Abstract
A role for the copper transporter, ATP7B, in secretion of copper from the human breast into milk has previously not been reported, although it is known that the murine ortholog of ATP7B facilitates copper secretion in the mouse mammary gland. We show here that ATP7B is expressed in luminal epithelial cells in both the resting and lactating human breast, where it has a perinuclear localization in resting epithelial cells and a diffuse location in lactating tissue. ATP7B protein was present in a different subset of vesicles from those containing milk proteins and did not overlap with Menkes ATPase, ATP-7A, except in the perinuclear region of cells. In the cultured human mammary line, PMC42-LA, treatment with lactational hormones induced a redistribution of ATP7B from a perinuclear region to a region adjacent, but not coincident with, the apical plasma membrane. Trafficking of ATP7B was copper dependent, suggesting that the hormone-induced redistribution of ATP7A was mediated through an increase in intracellular copper. Radioactive copper (64Cu) studies using polarized PMC42-LA cells that overexpressed mAtp7B protein showed that this transporter facilitates copper efflux from the apical surface of the cells. In summary, our results are consistent with an important function of ATP7B in the secretion of copper from the human mammary gland. (J Histochem Cytochem 56:389–399, 2008)
Keywords: ATP7B, copper, mammary gland, secretion, lactational hormones
An adequate supply of copper is essential during normal growth and development, and in mammals, this is delivered to the neonate in milk. During lactation in rats, there is a 20-fold increase in transport of plasma copper to the mammary gland (Donley et al. 2002). Copper concentrations in milk are high after birth (0.4–0.6 mg/liter in humans) but decrease during late lactation both in humans (0.2–0.3 mg/liter) (Perrone et al. 1993; Lönnerdal 1996; Wooten et al. 1996; Friel et al. 1999) and rodents (Linder et al. 1998). The supply of copper to milk may be regulated by hormonal control of copper fluxes into and out of the mammary gland during lactation.
The mechanisms by which copper enters the mammary gland and is secreted into milk are not established. Copper transporters including ATP7B have been implicated in the movement of copper into milk. ATP7B is a P-type ATPase with six transmembrane domains and six metal-binding sites responsible for the translocation of copper across cell membranes. ATP7B is predominantly located in the liver, but recently has been identified in breast and placental tissue along with another copper efflux protein, ATP7A (Michalczyk et al. 2000; Hardman et al. 2007). The clearest indication that ATP7B is involved in copper secretion is provided by the phenotype of the toxic milk (tx) mouse mutant. This autosomal recessive condition is caused by a mutation in Atp7b, the murine ortholog of ATP7B (Theophilos et al. 1996). Homozygous mice accumulate massive amounts of hepatic copper because of the failure of the biliary excretion of copper, mediated by Atp7b. A similar condition occurs in humans with Wilson disease. Paradoxically, the mutant tx dams produce copper-deficient milk that may lead to the death of the pups (Rauch 1983). In Atp7b knockout mice, the absence of ATP7b also results in the accumulation of copper within the mammary gland to a level twice that of the wild type (Buiakova et al. 1999). This is consistent with the concept that, whereas the copper uptake pathway into the mammary gland is functional, the secretion of copper into milk is impaired.
The tx mouse expresses normal levels of the mutant Atp7b, but this protein has been shown to be non-functional with regard to Cu transport (Voskoboinik et al. 2001). Moreover, Atp7b fails to relocalize from the trans-Golgi network to vesicles in response to copper, a phenomenon displayed by normal Atp7b (La Fontaine et al. 2001). Similarly, we have also shown in the tx homozygous mouse that Atp7b does not relocalize in the lactating mammary gland, thus contributing to the failure of normal copper delivery to milk (Michalczyk et al. 2000).
Although Atp7b is clearly involved in delivery of copper to milk in mice, a function for ATP7B in copper secretion into milk by human mammary epithelial cells has not been established. In patients with Wilson disease, copper concentrations in the milk of lactating mothers have not been tested. To determine the involvement of ATP7B in secretion of copper into milk, we used a cell culture model of the human breast, PMC42-LA (Ackland et al. 2001). PMC42-LA cells are unique in their capacity to express milk proteins when stimulated with lactogenic hormones in the presence of extracellular matrix. In these cells, expression of β-casein, the major human-specific milk protein, and milk-specific gene expression were induced through hormone and matrix interactions similar to those operating in vivo (Ackland et al. 2001).
In this study, we show for the first time that ATP7B is present in human breast epithelial cells and that its localization, as in mice, is altered during lactation. Using PMC42-LA cells, we show that lactational hormones induce relocalization of ATP7B, in a copper-dependent manner, and that overexpression of ATP7B increases apical secretion of copper, consistent with a key role of ATP7B in copper secretion into milk.
Materials and Methods
Human Tissue and Cell Line
Two samples of tissue from disease-free resting and lactating human breasts were obtained from breast biopsies performed for diagnosis of breast disease. Tissue was immediately frozen at –80C until use.
The breast adenocarcinoma cell line PMC42-LA, a variant of the PMC42 line, originally derived from a pleural effusion (Whitehead et al. 1983), was cultured in RPMI 1640 culture media (Trace Bioscience; Melbourne, Australia) supplemented with 10% FBS (CSL; Melbourne, Australia). The cells were passaged when confluent using 0.05% trypsin-versene solution in PBS (Sigma-Aldrich; Sydney, Australia). To induce cellular differentiation, PMC42-LA cells were grown on porous Transwell filters (Costar; EK Medical, Melbourne, Australia) coated with a thin layer of 1:5 diluted EHS matrix (from Engelbreth Holm-Swarm mouse sarcoma; Sigma-Aldrich) in RPMI 1640 media. Differentiated PMC42-LA cells were treated with lactogenic hormones to obtain cellular model of lactating epithelia as we previously described (Ackland et al. 2001,2003). Briefly, cells were grown to confluency, and they were treated with 2.7 ng/ml β-estradiol (Sigma-Aldrich) and 157 ng/ml progesterone (Sigma-Aldrich) for 3 days, followed by 3-day treatment of 1 μg/ml dexamethasone (Sigma-Aldrich), 0.6 μg/ml insulin (Sigma-Aldrich), and 200 ng/ml prolactin (Jomar Diagnostic; Melbourne, Australia). The cells grown on the porous Transwell filters were processed for immunofluorescence or harvested for protein analysis.
