Abstract
Budding yeast cells divide asymmetrically, giving rise to a mother and its daughter. Mother cells have a limited division potential, called their lifespan, which ends in proliferation-arrest and lysis. In this report we mutate telomerase in Saccharomyces cerevisiae to shorten telomeres and show that, rather than shortening lifespan, this leads to a significant extension in lifespan. This extension requires the product of the SIR3 gene, an essential component of the silencing machinery which binds to telomeres. In contrast, longer telomeres in a genotypically wild-type strain lead to a decrease in lifespan. These findings suggest that the length of telomeres dictates the lifespan by regulating the amount of the silencing machinery available to nontelomeric locations in the yeast genome.
Telomere shortening has been observed in somatic cells of aging humans and in primary human fibroblasts as they divide in culture (1, 2). This shortening is due to the absence of telomerase in these cells, which leads to a progressive loss of DNA sequences at the ends of replicated chromosomes. It has been suggested that telomere shortening serves as a mitotic clock, eventually triggering the senescence of cultured cells and aging in people (3, 4). Short telomeres could halt cell division by cell cycle arrest, damage to chromosomes in mitosis, or deletion of telomere-proximal genes (see ref. 4). In many tumor cell lines telomerase activity has been restored during immortalization (5).
Lifespan in budding yeast is defined by the finite number of times that the mother cell can divide (6, 7). Because cell division is asymmetrical, the larger mother cell can be followed through many rounds of cell division by microscopic removal of the smaller daughter cell, which arises from the bud. The number of cell divisions mother cells undergo before senescing is relatively constant, and the average number defines the mean lifespan of a yeast strain.
Yeast telomeres contain repeats of the sequence C1–3A which are maintained by telomerase (8, 9). Genes that are positioned at telomeres are subject to silencing by the SIR2/3/4 complex (10, 28). This complex binds to Rap1p, a protein positioned at yeast telomeres (11, 12). SIR2–4 along with SIR1 also silences mating-type genes at HML and HMR (13, 14). In this case RAP1, ABF1, and ORC play a role in recruiting the Sir complex (15–17). Silencing at all of these sites also involves the amino terminus of histone H4 and presumably results in a heterochromatic structure over the silenced region (18).
The RNA component of yeast telomerase is encoded by TLC1 (9). TLC1 was isolated on high-copy clones which can have truncations at the 3′ end of the RNA (9). These clones were identified because they exerted a dominant inhibition of telomerase, resulting in shorter telomeres and a loss of telomere silencing. The protein component of telomerase is thought to be encoded by EST2, which is homologous to a protein purified from Euplotes with telomerase activity (19). Both Est2 protein and the Euplotes protein bear homology to signature residues of reverse transcriptases.
In this report, we genetically manipulate telomeres in yeast and demonstrate an inverse correlation between telomere length and lifespan. Our findings are consistent with the idea that the redistribution of the Sir complex to nontelomeric sites promotes longevity. We suggest a revised view of the role of telomere shortening in human aging.
MATERIALS AND METHODS
Strain Manipulations.
All strains were in the standard W303 background (ade2-1 his3-11,15 trp1-1 leu2-3,112 can1-100; ref. 20). The W303 Δsir3 strain, RS862, was generously provided by S. Bell (Massachusetts Institute of Technology). The W303 Δrif1 strain, YLS531, was a gift from D. Shore (University of Geneva). To obtain the series of wild-type strains with different telomere lengths, strain W303 was transformed with a plasmid containing either the rap1t or the wild-type allele of RAP1 as a control (gifts of A. Lustig, Sloan–Kettering Cancer Institute, New York). Transformants containing the rap1t allele have lengthened telomeres (21). These transformants and controls were then cultivated in liquid cultures of rich medium (yeast extract/peptone/dextrose; YEPD) overnight to allow for plasmid loss and then plated on YEPD plates. Replica plating identified those clones that had lost their plasmids. Further passaging of these clones led to a gradual reduction of telomere length. Clones picked for lifespan and telomere length analysis were as follows: (i) Long telomeres–early passage cells were taken from a single chimeric colony which consisted of cells that had lost and cells that had retained the rap1t plasmid, suggesting that the loss of the plasmid had occurred during colony formation on the YEPD plate. (ii) Long telomeres–later passage cells were taken from a single colony which consisted only of cells which had lost the rap1t plasmid, suggesting that plasmid loss had occurred in liquid culture prior to plating. These cells were cultivated further on two YEPD plates to obtain intermediate telomere lengths. (iii) Wild-type telomere cells were cells that had evicted a wild-type RAP1 plasmid and therefore had wild-type telomere lengths. All mortality curves were obtained from two independent experiments, and lifespan and statistical analyses were done as previously described (22).
