Abstract
Fibroblasts are critical for tissue homeostasis, and their inappropriate proliferation and activation can result in common and debilitating conditions including fibrosis and cancer. We currently have a poor understanding of the mechanisms that control the growth and activation of fibroblasts in vivo, in part because of a lack of suitable fibroblast markers. We have taken advantage of an antibody previously shown to stain stromal cells in frozen tissues (TE-7) and identified conditions in which it can be used to stain fibroblasts and myofibroblasts in the paraffin-embedded tissue samples routinely collected for pathological analysis. We show that this antibody recognizes growing and quiescent fibroblasts and myofibroblasts by immunohistochemistry, immunofluorescence, and ELISA assays. We also present its staining patterns in normal tissue samples and in breast tumors. (J Histochem Cytochem 56:347–358, 2008)
Keywords: fibroblasts, immunohistochemistry, TE-7
Fibroblasts play a critical role in maintaining homeostasis of the microenvironment and in coordinating the complex physiological response to wounds (Martin 1997; Iyer et al. 1999). During early wound healing, growth factors released by inflammatory cells stimulate fibroblasts to migrate toward a wound, proliferate, and secrete a collagen-rich extracellular matrix (Martin 1997). During the wound healing process, fibroblasts differentiate into myofibroblasts, defined by their expression of smooth muscle actin, and actively close the wound by contraction (Garana et al. 1992). Normally, the myofibroblasts disappear at the end of the wound. The continued presence of myofibroblasts within a wound may be associated with fibrous neoplasms called fibromatoses (Fletcher 2000), fibrotic disease (Desmouliere et al. 2005), and a predisposition to cancer (Chang et al. 2004). In addition, epithelial tumors of a number of organs, including breast, are often surrounded by an activated stroma characterized by myofibroblasts that can promote tumorigenesis (van den Hooff 1988; Olumi et al. 1999; Tlsty 2001; Tlsty and Hein 2001; Tuxhorn et al. 2001; Bissell et al. 2002; Coussens and Werb 2002; Beacham and Cukierman 2005; Orimo et al. 2005).
To date, fibroblasts have been difficult to positively identify. In some cases, fibroblasts are identified based on their spindle shape combined with positive staining for the mesenchymal marker vimentin and the absence of staining for epithelial or other mesenchymal cell types, such as muscle cells, astrocytes, or hematopoietic cells (Chang et al. 2002). However, this approach is hardly definitive. Fibroblasts can take on a wide array of shapes in different tissues, whereas vimentin-positive cells that are not fibroblasts, including macrophages, can also have a spindle-shaped appearance. Furthermore, vimentin stains a large number of cell types, making it difficult to identify fibroblasts by elimination.
A few antibodies have been previously reported to recognize fibroblasts, some of which take advantage of fibroblasts as the major producer of collagen. One monoclonal antibody (anti-pC) is directed against the cleaved carboxy terminal propeptide of the triple-helical procollagen molecule. This antibody stains some of the fibroblasts from patients with active pulmonary fibrosis, but not fibroblasts that are not actively dividing (McDonald et al. 1986). 1B10, an IgM antibody that reacts with a surface membrane protein (Singer et al. 1989), has also been reported to be fibroblast-specific. The 1B10 antibody is reported to recognize human fibroblasts, tissue macrophages, and peripheral monocytes (Singer et al. 1989). Yet another reported anti-fibroblast antibody, 5B5, is directed against prolyl-4-hydroxylase. This enzyme catalyzes a hydroxylation reaction that is a prerequisite for triple helix formation of the collagen protein. The 5B5 antibody recognizes fibroblasts in loosely cellular regions, but false-negative staining was observed for resting, mature fibroblasts in dense connective tissue (Janin et al. 1990). Furthermore, the 5B5 antibody has been reported to recognize not only fibroblasts, but also myoepithelial cells (Janin et al. 1990), plasma and acinar cells (Janin et al. 1990), follicular dendritic cells (Bosseloir et al. 1994), and type II alveolar and bronchial epithelial cells (Kasper et al. 1994).
In an attempt to better understand epithelial–mesenchymal interactions during thymus development, Haynes et al. (1984) raised a monoclonal antibody against human thymic stroma, TE-7. Using immunofluorescence on fresh, acetone-fixed tissue, they found that TE-7 defined the mesodermal portion of the stroma and reacted with fibrous tissue and vessels in interlobular septae. TE-7 recognized stroma from every 1 of 16 tissues tested. TE-7 also reacted with fibrosarcomas but none of 10 other tumor types.
Formalin fixation followed by embedding in paraffin is widely used to achieve the best preservation of tissue biopsies before light microscopic analysis. The ability to identify fibroblasts in formalin-fixed, paraffin-embedded tissues would be extremely helpful for a wide variety of pathological analyses. We adopted the TE-7 antibody for identifying fibroblasts in formalin-fixed, paraffin-embedded tissue. We report here its application using immunohistochemistry, ELISA, and immunofluorescence. We also show the results when this antibody is used to stain cultured fibroblasts and other cell types, normal tissue samples, and the fibroblasts surrounding breast tumors.
Materials and Methods
Cell Culture
For ELISA assays and immunofluorescence, human dermal fibroblasts (strain 91FS5) were grown in Fibroblast Growth Medium (FGM)-2 (Cambrex BioScience; Walkersville, MD), which contains 2% FBS, insulin, and fibroblast growth factor (FGF). For cell pellets for immunohistochemistry, human foreskin fibroblasts (HFFs), WI-38 lung fibroblasts, osteoblast-like Saos cells, and glioblastoma U373 cells were grown in DMEM, 0.1 mM non-essential amino acids, 1.0 mM sodium pyruvate, 1% penicillin/streptomycin, and 15% FBS. For THP-1 cells, the same medium was used except the FBS concentration was 10% and 0.05 mM β-mercaptoethanol was added. For cell pellets, cells were rinsed twice with warm PBS and fixed with 10% neutral-buffered formalin at room temperature for 4 hr. Cells were scraped with a rubber policeman, pelleted, and washed with PBS. Pellets were processed and embedded in paraffin as for tissue samples. Alternatively, cells were plated in BD Biocoat poly-d-lysine–coated chamber slides (Thermo Fisher Scientific; Waltham, MA) in appropriate media and incubated overnight at 37C. After transferring the slides to room temperature, the medium was removed and washed twice with PBS. The cells were fixed with 10% neutral-buffered formalin at room temperature for 8 hr and transferred to PBS for immunohistochemistry.