Antibodies
A polyclonal antibody against the human ATP7B protein was prepared after the immunization of a rabbit (Institute of Medical and Veterinary Science; Adelaide, South Australia) with a peptide that spanned copper-binding sites 4 and 5 (MPEQERQITAREGASRKI) conjugated to Diphtheria toxoid (Chiron Technologies; Melbourne, Australia). ATP7B antiserum was precipitated with sodium sulfate. A polyclonal rabbit anti-mouse Atp7b antibody, sheep anti-human ATP7A (R17) antibody, and sheep anti-human ATP7B (NC36) antibody were raised as previously described (Michalczyk et al. 2000; Cater et al. 2004; Ke et al. 2006). A polyclonal rabbit antibody against total human milk proteins was a gift from Charles H. Streuli (Lee et al. 1985; Streuli et al. 1991). MUC1 (BC2 murine monoclonal anti-human mucin) antiserum was kindly supplied by Ian McKenzie (Xing et al. 1989). Monoclonal antibody to β-casein was purchased from Harlan Sera-Lab (Melbourne, Australia).
IHC
Tissue blocks (1 cm3) were immersed in OCT compound (Tissue Tek; Melbourne, Australia), frozen in liquid nitrogen for 3 min, and sectioned (CM 1800 cryostat; Leica, North Ryde, Australia) between −20C and −17C. Sections of 8–10 μm thickness were collected on gelatin (5%)-coated slides. Samples from resting and lactating breasts were processed concurrently. The sections were dried and fixed in 4% paraformaldehyde, rinsed twice for 10 min in PBS, permeabilized with 5% Triton X-100 in PBS for 5 min, and blocked with 3% BSA in PBS for 90 min. Primary antibody to human ATP7B diluted 1:500 in 1% BSA in PBS was applied to tissues for 2 hr at room temperature. After three PBS washes, second antibody AlexaFluor 488 donkey anti-rabbit IgG (Molecular Probes; Eugene, OR) 1:2000 dilution in 1% BSA in PBS was applied for 2 hr. Sections were washed in PBS and mounted in Fluoroguard antifade reagent (Bio-Rad Laboratories; Hercules, CA). Confocal images were collected using a Leica confocal microscope system TCS SP2 (Leica).
Immunocytochemistry
PMC42-LA cells grown on 1-cm2 glass coverslips or on EHS matrix–coated (diluted 1:5 in media) porous Transwell cell culture inserts were fixed in 4% paraformaldehyde for 10 min, permeabilized with 0.1% Triton X-100 in PBS for 10 min, and blocked with 3% BSA in PBS for 90 min. Primary antibodies diluted in 1% BSA in PBS [1 in 100 for hATP7B, 1 in 50 for mAtp7b, 1 in 4000 for hATP7B(NC36), 1 in 500 for hATP7A(R17), 1 in 50 for β-casein antibody, 1 in 100 for total milk proteins, and 1 in 200 for MUC1] were applied to cells overnight at 4C. After PBS washes, a secondary antibody AlexaFluor 488 donkey anti-rabbit IgG, AlexaFluor 488 goat anti-mouse IgG, or AlexaFluor 488 donkey anti-sheep IgG (Molecular Probes) 1 in 2000 dilution in 1% BSA in PBS was applied for 2 hr at room temperature. Additional AlexaFluor 568 donkey anti-sheep or AlexaFluor 568 donkey anti-mouse secondary antibodies (Molecular Probes) were added to cells at 1 in 2000 dilution for 2 hr, when double label was needed. After washing off excess secondary antibodies, ethidium bromide (10 mg/ml; diluted 1 in 10,000) was added to some cells for 3 min to enable visualization of the nuclei. Cells were washed in PBS and mounted in Fluoroguard antifade reagent (Bio-Rad; Sydney, Australia). Confocal images were collected using a Leica confocal microscope system TCS SP2 (Leica). When applicable, a colocalization analysis plug-in for ImageJ 1.38× (I. Collins and W. Rasband; National Institutes of Health, Bethesda, MD) was used to quantify colocalization of fluorescence from different channels. Threshold Manders colocalization coefficients (Manders et al. 1993) were applied for ATP7B (tM1; channel 1) and β-casein or total milk proteins (tM2; channel 2) using automatic threshold determination according to Costes et al. (2004). Zero/zero pixels were excluded. The values for percentage of pixels colocalized from different channels are presented.
Western Blot Analysis
Breast tissue (100 mg) was disrupted in liquid nitrogen, homogenized in a Dounce homogenizer, sonicated (15 pulses, 40% power output, 30% duty cycles; Microson XL2000 Ultrasonic Cell Disruptor, Misonix, Farmingdale, NY) on ice in 1 ml of EDTA-free inhibitor cocktail (Roche Diagnostics; Melbourne, Australia) containing 20 mg/ml SDS and 5 mM β-mercaptoethanol, and centrifuged for 5 min at 18,000 × g. PMC42-LA cells grown in 80-cm2 plastic flasks were washed twice with PBS, trypsinized, and collected as a cell pellet by centrifugation at 3000 × g for 5 min. The cell pellet was resuspended in 300–500 μl EDTA-free inhibitor cocktail containing 20 mg/ml SDS and 5 mM β-mercaptoethanol. Cells were lysed by repeated passages through a 21-gauge needle followed by sonication as for breast tissue. The total protein content of tissue and cells extracts was measured using the DC Protein Assay Kit (Bio-Rad Laboratories) calibrated against BSA standards. The cell extracts (40 μg protein) and breast tissue supernatants (100 μg protein) were fractionated by SDS-PAGE and transferred to nitrocellulose membranes (Pall Gelman & Whatman; Dassal, Germany). After 1 hr blocking in 1% (w/v) casein in TBS at room temperature, membranes were exposed overnight to anti-human ATP7B polyclonal antibodies or anti-mouse Atp7b polyclonal antibodies, both diluted 1/500 in 1% (w/v) casein in TBS. Membranes were rinsed in blocking buffer, and human ATP7B and mouse Atp7b were detected using 1/2000 dilution of horseradish peroxidase–conjugated goat anti-rabbit secondary antibody (Silenus; Melbourne, Australia) in 1% (w/v) casein in TBS for 2 hr at room temperature. After removal of excess secondary antibody, membranes were rinsed twice in TBS with 0.1% Tween 20 (Sigma-Aldrich). Proteins were detected by enhanced chemiluminescence (POD Chemiluminescence Blotting Substrate; Roche Diagnostics) and a LAS-3000 FujiFilm Lumino-Image Analyzer (Fuji Photo Film; Tokyo, Japan). To monitor protein loading, membranes were stripped in Re-Blot solution (Chemicon International; Temecula, CA) and reprobed with monoclonal β-actin primary antibody (Sigma-Aldrich) diluted 1/5000. Densitometry to quantify results was performed using Fuji Film Multi Gauge V2.3 computer software, and ratios for protein levels were calculated relative to β-actin controls.