DNA Manipulations.
The plasmid used to overexpress truncated TLC1 (a gift of D. McNabb; Massachusetts Institute of Technology) was constructed with PCR using the following primers: 5-TLC1, GGG AAG CTT GAG CTC AAT AAA ACT AGA GAG GAA GAT AGG TAC CC; 3-TTLC1, GGG AAG CTT TCT AAA TGC ATC GAA GGC ATT AGG AGA AGT AGC TGT G. A truncated clone of TLC1 was obtained from genomic yeast DNA, digested with HindIII, inserted into the HindIII site of plasmid pDB20 (23), and checked for proper orientation with respect to the ADH promoter. The anti-SIR4 plasmid (pJH3A) has been described (13). Integration of SIR4-42 was done as previously described (22). All integration events were checked by Southern analysis or PCR.
Telomere Length Determinations.
Total genomic DNA was isolated from an overnight culture of the appropriate yeast strain, digested with XhoI, and separated on a 1.5% agarose gel and transferred to a GeneScreenPlus hybridization transfer membrane (New England Nuclear). Hybridization and wash conditions were as suggested by the manufacturer of the membrane. A plasmid containing 600 bp located within the conserved Y′ region of yeast telomeres, generously supplied by V. Zakian (28), was used to generate a random primed probe which overlaps the XhoI site and thus hybridizes to fragments both telomere-proximal and telomere-distal to the restriction site. Most yeast telomeres contain the Y′ region.
RESULTS AND DISCUSSION
We wished to test the effects of telomere shortening on the lifespan of yeast mother cells, even though yeast telomeres do not shorten in normal aging (24, 29). Thus, we overexpressed the dominant truncated allele of TLC1 (tTLC1), which was shown to result in telomeres adopting a shorter steady-state length (ref. 9; Fig. 1A, lane 3).
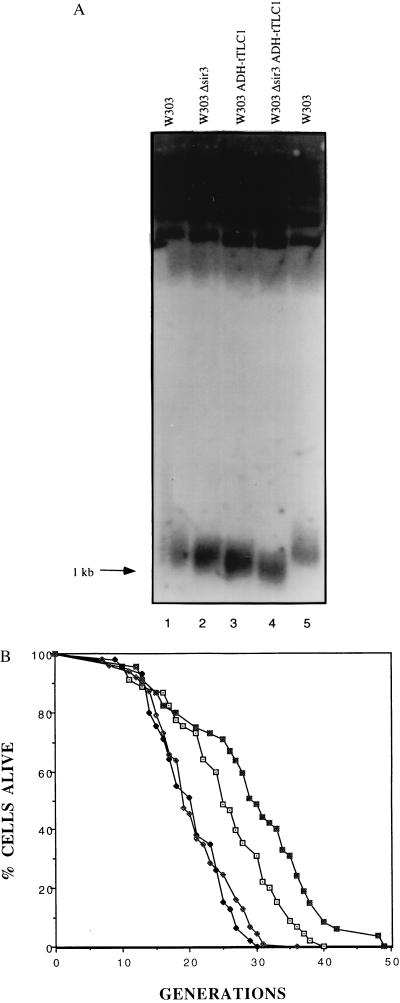
Figure 1.