Human Tissue Samples
Normal skin and breast tumors were obtained from the Breast Specimen Repository at Fred Hutchinson Cancer Research Center. Skeletal muscle was collected through the Fred Hutchinson tissue retrieval service. Young thymus tissue from a control tissue block was obtained from the University of Medicine and Dentistry of New Jersey. Informed consent was obtained as described in the Institutional Review Board protocols. Tissue samples were fixed in 10% neutral-buffered formalin and paraffin-embedded.
Immunohistochemistry
For immunohistochemistry, 4-μm sections were cut, baked at 60C for 30 min, and cooled. Sections were deparaffinized and rehydrated through graded alcohols to water. If antigen retrieval was required, it was done at this time as specified for each antibody below. Slides were placed in Tris-buffered saline with 0.1% Tween-20 and loaded onto a DakoCytomation Autostainer. Endogenous peroxidase was blocked with a 10-min incubation of 3% hydrogen peroxide. When using the Vectastain Universal Elite ABC kit (Vector Laboratories; Burlingame, CA), endogenous biotin was blocked using the DAKO Biotin Blocking System (DakoCytomation; Glostrup, Denmark). Slides were washed, and a serum block of 15% normal goat or swine serum plus 5% human serum (Jackson Immunoresearch Laboratories; West Grove, PA) in antibody diluent (TBS, 0.1% Tween-20, 1% BSA) was added for 10 min. The serum block was blown off, and the primary antibody was applied for 30 min at room temperature. A biotin-conjugated goat-derived secondary antibody (Jackson Immunoresearch Laboratories; Zymed, South San Francisco, CA; or Caltag/Invitrogen, Carlsbad, CA) was applied followed by the Vectastain Elite ABC for 30 min for detection unless noted. Slides were washed with wash buffer, incubated with liquid diaminobenzidine tetrahydrochloride plus substrate (DakoCytomation) for 7 min, and rinsed with water. They were counterstained for 2 min with hematoxylin (DakoCytomation), blued with wash buffer, rinsed with water, dehydrated, cleared, and coverslipped. Images were visualized with an Eclipse 50i microscope with Plan Fluor lenses (Nikon; Melville, NY). Images were taken with a Digital DS-L1 camera (Nikon).
Isotype control antibodies were used at the same concentration as primary antibodies. Isotype control antibodies were mouse IgG (I-2000; Vector Laboratories), mouse IgG1 (CBL600; Chemicon, Temecula, CA), mouse IgG2a (CBL601; Chemicon), mouse IgG3 (M9019; Sigma-Aldrich, St Louis, MO), mouse IgM (PP50; Chemicon), and rabbit IgG (011-000-003; Jackson Immunoresearch Laboratories). For mouse IgG antibodies, biotinylated goat anti-mouse IgG F(ab')2 was used as a secondary antibody (Code 115-066-003; Jackson Immunoresearch Laboratories). For mouse IgM antibodies, biotinylated goat anti-mouse IgG, IgA, and IgM (Cat. No. 65-6400; Zymed Laboratories) was used as a secondary antibody. To identify epithelial and glandular cells, we used clone AE1/AE3 (mouse monoclonal IgG1 at 1.27 μg/ml; DakoCytomation) with pH 6 citrate buffer target retrieval (DakoCytomation). To identify endothelial cells, megakaryocytes, and platelets, we used an antibody to von Willebrand factor (rabbit polyclonal IgG at 15.5 μg/ml; DakoCytomation) with pH 8 EDTA antigen retrieval (Trilogy; Cell Marque Corporation, Rocklin, CA) followed by the Envision Rabbit Polymer (DakoCytomation) instead of the Vectastain. To identify macrophages, monocytes, neutrophils, basophils, and natural killer (NK) cells, we used an antibody to the cell surface protein CD68 (mouse monoclonal IgG3 1.9 μg/ml; PG-M1, DakoCytomation) with pH 6 citrate buffer target retrieval (DakoCytomation). To identify smooth muscle cells, myofibroblasts, and myoepithelial cells, we used clone 1A4 against smooth muscle actin (mouse monoclonal IgG2a 1.4 μg/ml; DakoCytomation) with pH 6 citrate buffer target retrieval (DakoCytomation). To identify fibroblasts, we used clone TE-7 (mouse monoclonal IgG1 1.3 μg/ml; Chemicon) with pH 8 EDTA antigen retrieval (Trilogy). For samples on chamber slides, Triton X-100 was added for 20 min prior to the addition of the TE-7 antibody. To identify mesenchymal cells, we used an antibody to vimentin (mouse monoclonal IgG1 1.2 μg/ml; Clone V9, DakoCytomation) with pH 8 EDTA antigen retrieval (Trilogy). To identify proliferating cells, we used an antibody against Ki67 clone MIB1 (mouse monoclonal IgG1 0.8 μg/ml; DakoCytomation) with pH 6 citrate buffer target retrieval (DakoCytomation). For the 5B5 antibody (mouse monoclonal IgG1 at 3.1 μg/ml; DakoCytomation), we used pH 6 citrate buffer target retrieval (DakoCytomation). For the 1B10 antibody (mouse monoclonal IgM at 2 μg/ml; Sigma-Aldrich), we used pH 8 EDTA antigen retrieval (Trilogy).
For analysis of skin samples, we used a blue chromogen so as not to confuse brown antibody stain with melanin. We performed immunohistochemistry as described above up to the tertiary step. Instead of the Ready-to-use ABC-HRP reagent, the Vectastain ABC-AP kit was used according to the manufacturer's instructions and incubated for 30 min. We washed the slides in Dako Wash Buffer and then PBS for 5 min. We used the Vector Blue Alkaline Phosphatase Substrate Kit III (Vector Laboratories). The reagent was prepared as per the manufacturer's directions immediately before use. One half of the reagent was applied, slides were incubated in the dark for 20 min, the liquid was removed, and the remaining half of the solution was applied for another 20 min. Slides were rinsed in three changes of PBS, followed by three changes of dH2O. Sections were counterstained using Nuclear Fast Red (DakoCytomation) for 3 min, rinsed in dH2O, and mounted.