Plasmid Construction and Cell Transfections
The full-length mouse Atp7b cDNA construct was prepared as described in La Fontaine et al. (2001) and designated pCMB98. The empty pcDNA3.1 vector (Invitrogen; Melbourne, Australia) was used for cell transfections as a vector-control. For stable transfections, PMC42-LA cells were incubated in the presence of the construct pCMB98 or vector pcDNA3.1 and Lipofectamine 2000 transfection reagent (Life Technologies; Melbourne, Australia) following the manufacturer's instructions. The clone selection was carried out in 300 μg/ml of G418 (Life Technologies) for 3 months. Screening of G418-resistant transfectants for expression of mouse Atp7b protein was carried out by indirect immunofluorescence.
Accumulation of Radioactive Copper Assay
PMC42-LA cells transfected with pCMB98 plasmid (mouse Atp7b) or pcDNA3.1 vector (vector-control) were grown to confluency on EHS matrix–coated (diluted 1:10 in media), porous Transwell cell culture inserts. The monolayers integrity was determined by measuring transepithelial resistance using an ohmmeter. Radioactive copper (64Cu) was purchased from ARI, Australian Nuclear Science and Technology Organization (Lucas Heights, Australia). The average radioactive concentration of 64Cu was 2.61 GBq/ml of copper in the form of CuCl2 diluted in 0.1 M HCl. Growth media were supplemented with 1.0–2.0 μl 64Cu/ml and non-radioactive copper to final concentrations of 0.15 and 0.5 μM. Cells were incubated in this media for 6 hr. After 6 hr, the membranes were rinsed with cold HBSS and solubilized with 0.2 M KOH. The cell-associated radioactivity was measured with a Minaxi Auto Gamma counter (Canberra-Packard; Toronto, ON, Canada). Copper accumulation was normalized to the protein concentration of the cell lysate (quantified using a Bio-Rad Protein Assay Kit; Bio-Rad Laboratories).
64Cu Transport Assay
PMC42-LA cells transfected with pCMB98 plasmid (mouse Atp7b) or pcDNA3.1 vector (vector-control) were grown to confluency on EHS matrix–coated (diluted 1:10 in media), porous Transwell cell culture inserts. One to 2 μl 64Cu/ml (2.61 GBq/ml radioactive concentration of 64Cu) was added to the media containing non-radioactive copper to final concentrations of 0.15 and 0.5 μM. This medium was added to the basolateral chamber. Media aliquots were taken from the apical chamber after 0.5, 1, 3, and 6 hr and counted using a Minaxi Auto Gamma counter.
Statistical Analysis
All experiments were repeated in triplicate, and values are expressed as means ± SD. Student's t-tests were used to determine statistical differences between treatments using the statistical software SPSS 12; p<0.05 was considered significant.
Results
ATP7B Protein Is Present in Both Resting and Lactating Breasts
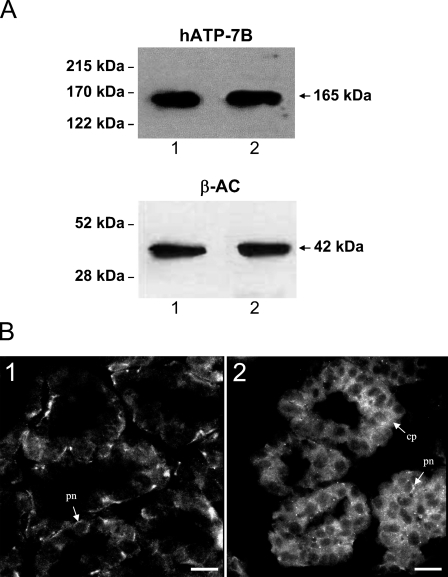
To determine whether ATP7B was expressed in human breast tissue and to study the effect of lactation, protein extracts from biopsies of human breasts were analyzed by Western blots. A polyclonal antibody to human ATP7B detected a single band of size 165 kDa consistent with the expected size of the ATP7B protein (Figure 1A). Comparison of the relative amounts of ATP7B protein to housekeeping protein β-actin (Figure 1A) by densitometry (5.9 ± 0.4 to 6.2 ± 0.5 relative density units) indicated there was no difference in the amounts of ATP7B in resting and lactating tissue.
Figure 1.
(A) Representative Western blot analysis of human breast tissue. Tissue extracts (100 μg) were separated on 7.5% SDS-PAGE, and ATP7B protein (165 kDa) was detected using a polyclonal antibody for human ATP7B. Lane 1, resting human breast tissue extract; Lane 2, lactating human breast tissue extract. β-actin protein (42 kDa) was used as a loading control in same tissue extracts. (B) IHC localization of human ATP7B in section of an alveolus from human breast tissues. (B1) ATP7B was localized to the perinuclear region (pn) of epithelial cells in resting breast tissue. (B2) Granular, diffuse cytoplasmic label (cp) of ATP7B with some perinuclear fraction (pn) was detected in lactating breast tissue. Bar = 12 μm.
Immunofluorescence of human breast tissue sections indicated that ATP7B was present in most luminal epithelial cells of both resting and lactating breast tissue. In the resting breast, ATP7B had a predominantly perinuclear location, with a small proportion of labeling in the cytoplasm (Figure 1B1), whereas in lactating tissue, ATP7B had a more diffuse granular cytoplasmic localization in addition to a perinuclear component (Figure 1B2).