Overexpression of truncated TLC1 results in loss of telomeric silencing, shortened telomeres, and lengthened lifespan. (A) Genomic DNA isolated from the indicated strains was digested with XhoI and electrophoresed on a 1.5% agarose gel and probed with a telomere probe. The broad band at the bottom of the gel migrating at about 1 kb consists of yeast telomeres. (B) Mortality curves are shown for the wild-type and Δsir3 strains containing either an empty vector or a vector overexpressing the truncated form of TLC1. The mean lifespans were 25.4, 20.3, 29.2, and 20.6 generations for the W303 (⊡), W303 Δsir3 (♦), W303 ADH-tTLC1 (▪), and W303 Δsir3 ADH-tTLC1 (⋄) strains, respectively. Corresponding sample sizes were 45, 45, 45, and 48 cells, respectively.
Lifespans were then determined for both strains and, strikingly, the tTLC1 mutant had a lifespan approximately 20% longer than the parent (Fig. 1B). The kinetics of aging were similar in the wild-type and tTLC1 strains.
Why is the lifespan extended in the strain with shorter telomeres? Earlier findings indicate that the yeast silencing machinery, encoded by SIR2, SIR3, and SIR4, is important in setting the lifespan. First, a mutation in SIR4, SIR4-42, that removes the C terminus of the protein prevents recruitment of the Sir complex to HM loci and telomeres (11, 12), thereby lengthening the lifespan (22). Second, null mutations in SIR4 resulted in a decrease in lifespan (22). Third, silencing of HML and HMR is lost in old mother cells, resulting in sterility (24). All these findings suggest the possibility that the redistribution of the Sir complex to some nontelomeric location may increase the lifespan.
We considered the possibility that the tTLC1 mutant increased lifespan by reducing the size of the target for the Sir complex at telomeres, thereby causing a redistribution of the Sir complex to nontelomeric sites. In this case, the extension in lifespan in the tTLC1 mutant would require the integrity of the Sir complex. Thus, we deleted SIR3 in the wild type and tTLC1 overexpressing strain and repeated lifespan assays (Fig. 1B). The lifespan in the sir3 tTLC1 strain was short; i.e., tTLC1 requires SIR3 to extend lifespan. We conclude that shortening yeast telomeres results in an increase in lifespan, which is due to redistribution of the Sir complex to nontelomeric sites.
By the above model, lengthening telomeres should shorten lifespan. In an initial experiment, we deleted the RIF1 gene, which was known to result in longer telomeres (ref. 25; data not shown). Strikingly, the Δrif1 strain had a lifespan that was reduced approximately 40% compared with the parent (Fig. 2). Because a shortening in lifespan may be due to nonspecific effects on cell growth, and because the precise function of RIF1 is not known, it is possible that the observed decrease in lifespan might not be specific. However, the Δrif1 strain grew as well as the parent (25).
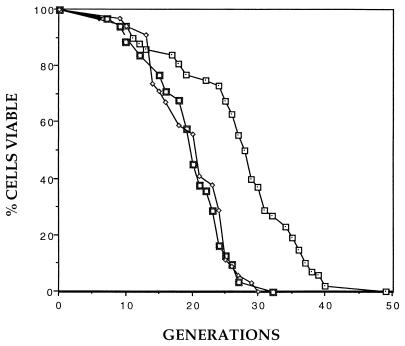
Figure 2.
Deletion of RIF1 decreases lifespan. Mortality curves are shown for the wild-type (⊡), Δrif1 (□), and Δsir3 (⋄) strains in the W303 strain background (20). Mean lifespans were 27.3, 19.1, and 20.0 generations, respectively. Corresponding sample sizes were 52, 31, and 34 cells.