Indirect Immunofluorescence
Quiescent 91FS5 cells were grown for 4 days in FGM-2 and either collected while proliferating or made quiescent by serum starvation for 6 days in Fibroblast Basal Medium containing 0.1% FBS and insulin. Growing or quiescent cells were removed from the plates with trypsin, and the trypsin was inhibited with soybean trypsin inhibitor. Cells were plated on glass coverslips with the appropriate medium and incubated for 24 hr at 37C. The coverslips were collected, washed twice with PBS and fixed with 3% paraformaldehyde for 15 min at room temperature. The cells were washed with PBS three times, permeabilized with 1% Triton X-100 for 30 min, and blocked with PBS containing 5% BSA for 1 hr at room temperature to prevent nonspecific binding of antibodies. Cells were incubated for 1 hr at room temperature with the indicated primary antibody diluted (1:50) in PBS, 1% BSA, 0.1% Tween 20, or in the same buffer in the absence of any antibody (no primary antibody control). Coverslips were washed five times with PBS and incubated for 1 hr at room temperature with 1:75 dilution of FITC-conjugated donkey anti-mouse IgG (Jackson Immunoresearch Laboratories) diluted in the same buffer used for the first antibodies. To visualize the nucleus, Draq 5 (Alexis Biochemicals; San Diego, CA) was added to the samples in a 1:750 dilution for the last half hour of incubation. The cells were washed five times with PBS and mounted with PBS/glycerol in a 1:1 dilution. Images were acquired with a PerkinElmer RS3 spinning disk microscope (PerkinElmer; Waltham, MA) mounted on a Nikon TE2000S microscope using a Plan Apochromat 40 × 1.3 NA objective. Using an UltraVIEW LCI confocal imaging system (PerkinElmer), image stacks (15–20 frames) were recorded every 0.3 μm, and the optical sections were merged. The same magnification and exposure time were used for all samples.
Whole Cell ELISA
ELISA assays were performed using a modified version of the protocol described by Wewetzer et al. (1996) for whole cell ELISA assays. 91FS5 dermal fibroblasts or U373 glioblastoma cells were plated in triplicate into 96-well plates in an appropriate medium with a total volume of 200 μl. Cells were plated at 5000, 8000, and 30,000 cells/well. Cells were incubated overnight at 37C. After transferring the plates to room temperature, the medium was removed and washed twice with PBS. Cells were fixed with 3% paraformaldehyde in PBS for 15 min and washed twice. Cells were permeabilized with 1% Triton X-100 for 30 min and blocked with PBS containing 5% BSA for 1 hr at room temperature. Primary antibody was added in the indicated dilution, and the mixture was incubated at room temperature for 30 min. Plates were washed three times with PBS and treated with 50 μl of horseradish peroxidase (HRP)-conjugated secondary antibody (goat anti-mouse IgG; Sigma), diluted 1:3000 in PBS, 1% BSA, and 0.1% Tween-20, and incubated for 1 hr at room temperature. Plates were washed four times with PBS. To detect the reaction, 100 μl of 2,2′-azino-bis(3-ethylbenzthiazoline-6-sulfonic acid) (ABTS; Invitrogen) was added to each well. After 15–30 min, when the substrate had produced a green color on reaction with peroxides, samples were read with a plate reader at 415 nm. For the primary antibody, we used monoclonal antibodies 1B10 (Sigma-Aldrich), TE-7 (Millipore; Billerica, MA), and 5B5 (GeneTex; San Antonio, TX). Antibodies were diluted to the appropriate concentration with PBS and 1% BSA. As a control, we incubated the cells with Concanavalin A (Con A), which recognizes carbohydrate moieties on cell surface proteins, to normalize for the number of cells in each well. Fixed cells were incubated for 1 hr at room temperature with peroxidase-conjugated Con A (Sigma) and washed three times with PBS. Color development with ABTS was performed as described above.
Results
Immunohistochemical Staining of Cultured Cell Pellets Shows That TE-7 Specifically Recognizes Fibroblasts
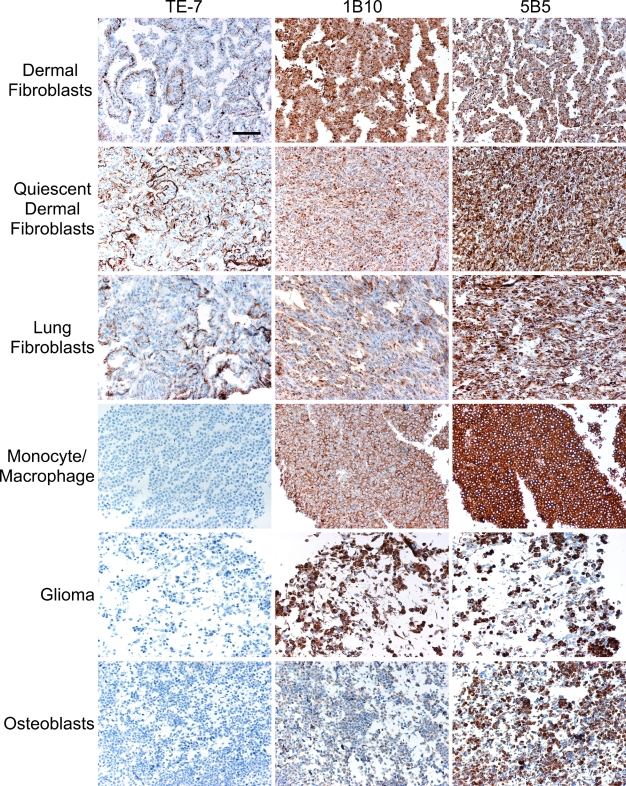
Our goal was to identify growing and quiescent fibroblasts in formalin-fixed, paraffin-embedded tissue samples. Formalin fixation is the method of choice for preservation of tissue morphology and architecture and is routinely used for collection and storage of patient samples. We tested the TE-7, 1B10, and 5B5 antibodies on formalin-fixed cell pellets from cultured cells to determine which antibody showed the best specificity for fibroblasts (Figure 1; Table 1). Cells and their surrounding extracellular matrix were fixed on tissue culture plates, scraped off the plate, pelleted, paraffin-embedded, and analyzed by immunohistochemistry. As controls, we included an antibody to smooth muscle actin as a marker for smooth muscle cells and myofibroblasts, an antibody to CD68 as a marker for monocyte/macrophage lineages, and an antibody to vimentin as a marker for mesenchymal cells. Isotype controls for all antibodies were included. All antibodies were tested on two types of fibroblasts: normal lung fibroblasts (WI38) and normal dermal fibroblasts from foreskin. The dermal fibroblasts were tested in both growing and quiescent conditions. For quiescence, the cells were grown to confluence and maintained under low serum conditions for 1 week. The same antibodies were also tested on glioblastoma U373 cells, monocyte-like THP-1 cells, and osteoblast-like Saos cells. Staining for each antibody on each cell type was scored as negative, weakly positive, or positive. In cases of positive staining, the intensity of the staining was graded on a scale of 1–3, and the fraction of positive cells was noted.