Lactational Hormones Alter the Intracellular Distribution of ATP7B Protein in the Breast Cell Model
To study whether the redistribution of ATP7B in the lactating breast was because of the influence of lactational hormones, we used PMC42-LA human breast epithelial cells (Ackland et al. 2001). Our initial experiments were carried on non-polarized cells grown on a glass substrate using standard cell culture medium: RPMI with 10% FBS. In these cells, ATP7B was located exclusively in the perinuclear regions (Figure 2A1).
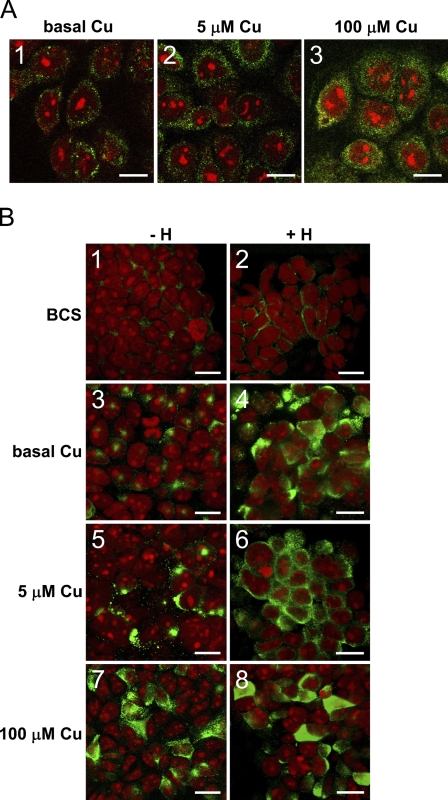
Figure 2.
(A) Immunocytochemical localization of human ATP7B (green) in non-differentiated PMC42-LA cells grown on glass in (A1) basal copper concentration (0.3 μM), (A2) 5 μM copper, and (A3) 100 μM copper for 3 days. (B) Immunocytochemical localization of human ATP7B (green) in differentiated PMC42-LA grown on EHS matrix–coated Transwell filters. Cells were cultured in (B1,B2) 50 μM bathocuproine disulfate, (B3,B4) basal copper, (B5,B6) 5 μM copper, and (B7,B8) 100 μM copper for 3 days. Cells (B2,B4,B6,B8) were subjected to lactogenic hormone treatment as described in the Materials and Methods section. Nuclei of the cells were stained with ethidium bromide (red), and all images were collected using Leica confocal system. Bars: A = 10 μm; B = 12 μm.
Atomic absorption spectrophotometric analysis of cell culture medium (RPMI with 10% FBS) indicated that the copper level was 0.3 μM, which is considerably lower than total human plasma copper levels (11–22 μM), even considering that only ∼25–35% of plasma copper may be available to cells (Linder 1991,2002). Cells growing in RPMI with 10% FBS may therefore have quite low cytoplasmic copper levels. ATP7B would be predicted to remain in the trans-Golgi network (La Fontaine et al. 2001). When we grew cells in increasing concentrations of copper from basal (0.3 μM) to 100 μM, the intracellular distribution of ATP7B changed from perinuclear to a granular cytoplasmic distribution following increases in copper concentration (Figures 2A1–2A3).
To provide a more physiologically relevant model of the lactating human gland, we grew PMC42-LA cells on EHS matrix on a porous membrane, which induced epithelial cell differentiation and polarization (Ackland et al. 2001,2003). The cells were treated with estrogen and progesterone followed by dexamethasone, insulin, and prolactin to mimic the physiological hormonal changes within the breast during lactation. In the presence of copper chelator bathocuproine disulfonate (BCS), a perinuclear localization of ATP7B was found in both hormone-treated and non-treated cells (Figures 2B1 and 2B2). In standard cell culture medium (0.3 μM copper), ATP7B was located in the perinuclear regions of the non-treated PMC42-LA cells (Figure 2B3). Hormone treatment induced a more diffuse cytoplasmic staining of ATP7B (Figure 2B4). When the hormone treatment was repeated in the presence of 5 μM copper, ATP7B changed from a mainly perinuclear localization (Figure 2B5) to a predominantly punctate cytoplasmic label in hormone-treated cells (Figure 2B6). Treatment of cells with 100 μM copper resulted in a general punctate cytoplasmic distribution of ATP7B in both non-treated and hormone-treated cells (Figures 2B7 and 2B8).
In hormone-treated PMC42-LA cells, ATP7B protein was located near the apical membrane.
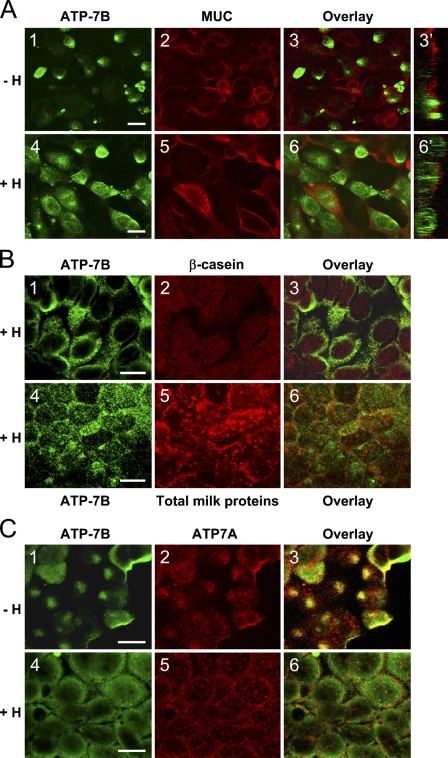
Double-labeling immunofluorescence was used to further study the localization of ATP7B in differentiated PMC42-LA cells. PMC42-LA cells were grown on EHS matrix on a porous membrane in the presence of 5 μM copper with and without hormones. Cells were labeled with anti-ATP7B antibody (Figures 3A1 and 3A4) and anti-MUC1 antibody to mucin glycoprotein. MUC1 had an apical localization (Figures 3A2 and 3A5), consistent with its presence as a resident of the apical membrane in breast epithelial cells (Patton et al. 1995). ATP7B labeling did not overlap with the MUC1 in either the non–hormone-treated PMC42-LA cells (Figure 3A3) or in the hormone-treated cells (Figure 3A6), indicating that most of the ATP7B protein was not present in the vicinity of the apical membrane. The XZ sections further support the absence of ATP7B from the apical membrane (Figure 3A3′); however, they showed that hormones caused redistribution of ATP7B toward the apical region of the PMC42-LA cytoplasm (Figure 3A6').