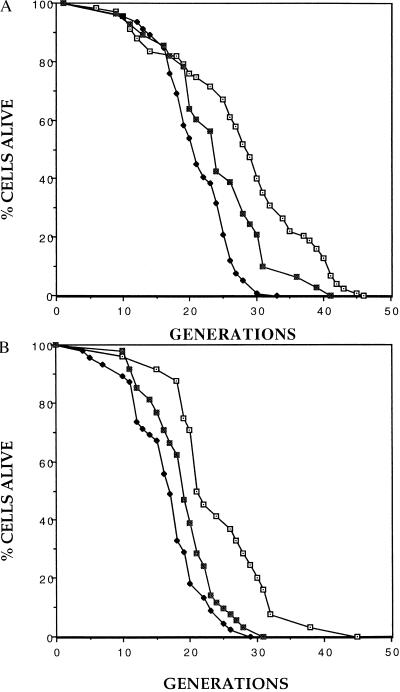
By analogy to the telomere shortening experiment described above, we reasoned that shortened lifespan in the Δrif1 strain may be due to enhanced binding of the Sir complex to longer telomeres, resulting in inadequate recruitment of the complex to a nontelomeric site. Consistent with this possibility, the lifespan of the Δrif1 strain is approximately equal to that of a Δsir3 strain which completely lacks silencing (Fig. 2). To test this model further, we constructed Δrif1 strains in which recruitment of the Sir complex to telomeres was inhibited. First, we expressed the dominant-interfering fragment of SIR4 that prevents recruitment of the complex to HM loci and telomeres (13), and we found that it partially restored normal lifespan (Fig. 3A). Next, we inserted the SIR4-42 gene into the Δrif1 strain and, again, found a partial recovery in lifespan (Fig. 3B). Because this mutation is semidominant (22), a partial effect is the maximum that could be expected in this SIR4/SIR4-42 strain. The wild-type allele of SIR4 was retained to ensure maintenance of long telomeres in the Δrif1 mutant. In summary, the effects of the Δrif1 mutation suggest that telomere lengthening decreases lifespan and that this decrease is due, at least in part, to greater recruitment of the Sir complex by the long telomeres.
Figure 3.
Mutations in SIR4 that disrupt recruitment of the SIR proteins to telomeres suppress the short-lifespan phenotype of the Δrif1 mutant. (A) Mortality curves are shown for the wild type (⊡), the Δrif1 (♦) strain, and the Δrif1 strain containing the anti-SIR4 construct (▪). Controls contained an empty vector. Mean lifespans were 27.1, 20.2, and 23.4 generations, respectively. Corresponding sample sizes were 67, 46, and 28 cells. (B) Mortality curves are shown for the wild type (⊡), the Δrif1 (♦) strain, and the Δrif1 strain carrying an integrated SIR4-42 allele (▪). Mean lifespans were 24.5, 16.8, and 19.3 generations, respectively. Corresponding sample sizes were 24, 45, and 48 cells. All mortality curves were obtained from two independent experiments, and lifespan analysis was done as described (22).
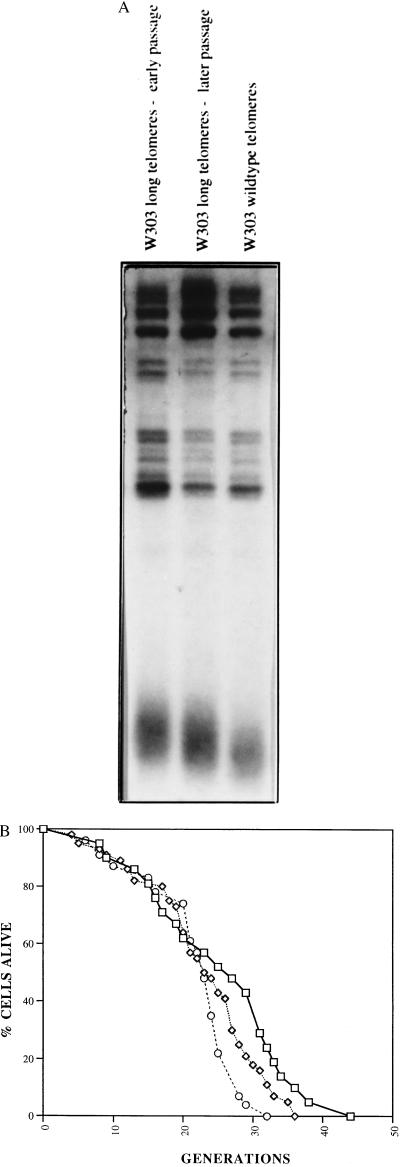
While the above experiments suggested that telomere length was inversely related to lifespan, it was possible that the mutations in TLC1 or RAP1 used to alter telomere length affected lifespan in some other way. To obtain more definitive evidence for the directness of these effects on lifespan, we took advantage of the rap1t shuffling technique described previously (21) to obtain strains with different telomere lengths but with completely wild-type genotype. As the strain was passaged telomeres gradually shortened, enabling us to determine lifespan of cells with long, intermediate-, or normal-length telomeres (Fig. 4A). Lifespan assays show an excellent inverse correlation with telomere length (Fig. 4B). The earliest passaged strain had the longest telomeres and the shortest lifespan. Continued passaging led to shorter telomeres and longer lifespan. These findings support the prior experiments, in this case, in a genotypically wild-type strain.