Figure 1.
The TE-7 antibody, but not 1B10 or 5B5, is specific for formalin-fixed cultured fibroblasts. Growing THP-1 monocytes/macrophages, U373 glioblastoma cells, osteoblast-like Saos cells, lung fibroblasts, and dermal fibroblasts in both growing and quiescent states were fixed with formalin and embedded in paraffin. Slides of each cell type (shown in rows) were stained with the TE-7, 1B10, and 5B5 antibodies (shown in columns). Nuclei are stained blue with hematoxylin. Antibody staining is shown in brown from DAB plus substrate. TE-7 but not 1B10 or 5B5 displayed fibroblast-specific staining. Bar = 10 μm.
Table 1.
Immunohistochemical staining of cell pellets from a variety of cell types with TE-7 and other antibodies
| Dermal fibroblasts | Quiescent dermal fibroblasts | Lung fibroblasts WI-38 | Monocyte/macrophage THP1 | Glioma U373 | Osteosarcoma Saos | |
|---|---|---|---|---|---|---|
| IgG1 | Na | +/−b | N | N | N | N |
| IgG2 | N | N | N | +/− | N | 2c, 40%d |
| IgG3 | N | N | N | N | N | N |
| IgM | N | N | N | N | N | N |
| Vimentin | 3, 100% | 3, 100% | 3, 100% | 2, 10% | 3, 100% | 3, 100% |
| CD68 | N | N | N | 2, 50% | N | N |
| Smooth muscle actin | 2, 10% | 2, 30% | 3, 100% | N | +/− | 1, 80% |
| TE-7 | 2, 50% | 2, 50% | 2, 50% | N | N | N |
| 1B10 | 2, 100% | 1, 100% | 1, 100% | 2, 100% | 1, 100% | +/− |
| 5B5 | 2, 90% | 3, 100% | 2, 90% | 3, 100% | 3, 50% | 3, 60% |
N, negative for staining.
+/−, weak staining.
Staining intensity on a scale from 1 to 3.
Percent of cells that are positive.
The three isotype control antibodies did not positively stain any of the various cell types (Table 1). The antibody to vimentin stained the fibroblasts, the glioblastoma cells, the osteoblast-like cells, and a subset of the monocyte-like cells. The antibody to cell surface marker CD68 recognized the monocyte-like line THP-1. The antibody to smooth muscle actin was negative on the THP-1 monocytes as expected, but stained the glioblastoma U373 and Saos osteoblast-like cells weakly. Stronger staining was observed with smooth muscle actin on a subset of dermal fibroblasts in both the growing and quiescent conditions. Intense smooth muscle actin staining was observed in essentially all of the WI-38 lung fibroblasts, suggesting that these cells have a myofibroblast character.
The 5B5 antibody and the 1B10 antibody positively stained dermal and lung fibroblasts (Table 1; Figure 1) as would be expected for fibroblast markers. However, these antibodies also positively stained non-fibroblast cell types: the osteoblasts, monocytes/macrophages, and glioblastoma cells. The TE-7 antibody, in contrast, was negative for staining in the monocyte THP-1, the glioblastoma U373, and the osteoblastoma Saos cells. Approximately 50% of the WI-38 lung fibroblasts and 50% of the growing and quiescent dermal fibroblasts appeared positive for TE-7 staining. TE-7 stained these cell pellets with moderate intensity. We thus conclude that, in formalin-fixed, paraffin-embedded tissues, the TE-7 antibody, but not 5B5 or 1B10, is specific for fibroblasts. Furthermore, the TE-7 antibody recognized dermal and lung fibroblasts, growing and quiescent fibroblasts, and fibroblasts with and without a myofibroblast phenotype.
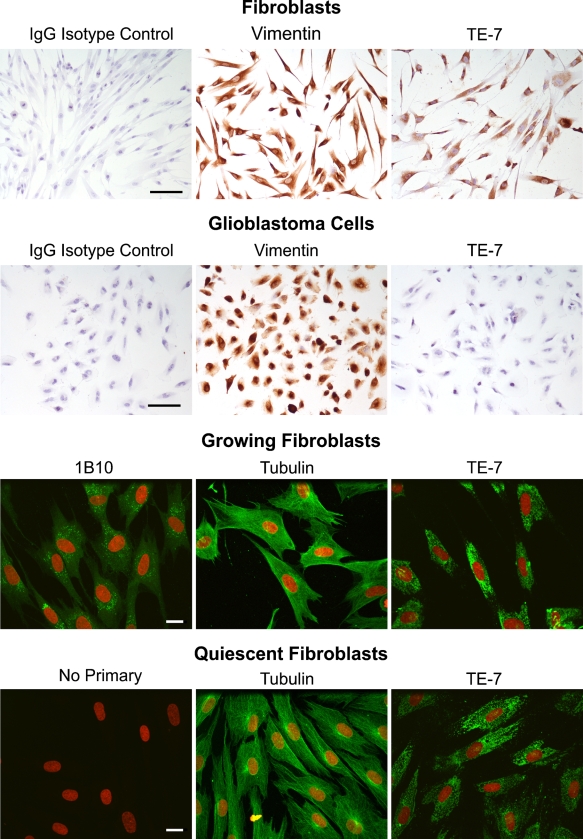
Immunohistochemistry and Immunofluorescence on Cultured Fibroblasts Shows That TE-7 Stains Growing and Quiescent Fibroblasts
When we used TE-7 to stain cell pellets, we discovered that only 50% of the fibroblasts were clearly positive for TE-7 staining. We hypothesized that this might be because the cells within the cell pellet are not a monolayer and that slicing through the three-dimensional cell pellet in a single thin plane missed the staining actually present in a different focal plane of the same cell. To test this hypothesis, we plated fibroblasts in a monolayer on chamber slides, fixed them with neutral-buffered formalin, and stained them with TE-7. As shown in Figure 2, each and every fibroblast stained with TE-7, showing that TE-7 does not stain only a subpopulation of cultured fibroblasts. Fibroblasts also stained with an antibody to vimentin, but not with an isotype control antibody. Glioblastoma cells stained under the same conditions did not stain with TE-7 (Figure 2). To further characterize TE-7 staining of fibroblasts, we performed immunofluorescence followed by confocal microscopy on growing and quiescent dermal fibroblasts with the TE-7 antibody (Figure 2). Negative controls incubated in the absence of primary antibody did not stain. As a positive control, we used an antibody to α-tubulin. 1B10 stained essentially every growing cell. TE-7 displayed a punctate staining pattern in essentially every fibroblast in both growing and quiescent conditions.