Figure 3.
(A) Double-label immunofluorescence of ATP7B (green) and mucin glycoprotein MUC1 (red) in differentiated PMC42-LA cells. Cells were grown in the presence of 5 μM copper with (A4,A5,A6,A6′) and without (A1,A2,A3,A3′) hormones. XZ sections of staining overlay are shown on the right side. (B) Localization of hATP7B (green) (B1,B4) in differentiated, hormone-treated PMC42-LA cells in comparison to β-casein (red) (B2) and total human milk proteins (red) (B5). Overlays of the images are shown (B3,B6). (C) Double-label immunofluorescence of ATP7B (green) and hATP7A (red) in differentiated PMC42-LA cells. Cells were grown in the presence of 5 μM copper with (C4–C6) and without (C1–C3) hormones. Bar = 10 μm.
In cultured PMC42-LA cells, ATP7B protein did not colocalize with milk proteins and only partially overlapped with ATP7A.
Colocalization studies using an antibody to β-casein and an antibody to total milk proteins in humans showed that, in differentiated hormone-treated PMC42-LA cells, ATP7B did not entirely overlap with either β-casein or milk proteins, suggesting that some ATP7B was not in the same secretory vesicles (Figures 3B1–3B3). Threshold Manders coefficients were 12% and 34% for ATP7B and β-casein, respectively, indicating that only 12% of the ATP7B label overlapped with β-casein and 34% of β-casein overlapped with ATP7B. When an antibody raised to total human milk proteins was used, only partial colocalization with ATP7B protein was detected (Figures 3B4–3B6) with threshold Manders coefficients of 57% for ATP7B and 52% for total milk protein. This suggested that approximately one half the ATP7B may co-reside in vesicles that are associated with milk proteins, whereas the remainder is not associated with the milk secretory pathway.
Double-label immunofluorescence was carried out to determine the extent of intracellular colocalization between ATP7B and ATP7A. Overlap of these ATPases occurred in the perinuclear region, seen in non–hormone-treated cells (Figures 3C1–3C3). In hormone-treated cells, ATP7B had a granular label that extended further from the nucleus compared with ATP7A (Figures 3C4–3C6).
ATP7B Mediates Transport of Copper From the Cell Through the Apical Membrane in Cultured PMC42-LA Cells
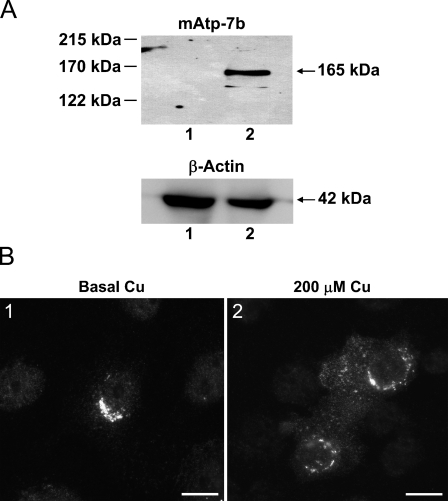
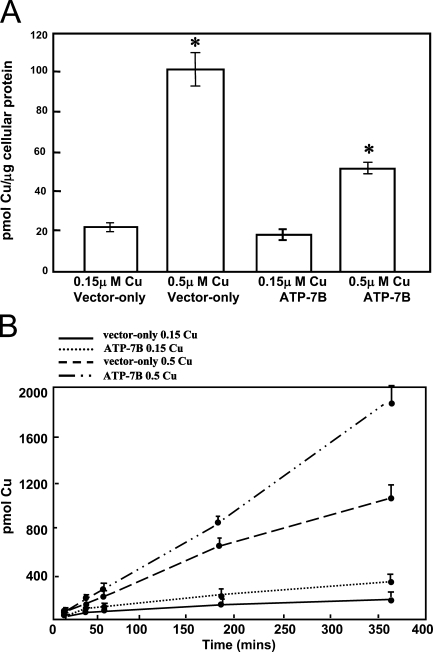
To establish whether ATP7B functioned as a copper transporter in breast epithelial cells, an ATP7B construct pCMB98 (La Fontaine et al. 2001) was introduced into PMC42-LA cells, and 64Cu transport studies were carried out. Expression of mouse Atp7b protein in PMC42-LA cells was confirmed by the presence of a band of 165 kDa corresponding to the expected size of the mouse protein (Figure 4A), using an antibody raised to mouse Atp7b. The band was detected only in transfected cells and was absent from vector-only transfected cells, because this antibody is specific to mouse Atp7b and does not cross-react with human ATP7B (Figure 4A). A strong perinuclear Atp7b stain was observed in transfected cells after immunofluorescence using the mouse-specific antibody (Figure 4B1). This pattern was similar to that observed for the endogenous protein (Figure 2A1). After treatment of transfected cells with 200 μM copper for 2 hr, Atp7b had a granular cytoplasmic distribution in addition to perinuclear localization (Figure 4B2). The effect of expression of Atp7b on copper transport in mammary epithelial cells was determined using transfected and non-transfected PMC42-LA cells grown on EHS-coated porous filters. The integrity of the monolayer was indicated by a transepithelial resistance of 300 Ω/cm2, within the normal range of 150–600 Ω/cm2 (Artursson et al. 1996). 64Cu was added to the basal compartment of the PMC42-LA cells, and the accumulation of copper in the cells together with copper transport from basal into the apical compartment was determined in the presence of 0.15 and 0.5 μM total copper concentration. In the presence of 0.15 μM copper, there was no difference in 64Cu accumulation in control and in cells expressing mouse Atp7b. In the presence of 0.5 μM copper, cells expressing the Atp7b accumulated less copper than control cells in 6 hr (p<0.001; Figure 5A). In addition, in cells expressing the Atp7b, significantly more 64Cu was transported across the cell monolayer to the apical compartment than in control cells in the presence of copper concentrations of 0.5 μM (p<0.001; Figure 5B).
Figure 4.