Figure 4.
Lifespan is inversely correlated with telomere length in wild-type yeast cells. (A) Wild-type yeast strains which have different telomere lengths were isolated as described in the text. Genomic DNA isolated from the indicated strains was digested with XhoI, electrophoresed on a 1.5% agarose gel, and probed with a telomere probe. The broad band at the bottom of each lane in the gel consists of yeast telomeres, which are heterogeneous in length. (B) Mortality curves are shown for the wild-type (□), long telomeres–early passage (○), and long telomeres–later passage (⋄) strains in the W303 strain background (20). Mean lifespans were 24.9, 21.6, and 22.8 generations, respectively. Corresponding sample sizes were 21, 44, and 23 cells.
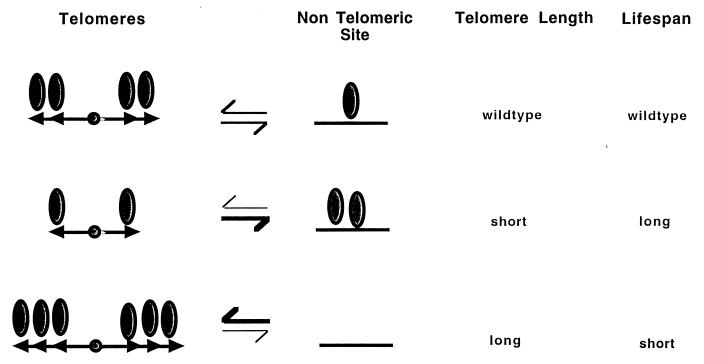
Our findings show that shortening yeast telomeres by a hypomorphic mutation in telomerase lengthens lifespan. We interpret this finding in terms of distribution of the Sir complex to genomic sites (Fig. 5). By this model, telomere shortening results in less recruitment of the Sir complex to telomeres and, by default, a greater recruitment to a nontelomeric locus. Conversely, lengthening of telomeres has the opposite effect and prevents recruitment to the nontelomeric site. The recruitment of the Sir complex to this site is a key event in promoting a long lifespan in yeast cells. This model is also consistent with the idea that telomeres may serve as a reservoir of the silencing machinery and regulate its release to nontelomeric sites (26, 27).
Figure 5.
Telomeres regulate lifespan by modulating genomic silencing. In this model, we propose that shortening of telomeres (arrowheads) results in less recruitment of the SIR silencing machinery (ovals) to telomeres and a concomitant redistribution of this machinery to nontelomeric sites. This redistribution causes a delay in senescence. Conversely, lengthening of telomeres results in greater recruitment of the silencing machinery and an acceleration of senescence.
Finally, our findings may have implications for the telomere shortening which normally occurs in human somatic cells (1–4). Although telomere shortening does not normally occur in yeast (24, 29), our findings in manipulated yeast strains are consistent with the idea that telomere shortening in humans could function as a mitotic clock. However, in contrast with the proposal that this mitotic clock causes aging, our findings raise the possibility that telomere shortening in humans is, rather, a homeostatic mechanism to promote longevity. It will be of interest to determine whether telomere shortening occurs in other very long-lived animals.