Figure 2.
TE-7 recognizes growing and quiescent cultured fibroblasts. Human diploid fibroblasts (strain 91FS5) were analyzed with immunohistochemistry and immunofluorescence. Fibroblasts were plated onto chamber slides, fixed in formalin, and stained with the indicated antibody using immunohistochemistry (top). An anti-vimentin antibody and TE-7 recognized essentially every cell. An IgG isotype control was negative. As a control, glioblastoma cells were also analyzed, and TE-7 was negative on these cells, whereas an anti-vimentin antibody stained them. Growing and quiescent fibroblasts were also grown on coverslips and analyzed by immunofluorescence (bottom). Growing cells were incubated with 1B10, anti-α-tubulin, or TE-7. All three antibodies recognized antigen in the growing cells. In quiescent cells, no staining was observed in the absence of primary antibody, as expected. The expected pattern for α-tubulin was observed and the TE-7 antibody recognized essentially every quiescent fibroblast. Black bar = 10 μm. White bar = 20 μm.
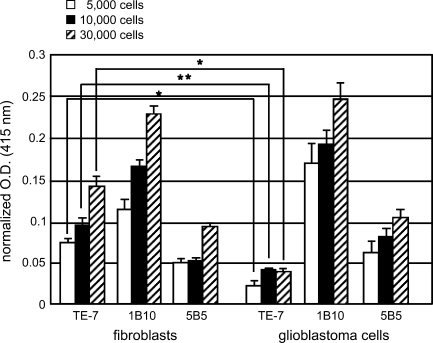
ELISA Assays Show That TE-7 Has High Sensitivity and Specificity for Fibroblasts
To further characterize the sensitivity and specificity of the TE-7 antibody and to compare it with the 1B10 and 5B5 antibodies, we performed a whole cell ELISA assay on fibroblasts and the glioblastoma U373 cells. As shown in Figure 3, TE-7 produced a significantly stronger signal for the same number of cells in fibroblasts compared with glioblastoma cells. This was not the case for 5B5 or 1B10, which gave similar signals for the fibroblasts and glioma cells, consistent with the immunohistochemistry results on cell pellets. When the assay was performed on as few as 5000 fibroblasts per well, increased TE-7 signal was observed compared with the glioblastoma cells.
Figure 3.
TE-7, but not 5B5 or 1B10, specifically reacts with fibroblasts using ELISA. Whole cell ELISA assays were performed using the TE-7, 5B5, and 1B10 antibodies on fibroblasts and glioblastoma cells. Cells were plated at 5000, 10,000, and 30,000 cells/well in triplicate. Optical densities at 415 nm were determined for each antibody and were normalized to concanavalin A levels for cells in the same condition. The average signal intensities and SDs are plotted. TE-7 staining on fibroblasts vs glioblastoma cells was statistically significantly different for 5000, 10,000, and 30,000 cells/well. An asterisk indicates p<0.05. Two asterisks indicate p<0.0005.
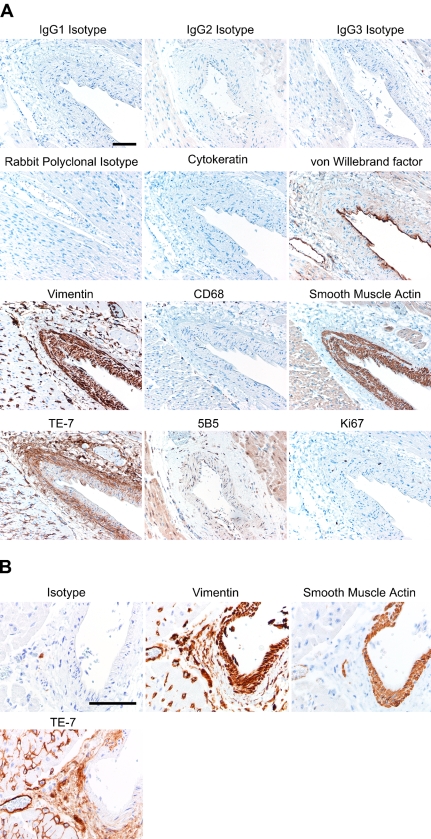
TE-7 Recognizes the Fibroblasts Within the Adventitia Surrounding Blood Vessels in Skeletal and Heart Muscle
We tested TE-7 in formalin-fixed, paraffin-embedded normal human tissue samples. In Figure 4A, we show the staining pattern with a number of antibodies, including TE-7, on a large blood vessel in skeletal muscle. Isotype controls were negative as expected. Staining with the AE1/AE3 antibody that recognizes epithelial cells was negative in skeletal muscle. Endothelial cells lining the large blood vessels and smaller blood vessels were recognized by an antibody against von Willebrand factor. An anti-vimentin antibody recognized all mesenchymal cells, including the smooth muscle cells and fibroblasts in the tunica media and the adventitia surrounding the large blood vessel. The anti-vimentin antibody also recognized the smooth muscle cells lining smaller capillaries scattered throughout the skeletal muscle. A small number of vimentin-positive cells scattered throughout the tissue are most likely cells of monocyte/macrophage origin because they were recognized by an antibody against the monocyte/macrophage surface marker CD68. Smooth muscle cells of the tunica media surrounding the endothelial cells were recognized by an antibody against smooth muscle actin. Smooth muscle actin was also expressed by cells that are likely myofibroblasts in the adventitia of the large vessel. The TE-7 antibody stained essentially all of the spindle-shaped cells in the adventitia of the large blood vessel, as would be expected for a marker that recognizes fibroblasts. TE-7 also stained regions surrounding smaller vessels throughout the skeletal muscle area. In addition, the protein recognized by TE-7 is a constituent of the endomysium that surrounds individual muscle fibers. Staining with the 5B5 antibody previously reported to recognize fibroblasts (Janin et al. 1990) resulted in staining of the muscle cells themselves, which would not be consistent with fibroblast-specific staining. Therefore, in a complex human tissue containing a wide variety of cell types, TE-7 antibody staining is consistent with it recognizing fibroblasts. Finally, staining with an antibody to the proliferation marker Ki-67 (Figure 4) showed that only a small fraction of the cells were actively dividing. Thus, our observations on human tissues support our conclusion that the TE-7 antibody recognizes a protein synthesized by fibroblasts and that it can be used to recognize non-dividing as well as proliferating fibroblasts.
