Virology 12:316–317 [DOI] [PubMed] [Google Scholar]
- Vogt L, Schrimpf SP, Meskenaite V, Frischknecht R, Kinter J, Leone DP, Ziegler U, et al. (2001) Calsyntenin-1, a proteolytically processed postsynaptic membrane protein with a cytoplasmic calcium-binding domain. Mol Cell Neurosci 17:151–166 [DOI] [PubMed] [Google Scholar]