(A) Representative Western blot analysis of PMC42-LA cells transfected with pCMB98 plasmid (mouse Atp7b) or pcDNA3.1 vector (vector-control). Sixty μg of cell extracts was separated on 7.5% SDS-PAGE, and Atp7b protein (165 kDa) was detected using a polyclonal antibody for mouse Atp7b. Lane 1, pcDNA3.1 vector transfected cell extract; Lane 2, pCMB98 plasmid transfected cell extract. β-actin protein (42 kDa) was used as a loading control in same cell extracts. (B) Immunocytochemical localization of mouse Atp7b in PMC42-LA cells transfected with pCMB98 plasmid. (B1) Atp7b was localized to the perinuclear region of PMC42-LA cells when grown in basal copper levels. (B2) Granular, diffuse cytoplasmic label of Atp7b with some perinuclear fraction was detected in PMC42-LA cultured in 200 μM copper. Bar = 10 μm.
Figure 5.
(A) The effect of expression of Atp7b on copper accumulation in PMC42-LA cells. Cells transfected with pCMB98 plasmid or pcDNA3.1 vector were grown to confluency on EHS matrix–coated, porous Transwell cell culture inserts. Growth media were supplemented with 64Cu and non-radioactive copper to final concentrations of 0.15 and 0.5 μM, and cells were cultured in it for 6 hr. Copper accumulation was normalized to the protein concentration of the cell lysate. Bars represent mean 64Cu accumulation ± SD from three experiments. Asterisks denote a significant difference (p<0.001). (B) The effect of expression of Atp7b on copper transport in PMC42-LA cells. PMC42 cells, transfected with pCMB98 plasmid or pcDNA3.1 vector, were treated with media containing 64Cu and non-radioactive copper to final concentrations of 0.15 and 0.5 μM. This medium was added to the basolateral chamber. Media aliquots were taken from the apical chamber after 0.5, 1, 3, and 6 hr and were counted using a Minaxi Auto Gamma counter. Copper accumulation was normalized to the protein concentration of the cell lysate. Bars represent mean 64Cu levels ± SD from three experiments.
Discussion
A function for ATP7B in the transport of copper from human mammary epithelial cells into milk has not previously been reported. In this study, we analyzed the expression and cellular localization of ATP7B in the resting and lactating human mammary gland. ATP7B protein had a perinuclear localization in cells from the resting breast, whereas in the lactating breast, the protein was redistributed throughout the cytoplasm of the luminal epithelial cells. This observation is consistent with our previous studies of the mammary gland in non-lactating and lactating mice (Michalczyk et al. 2000) and data from lactating rats showing punctate staining of Atp7b in a region of the cell adjacent to the luminal surface of the epithelial cells (Kelleher and Lönnerdal 2003,2006). The data suggest that the intracellular relocalization of ATP7B could be associated with physiological secretion of copper into milk during lactation. This conclusion is supported by our previous data from the tx mouse showing that the defect in delivery of copper into milk is accompanied by a failure of the ATP7B protein to relocalize (Michalczyk et al. 2000). It is not known if there is a defect in copper secretion into milk in mothers with Wilson disease, caused by mutations in the ATP7B gene, because there are no reports in the literature of copper levels in the milk of patients.
Analysis of ATP7B in the human mammary gland indicated no differences between the amount of protein in the resting and lactating breast. This suggests that the capacity of ATP7B to facilitate copper transport into milk does not need increased amounts of the protein. This finding is consistent with a previous study in mouse mammary tissue where we also found no differences in ATP7B protein levels between the resting and lactating glands. (Michalczyk et al. 2000) but different from another study where a reduction in Atp7b protein of ∼25% was seen after the decline in the milk copper levels during lactation in rats (Kelleher and Lönnerdal 2006). It is possible that these differences in results may reflect species differences.
Although similar levels of ATP7B protein were seen in lactating and resting breast tissue, this pattern is in contrast to the ATP7A protein previously found in human and mouse mammary tissue. ATP7A was more highly expressed in lactating tissue compared with resting tissue in the human gland (Ackland et al. 1999), as well as in the mouse gland (Michalczyk et al. 2000). This suggests a fundamental difference in the physiological regulation and possibly the function of these two ATPases in lactation, despite the fact that ATP7B can partially substitute for ATP7A in the Menkes-deficient mottled fibroblast cell line (Payne et al. 1998). The fact that some copper is transported into milk in the absence of ATP7B (Michalczyk et al. 2000) suggests the presence of additional copper transporters or mechanisms of secretion in the mammary gland. ATP7A has been considered for this role, but there is contradictory evidence relating to localization of this protein in the breast tissue. In the mammary gland of lactating rats, Atp7a was found to be associated with the apical and basolateral membranes of epithelial cells, suggesting that Atp7a localization is compatible with its role in lactogenic hormone stimulated copper secretion (Kelleher and Lönnerdal 2006). Conversely, our current study in mice (unpublished data) and a previous study on transfected MDCK cells showed basolateral membrane localization of ATP7A (Greenough et al. 2004), which may not be consistent with efflux of copper from the apical surface.
We used the PMC42-LA human mammary epithelial cell line to further study the intracellular location of ATP7B and to determine the effects of hormones. We first grew PMC42-LA in RPMI with 10% FBS on a normal glass substrate. Cells grown on glass showed a very tight perinuclear staining of ATP7B, different from the perinuclear and cytoplasmic label found in the resting breast. We speculated that the tight perinuclear label could be caused by lower than physiological levels of copper present in standard RPMI culture medium with 10% FBS (0.3 μM) and that the lack of copper in the culture medium could explain why ATP7B was somewhat refractory to hormone-induced relocalization relative to cells grown in 5 and 100 μM copper. In support of this hypothesis, we found that, in hormone-treated PMC42-LA cells grown in 5 μM copper, the localization of ATP7B changed from perinuclear to granular cytoplasmic. In response to increased extracellular copper levels (100 μM), only a punctated cytoplasmic ATP7B pattern was found, and there was no difference between hormone-treated and non-treated cells. Additionally, in the presence of a copper chelator (BCS), ATP7B did not relocalize from the perinuclear region in response to hormones. These data suggest that hormones may act to change the localization of ATP7B through an increase in intracellular copper levels, to facilitate the efflux of copper from mammary epithelial cells into milk.