Figure 4.
TE-7 recognizes adventitial fibroblasts surrounding blood vessels. (A) A portion of skeletal muscle from a healthy individual was formalin-fixed and paraffin-embedded. Serial sections were processed with a different primary antibody with its associated antigen retrieval method and secondary antibody. Results are shown for isotype controls (IgG1, IgG2, IgG3, and rabbit polyclonal). Staining with the AE1/AE3 antibody against epithelial cytokeratin was negative. Endothelial cells lining the blood vessels were recognized by an antibody against von Willebrand factor. All cells of mesenchymal origin, including fibroblasts and smooth muscle cells, were recognized by an anti-vimentin antibody. Cells of monocyte/macrophage lineage were scattered through the tissue and are identified by an anti-CD68 antibody. Smooth muscle cells of the tunica media labeled positively with an antibody to smooth muscle actin. Staining with the TE-7 antibody identified fibroblasts in the adventitia. The 5B5 antibody stained mostly surrounding muscle rather than fibroblasts. A small fraction of the cells are actively proliferating as determined by staining with an antibody that recognizes the proliferation marker Ki-67. (B) Heart muscle was formalin-fixed and paraffin-embedded. Serial sections were processed with antibodies as described in Materials and Methods. Staining with an isotype control IgG antibody resulted in little staining. An antibody to vimentin stained the tunica media, the adventitia, and cells within the heart muscle. An antibody to smooth muscle actin recognized the smooth muscle cells of the tunica media. Staining with the TE-7 antibody identified fibroblasts in the adventitia. TE-7 was largely negative on the tunica media smooth muscle cells. TE-7 also stained scattered cells and possibly a portion of the endomysium in the heart muscle. Bar = 10 μm.
In Figure 4B, we show further staining of an artery within heart muscle. Staining with an isotype control is negative as expected. An antibody to vimentin recognizes the smooth muscle cells surrounding the vessel as well as the fibroblasts in the adventitia and cells within the heart muscle itself. An antibody to smooth muscle actin recognizes the smooth muscle cells surrounding the artery. TE-7 is largely negative for the smooth muscle cells while staining the fibroblasts within the adventitial region and scattered fibroblasts in the heart muscle. Thus, TE-7 does not routinely stain smooth muscle cells surrounding vessels.
TE-7 Recognizes Fibroblasts Below the Epidermal Layer in Normal Skin
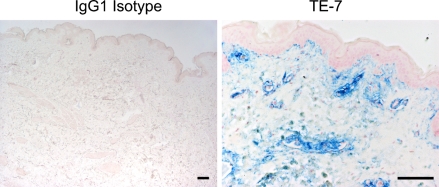
Fibroblasts are prominent in the layer of tissue below the epidermis in skin. We tested whether TE-7 recognizes fibroblasts in this anatomical region. We used the TE-7 antibody to identify fibroblasts in normal breast skin tissue (Figure 5). The TE-7 antigen was present in the fibroblast-rich region below the epidermis as expected. For staining of the skin, we used a blue chromogen to distinguish antibody staining from melanin.
Figure 5.
TE-7 stains cells below the epidermis in normal skin tissue. Human dermis was collected from breast tissue, fixed, and paraffin-embedded. Slides were cut and stained with an IgG1 isotype control or the TE-7 antibody as described in Materials and Methods. An image of the isotype control was taken to show that there was no background staining. TE-7 staining is shown in blue so as not to confuse antibody staining with melanin. The TE-7 antibody stains cells just below the epidermis as expected for fibroblasts. Bar = 10 μm.
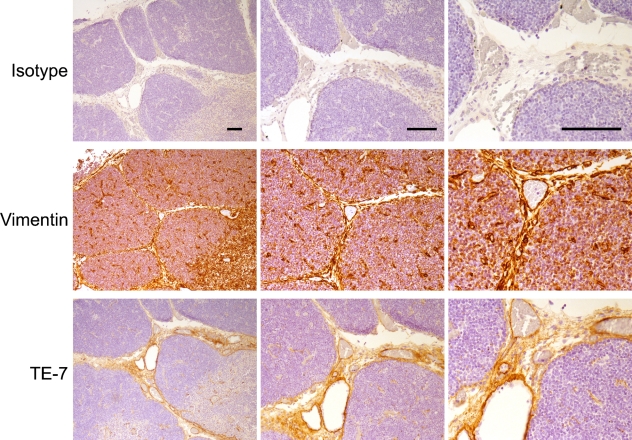
TE-7 Recognizes Stromal Regions in Normal Thymus
We also used immunohistochemistry with TE-7 to stain paraffin-embedded young human thymus. We discovered that TE-7 did not stain the epithelial cells but did recognize the vimentin-positive fibrous tissue and vessels of the interlobular septae (Figure 6). These results are similar to those reported by Haynes et al. (1984) in their original report describing the production and characterization of the TE-7 antibody. Using indirect immunofluorescence, they came to a similar conclusion that TE-7 stains the fibrous interlobular septae in acetone-fixed frozen thymus from a 3-month-old subject (Haynes et al. 1984).