The PMC42-LA cell line can be manipulated to represent both resting and lactating secretory breast epithelia. The resting model was produced by growing PMC42-LA cells on EHS matrix above a permeable basal membrane. Under these conditions, cells have tight junctions and are polarized but do not synthesize milk proteins. The lactating model was induced by hormone treatment of PMC42-LA cells on the EHS matrix, which stimulated β-casein synthesis (Ackland et al. 2001). We used confocal microscopy to determine whether ATP7B was present in vesicles containing milk proteins that are destined for secretion during lactation. After hormone treatment, most of the ATP7B did not colocalize with the major human milk protein, β-casein; however, ∼50% overlapped with total milk protein, suggesting some ATP7B may be in the milk secretory pathway. ATP7B migrated toward the apical region of the cell but did not overlap with the apical marker, MUC1, indicating that it was not at the plasma membrane itself. This is similar to observations made in primary rat liver hepatocytes and HepG2 cells, where ATP7B trafficked from the trans-Golgi to cytoplasmic vesicular compartment in response to copper administration (Schaefer et al. 1999; Cater et al. 2006). Cell fractionation of HepG2 cells showed that ATP7B was present in an intracellular compartment pathway and not associated with the plasma membrane after copper treatment (Hung et al. 1997). Similarly, also in HepG2 cells, a plasma membrane lawn assay showed that ATP7B was not localized to plasma membrane (Yang et al. 1997). In contrast to these reports, there is also evidence that ATP7B is associated with the plasma membrane in copper-treated HepG2 cells (Roelofsen et al. 2000; Guo et al. 2005).
To prove that ATP7B mediated cellular copper fluxes, 64Cu studies were carried out on polarized PMC42-LA cells grown on EHS on a porous filter. In cells expressing mouse Atp7b in addition to the endogenous human ATP7B, in the presence of 0.5 μM copper, there was a significant decrease in cellular copper accumulation and an increase in copper in the apical compartment relative to control cells. In the presence of 0.15 μM copper, the overexpressing cells did not accumulate more copper compared with the controls. This observation supports the concept that low levels of extracellular copper are insufficient to induce the copper trafficking of ATP7B that is needed for copper efflux from cells. The 64Cu data also confirm that ATP7B is involved in copper efflux through the apical surface of the cells, possibly with assistance of some other, yet unidentified, protein.
In summary, ATP7B is expressed in the resting and lactating human breast, where it is located in the luminal epithelial cells. ATP7B has a perinuclear localization in resting epithelial cells and a diffuse location in lactating tissue. Experiments using the PMC42-LA human mammary model showed that hormones induce a redistribution of ATP7B from a perinuclear region to a compartment adjacent but not at the apical plasma membrane. Physiological concentrations of extracellular copper are needed for lactational hormones to induce trafficking of ATP7B, suggesting that hormones act indirectly through increased intracellular copper to mediate copper secretion from mammary epithelial cells during lactation. 64Cu studies with cells overexpressing ATP7B showed that it facilitates copper efflux from mammary epithelial cells from the apical surface.
Acknowledgments
The authors thank the National Institutes of Health Department of Health and Human Services, Bethesda, MD, for support.
References
- Ackland ML, Anikijenko P, Michalczyk A, Mercer JF (1999) Expression of menkes copper-transporting ATPase, MNK, in the lactating human breast: possible role in copper transport into milk. J Histochem Cytochem 47:1553–1562 [DOI] [PubMed] [Google Scholar]
- Ackland ML, Michalczyk A, Whitehead RH (2001) PMC42, a novel model for the differentiated human breast. Exp Cell Res 263:14–22 [DOI] [PubMed] [Google Scholar]
- Ackland ML, Ward J, Ackland CM, Greaves M, Walker M (2003) Extracellular matrix induces formation of organoids and changes in cell surface morphology in cultured breast carcinoma cells PMC42-LA. In Vitro Cell Dev Biol Anim 39:428–433 [DOI] [PubMed] [Google Scholar]
- Artursson P, Karlsson J, Ocklind G, Schipper N (1996) Studying transport processes in absorptive epithelia. In Shaw AJ, ed. Epithelial Cell Culture: A Practical Approach. New York, Oxford University Press, 111–132
- Buiakova OI, Xu J, Lutsenko S, Zeitlin S, Das K, Das S, Ross BM, et al. (1999) Null mutation of the murine ATP7B (Wilson disease) gene results in intracellular copper accumulation and late-onset hepatic nodular transformation. Hum Mol Genet 8:1665–1671 [DOI] [PubMed] [Google Scholar]
- Cater MA, Forbes J, La Fontaine S, Cox D, Mercer JF (2004) Intracellular trafficking of the human Wilson protein: the role of the six N-terminal metal-binding sites. Biochem J 380:805–813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cater MA, La Fontaine S, Shield K, Deal Y, Mercer JF (2006) ATP7B mediates vesicular sequestration of copper: insight into biliary copper excretion. Gastroenterology 130:493–506 [DOI] [PubMed] [Google Scholar]
- Costes SV, Daelemans D, Cho EH, Dobbin Z, Pavlakis G, Lockett S (2004) Automatic and quantitative measurement of protein-protein colocalization in live cells. Biophys J 86:3993–4003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donley SA, Ilagan BJ, Rim H, Linder MC (2002) Copper transport to mammary gland and milk during lactation in rats. Am J Physiol Endocrinol Metab 283:E667–675 [DOI] [PubMed] [Google Scholar]
- Friel JK, Andrews WL, Jackson SE, Longerich HP, Mercer C, McDonald A, Dawson B, et al. (1999) Elemental composition of human milk from mothers of premature and full-term infants during the first 3 months of lactation. Biol Trace Elem Res 67:225–247 [DOI] [PubMed] [Google Scholar]