Figure 6.
TE-7 stains stromal cells in the interlobular region within young thymus Young thymus tissue was stained with isotype controls, TE-7, or vimentin. Although staining with an isotype control results in negligible staining, TE-7 recognizes the fibrous tissue of the interlobular septae also recognized by vimentin. Bar = 10 μm.
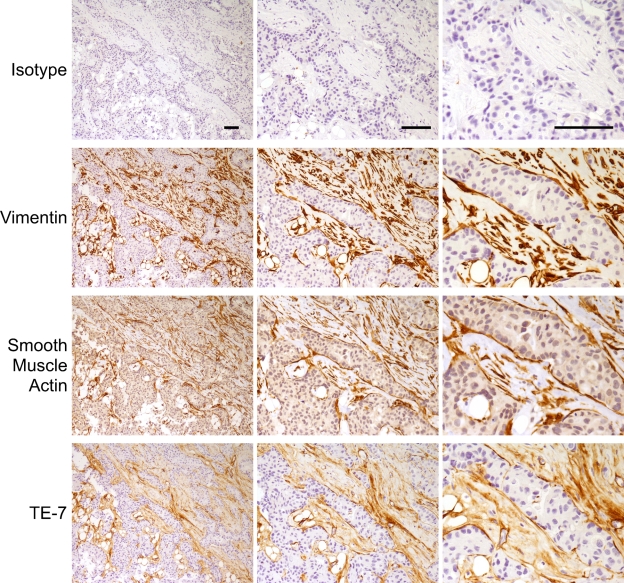
TE-7 Recognizes Myofibroblast-like Cells Within a Breast Tumor
Myofibroblast-like cells surround breast tumors and likely contribute to the tumorigenic process (van den Hooff 1988; Olumi et al. 1999; Tlsty 2001; Tlsty and Hein 2001; Tuxhorn et al. 2001; Bissell et al. 2002; Coussens and Werb 2002; Beacham and Cukierman 2005; Orimo et al. 2005). We asked whether the TE-7 antibody and an antibody to smooth muscle actin recognizes these cells, as would be expected for myofibroblasts. In Figure 7, we show staining of a high-grade infiltrating ductal breast carcinoma with a range of antibodies including TE-7. Isotype controls did not stain. Vimentin, smooth muscle actin, and TE-7 all stained the stromal regions strongly. At high magnification, it is clear that these antibodies are staining the same cell population. Thus, in this tumor, TE-7 stained the stromal fibroblasts and myofibroblasts.
Figure 7.
TE-7 recognizes the fibroblast-like cells in the stromal regions of a breast tumor. Serial sections of a high-grade infiltrating ductal breast carcinoma were stained with an isotype control antibody, an antibody to vimentin, an antibody to smooth muscle actin, or TE-7. Each row represents a different antibody. Three magnifications of the same image are shown. The antibody to vimentin, the antibody to smooth muscle actin, and the TE-7 antibody all stained the stromal myofibroblasts but not the tumor cells themselves. Bar = 10 μm.
Discussion
Fibroblast proliferation and activation is critical for wound healing (Martin 1997; Iyer et al. 1999) and goes awry in a number of pathologies including fibromatosis, sarcomas, and fibrosis (Fletcher 2000; Desmouliere et al. 2005). Activated fibroblasts stimulate tumor progression of adjacent epithelial cells (van den Hooff 1988; Olumi et al. 1999; Tlsty 2001; Tlsty and Hein 2001; Tuxhorn et al. 2001; Bissell et al. 2002; Coussens and Werb 2002; Beacham and Cukierman 2005). A better understanding of these complex pathologies would be aided by methods to positively identify fibroblasts under a wide range of cellular states. At present, identification of fibroblasts is often based on positive staining with vimentin followed by elimination of other non-fibroblast mesenchymal cells (Chang et al. 2002). We report here an immunohistochemical method to positively identify fibroblasts in formalin-fixed, paraffin-embedded tissue. A pH 8 EDTA antigen retrieval allowed specific, reproducible, and clear detection of the TE-7 antigen in formalin-fixed tissues. Importantly, we discovered that TE-7 staining must be performed on freshly cut slides to get reliable and reproducible results.
In formalin-fixed cell pellets, the TE-7 antibody was more specific than the 1B10 or 5B5 antibodies on the cell types tested (Figure 1; Table 1). The TE-7 antibody recognized both dermal and lung fibroblasts and both growing and quiescent fibroblasts, but did not react with osteosarcoma, glioblastoma, or monocyte/macrophage cells. The 1B10 and 5B5 antibodies were less specific; they recognized fibroblasts, but also recognized the non-fibroblast cell types tested. Other anti-fibroblast antibodies have been found to recognize only proliferating fibroblasts. The 5B5 antibody and the (anti-pC) fibroblast-specific antibody, which recognizes the portion of the procollagen molecule that is cleaved before secretion, recognize only fibroblasts that are actively dividing (McDonald et al. 1986; Janin et al. 1990). This limits their usefulness for identifying fibroblasts in quiescent conditions. In contrast, the TE-7 antibody recognizes growing and quiescent cultured fibroblasts by immunohistochemistry on cell pellets (Figure 1) and immunofluorescence (Figure 2). TE-7 recognizes fibroblasts in tissues with little Ki-67 staining (Figure 4A).
TE-7 also recognized myofibroblasts. In several examples of fibroblasts surrounding blood vessels, including the skeletal muscle blood vessel shown in Figure 4A, TE-7 stained both fibroblasts and myofibroblasts in the adventitia. TE-7 also stained myofibroblasts surrounding breast tumors (Figure 7). We conclude that TE-7 recognizes both fibroblasts and myofibroblasts and fibroblasts in both proliferating and quiescent conditions. We expect the TE-7 antibody will prove a valuable tool for unraveling the role of fibroblasts in development, homeostasis, fibrotic disease, soft tissue tumors, and epithelial carcinomas.
Acknowledgments
H.A.C. is the Milton E. Cassel Scholar of the Rita Allen Foundation. This work was also supported by grants from the Avon Opportunity Breast Cancer Fund, the Leukemia Lymphoma Society, and the New Jersey Commission on Cancer Research to H.A.C.