- Greenough M, Pase L, Voskobionik I, Petris MJ, O'Brien AW, Camakaris J (2004) Signals regulating trafficking of Menkes (MNK; ATP7A) copper translocating P-type ATPase in polarized MDCK cells. Am J Physiol Cell Physiol 287:C1463–1471 [DOI] [PubMed] [Google Scholar]
- Guo Y, Nyasae L, Braiterman LT, Hubbard AL (2005) NH2-terminal signals in ATP7B Cu-ATPase mediate its Cu-dependent anterograde traffic in polarized hepatic cells. Am J Physiol Gastrointest Liver Physiol 289:G904–916 [DOI] [PubMed] [Google Scholar]
- Hardman B, Michalczyk A, Greenough M, Camakaris J, Mercer J, Ackland L (2007) Distinct functional roles for the Menkes and Wilson copper translocating P-type ATPases in human placental cells. Cell Physiol Biochem 20:1073–1084 [DOI] [PubMed] [Google Scholar]
- Hung IH, Suzuki M, Yamaguchi Y, Yuan DS, Klausner RD, Gitlin JD (1997) Biochemical characterization of the Wilson disease protein and functional expression in the yeast Saccharomyces cerevisiae. J Biol Chem 272:21461–21466 [DOI] [PubMed] [Google Scholar]
- Ke BX, Llanos RM, Wright M, Deal Y, Mercer JF (2006) Alteration of copper physiology in mice overexpressing the human Menkes protein ATP7A. Am J Physiol Regul Integr Comp Physiol 290:R1460–1467 [DOI] [PubMed] [Google Scholar]
- Kelleher SL, Lönnerdal B (2003) Marginal maternal Zn intake in rats alters mammary gland Cu transporter levels and milk Cu concentration and affects neonatal Cu metabolism. J Nutr 133:2141–2148 [DOI] [PubMed] [Google Scholar]
- Kelleher SL, Lönnerdal B (2006) Mammary gland copper transport is stimulated by prolactin through alterations in Ctr1 and Atp7A localization. Am J Physiol Regul Integr Comp Physiol 291:R1181–1191 [DOI] [PubMed] [Google Scholar]
- La Fontaine S, Theophilos MB, Firth SD, Gould R, Parton RG, Mercer JF (2001) Effect of the toxic milk mutation (tx) on the function and intracellular localization of Wnd, the murine homologue of the Wilson copper ATPase. Hum Mol Genet 10:361–370 [DOI] [PubMed] [Google Scholar]
- Lee EY, Lee WH, Kaetzel CS, Parry G, Bissell MJ (1985) Interaction of mouse mammary epithelial cells with collagen substrata: regulation of casein gene expression and secretion. Proc Natl Acad Sci USA 82:419–423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linder MC (1991) Biochemistry of Copper. New York, Plenum Press
- Linder MC (2002) Biochemistry and molecular biology of copper in mammals. In Massaro EJ, ed. Handbook of Copper Pharmacology and Toxicology. Totowa, NJ, Humana Press, 2–32
- Linder MC, Wooten L, Cerveza P, Cotton S, Shulze R, Lomeli N (1998) Copper transport. Am J Clin Nutr 67(suppl 5):965S–971S [DOI] [PubMed] [Google Scholar]
- Lönnerdal B (1996) Bioavailability of copper. Am J Clin Nutr 63:821S–829S [DOI] [PubMed] [Google Scholar]
- Manders E, Verbeek F, Aten J (1993) Measurement of co-localization of objects in dual-colour confocal images. J Microsc 169:375–382 [DOI] [PubMed] [Google Scholar]
- Michalczyk AA, Rieger J, Allen KJ, Mercer JF, Ackland ML (2000) Defective localisation of the Wilson disease protein (ATP7B) in the mammary gland of the toxic milk mouse and the effects of copper supplementation. Biochem J 352:565–571 [PMC free article] [PubMed] [Google Scholar]
- Patton S, Gendler SJ, Spicer AP (1995) The epithelial mucin, MUC1, of milk, mammary gland and other tissues. Biochim Biophys Acta 1241:407–423 [DOI] [PubMed] [Google Scholar]
- Payne AS, Kelly EJ, Gitlin JD (1998) Functional expression of the Wilson disease protein reveals mislocalization and impaired copper-dependent trafficking of the common H1069Q mutation. Proc Natl Acad Sci USA 95:10854–10859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrone L, Di Palma L, Di Toro R, Gialanella G, Moro R (1993) Trace element content of human milk during lactation. J Trace Elem Electrolytes Health Dis 7:245–247 [PubMed] [Google Scholar]
- Rauch H (1983) Toxic milk, a new mutation affecting copper metabolism in the mouse. J Hered 74:141–144 [DOI] [PubMed] [Google Scholar]
- Roelofsen H, Wolters H, Van Luyn MJ, Miura N, Kuipers F, Vonk RJ (2000) Copper-induced apical trafficking of ATP7B in polarized hepatoma cells provides a mechanism for biliary copper excretion. Gastroenterology 119:782–793 [DOI] [PubMed] [Google Scholar]
- Schaefer M, Hopkins RG, Failla MA, Gitlin JD (1999) Hepatocyte-specific localization and copper-dependent trafficking of the Wilson's disease protein in the liver. Am J Physiol 276:G639–646 [DOI] [PubMed] [Google Scholar]
- Streuli CH, Bailey N, Bissell MJ (1991) Control of mammary epithelial differentiation: basement membrane induces tissue-specific gene expression in the absence of cell-cell interaction and morphological polarity. J Cell Biol 115:1383–1395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theophilos MB, Cox DW, Mercer JF (1996) The toxic milk mouse is a murine model of Wilson disease. Hum Mol Genet 5:1619–1624 [DOI] [PubMed] [Google Scholar]
- Voskoboinik I, Greenough M, La Fontaine S, Mercer JF, Camakaris J (2001) Functional studies on the Wilson copper P-type ATPase and toxic milk mouse mutant. Biochem Biophys Res Commun 281:966–970 [DOI] [PubMed] [Google Scholar]
- Whitehead RH, Bertoncello I, Webber LM, Pedersen JS (1983) A new human breast carcinoma cell line (PMC42) with stem cell characteristics. I. Morphologic characterization. J Natl Cancer Inst 70:649–661 [PubMed] [Google Scholar]
- Wooten L, Shulze R, Lancey R, Lietzow M, Linder M (1996) Ceruloplasmin is found in milk and amniotic fluid and may have a nutritional role. J Nutr Biochem 7:632–639 [Google Scholar]
- Xing PX, Tjandra JJ, Stacker SA, Teh JG, Thompson CH, McLaughlin PJ, McKenzie IF (1989) Monoclonal antibodies reactive with mucin expressed in breast cancer. Immunol Cell Biol 67:183–195 [DOI] [PubMed] [Google Scholar]
- Yang XL, Miura N, Kawarada Y, Terada K, Petrukhin K, Gilliam T, Sugiyama T (1997) Two forms of Wilson disease protein produced by alternative splicing are localized in distinct cellular compartments. Biochem J 326:897–902 [DOI] [PMC free article] [PubMed] [Google Scholar]