The authors thank David Madtes, Keith Loeb, Harry Hwang, Robert Hackman, and Sue Knoblaugh [Fred Hutchinson Cancer Research Center (FHCRC)], and Leroy Sharer (New Jersey Medical School) for helpful discussions; Louise Soltow and Eric Vallieres (University of Washington), and Peggy Porter, Lindi Farrell, and Barbara Stein (FHCRC) for assistance with tissue collection; Carrie Francek, Elizabeth Pollina, Joe Goodhouse, and Christina DeCoste (PU), and Lisa McLemore, Sharon McHale, and Liyun Sang (FHCRC), for technical assistance; and Ole William Petersen (The Panum Institute, Copenhagen, Denmark), Tom Norwood and Tony Subaru (University of Washington), and Patricia Robinson, Dai Wang, and Tom Shenk (Princeton University) for reagents.
References
- Beacham DA, Cukierman E (2005) Stromagenesis: the changing face of fibroblastic microenvironments during tumor progression. Semin Cancer Biol 15:329–341 [DOI] [PubMed] [Google Scholar]
- Bissell MJ, Radisky DC, Rizki A, Weaver VM, Petersen OW (2002) The organizing principle: microenvironmental influences in the normal and malignant breast. Differentiation 70:537–546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosseloir A, Heinen E, Defrance T, Bouzhazha F, Antoine N, Simar LJ (1994) Moabs MAS516 and 5B5, two fibroblast markers, recognize human follicular dendritic cells. Immunol Lett 42:49–54 [DOI] [PubMed] [Google Scholar]
- Chang HY, Chi JT, Dudoit S, Bondre C, van de Rijn M, Botstein D, Brown PO (2002) Diversity, topographic differentiation, and positional memory in human fibroblasts. Proc Natl Acad Sci USA 99:12877–12882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang HY, Sneddon JB, Alizadeh AA, Sood R, West RB, Montgomery K, Chi JT, et al. (2004) Gene expression signature of fibroblast serum response predicts human cancer progression: similarities between tumors and wounds. PLoS Biol 2:E7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coussens LM, Werb Z (2002) Inflammation and cancer. Nature 420:860–867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desmouliere A, Chaponnier C, Gabbiani G (2005) Tissue repair, contraction, and the myofibroblast. Wound Repair Regen 13:7–12 [DOI] [PubMed] [Google Scholar]
- Fletcher CD (2000) Diagnostic Histopathology of Tumors. 2nd ed. London, Harcourt Publishers
- Garana RM, Petroll WM, Chen WT, Herman IM, Barry P, Andrews P, Cavanagh HD, et al. (1992) Radial keratotomy. II. Role of the myofibroblast in corneal wound contraction. Invest Ophthalmol Vis Sci 33:3271–3282 [PubMed] [Google Scholar]
- Haynes BF, Scearce RM, Lobach DF, Hensley LL (1984) Phenotypic characterization and ontogeny of mesodermal-derived and endocrine epithelial components of the human thymic microenvironment. J Exp Med 159:1149–1168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyer VR, Eisen MB, Ross DT, Schuler G, Moore T, Lee JC, Trent JM, et al. (1999) The transcriptional program in the response of human fibroblasts to serum. Science 283:83–87 [DOI] [PubMed] [Google Scholar]
- Janin A, Konttinen YT, Gronblad M, Karhunen P, Gosset D, Malmstrom M (1990) Fibroblast markers in labial salivary gland biopsies in progressive systemic sclerosis. Clin Exp Rheumatol 8:237–242 [PubMed] [Google Scholar]
- Kasper M, Schuh D, Muller M (1994) Immunohistochemical localization of the beta subunit of prolyl 4-hydroxylase in human alveolar epithelial cells. Acta Histochem 96:309–313 [DOI] [PubMed] [Google Scholar]
- Martin P (1997) Wound healing: aiming for perfect skin regeneration. Science 276:75–81 [DOI] [PubMed] [Google Scholar]
- McDonald JA, Broekelmann TJ, Matheke ML, Crouch E, Koo M, Kuhn C 3rd (1986) A monoclonal antibody to the carboxyterminal domain of procollagen type I visualizes collagen-synthesizing fibroblasts. Detection of an altered fibroblast phenotype in lungs of patients with pulmonary fibrosis. J Clin Invest 78:1237–1244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olumi AF, Grossfeld GD, Hayward SW, Carroll PR, Tlsty TD, Cunha GR (1999) Carcinoma-associated fibroblasts direct tumor progression of initiated human prostatic epithelium. Cancer Res 59:5002–5011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orimo A, Gupta PB, Sgroi DC, Arenzana-Seisdedos F, Delaunay T, Richardson AL, Weinberg RA (2005) Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1/CXCL12 secretion. Cell 121:335–348 [DOI] [PubMed] [Google Scholar]
- Singer KH, Scearce RM, Tuck DT, Whichard LP, Denning SM, Haynes BF (1989) Removal of fibroblasts from human epithelial cell cultures with use of a complement fixing monoclonal antibody reactive with human fibroblasts and monocytes/macrophages. J Invest Dermatol 92:166–170 [DOI] [PubMed] [Google Scholar]
- Tlsty TD (2001) Stromal cells can contribute oncogenic signals. Semin Cancer Biol 11:97–104 [DOI] [PubMed] [Google Scholar]
- Tlsty TD, Hein PW (2001) Know thy neighbor: stromal cells can contribute oncogenic signals. Curr Opin Genet Dev 11:54–59 [DOI] [PubMed] [Google Scholar]
- Tuxhorn JA, Ayala GE, Rowley DR (2001) Reactive stroma in prostate cancer progression. J Urol 166:2472–2483 [PubMed] [Google Scholar]
- van den Hooff A (1988) Stromal involvement in malignant growth. Adv Cancer Res 50:159–196 [DOI] [PubMed] [Google Scholar]
- Wewetzer K, Heiniger C, Seilheimer B (1996) An improved cell-ELISA for the differential screening of antibodies against cell surface molecules of viable adherent Schwann cells. J Immunol Methods 191:171–178 [DOI] [PubMed] [Google Scholar]