Acknowledgments
We thank S. Bell, D. Gottschling, B. Kennedy, A. Lustig, D. McNabb, D. Shore, and V. Zakian for plasmids and strains; E. Donaldson, S. Mah, K. Mills, and P. van Heyningen for comments on the manuscript; D. M. Pierre for help with Fig. 4A; and D. Shore and the members of the Guarente Laboratory for helpful discussion. This work was supported by a research grant from the National Institutes of Health and a predoctoral fellowship from the Howard Hughes Medical Institute to N.R.A.
References
- 1.Harley C B, Futcher A B, Greider C W. Nature (London) 1990;345:458–460. doi: 10.1038/345458a0. [DOI] [PubMed] [Google Scholar]
- 2.Hastie N D, Dempster M, Dunlop M G, Thompson A M, Green D K, Allshire R C. Nature (London) 1990;346:866–868. doi: 10.1038/346866a0. [DOI] [PubMed] [Google Scholar]
- 3.Vaziri H, Dragowska W, Allsopp R C, Thomas T E, Harley C B, Lansdorp P M. Proc Natl Acad Sci USA. 1994;91:9857–9860. doi: 10.1073/pnas.91.21.9857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Greider C W, Harley C B. In: Cellular Aging and Cell Death. Holbrook N J, Martin G R, Lockshin R A, editors. New York: Wiley–Liss; 1996. pp. 123–138. [Google Scholar]
- 5.Kim N W, Piatyszek M A, Prowse K R, Harley C B, West M D, Ho P L, Coviello G M, Wright W E, Weinrich S L, Shay J W. Science. 1994;266:2011–2014. doi: 10.1126/science.7605428. [DOI] [PubMed] [Google Scholar]
- 6.Mortimer R K, Johnston J R. Nature (London) 1959;183:1751–1752. doi: 10.1038/1831751a0. [DOI] [PubMed] [Google Scholar]
- 7.Jazwinski S M. Mol Microbiol. 1990;4:337–343. doi: 10.1111/j.1365-2958.1990.tb00601.x. [DOI] [PubMed] [Google Scholar]
- 8.Shampay J, Szostak J W, Blackburn E H. Nature (London) 1984;310:154–157. doi: 10.1038/310154a0. [DOI] [PubMed] [Google Scholar]
- 9.Singer M S, Gottschling D E. Science. 1994;266:404–409. doi: 10.1126/science.7545955. [DOI] [PubMed] [Google Scholar]
- 10.Aparicio O M, Billington B L, Gottschling D E. Cell. 1991;66:1279–1287. doi: 10.1016/0092-8674(91)90049-5. [DOI] [PubMed] [Google Scholar]
- 11.Moretti P, Freeman K, Coodly L, Shore D. Genes Dev. 1994;8:2257–2269. doi: 10.1101/gad.8.19.2257. [DOI] [PubMed] [Google Scholar]
- 12.Cockell M, Palladino F, Laroche T, Kyrion G, Liu C, Lustig A J, Gasser S M. J Cell Biol. 1995;129:909–924. doi: 10.1083/jcb.129.4.909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ivy J M, Klar A J S, Hicks J B. Mol Cell Biol. 1986;6:688–702. doi: 10.1128/mcb.6.2.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rine J, Herskowitz I. Genetics. 1987;116:9–22. doi: 10.1093/genetics/116.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bell S P, Kobayashi R, Stillman B. Science. 1993;262:1844–1849. doi: 10.1126/science.8266072. [DOI] [PubMed] [Google Scholar]
- 16.Foss M, McNally F J, Laurenson P, Rine J. Science. 1993;262:1838–1844. doi: 10.1126/science.8266071. [DOI] [PubMed] [Google Scholar]
- 17.Kurtz S, Shore D. Genes Dev. 1991;5:616–628. doi: 10.1101/gad.5.4.616. [DOI] [PubMed] [Google Scholar]
- 18.Hecht A, Laroche T, Strahl-Bolsinger S, Gasser S M, Grunstein M. Cell. 1995;80:583–592. doi: 10.1016/0092-8674(95)90512-x. [DOI] [PubMed] [Google Scholar]
- 19.Linger J, Hughes T R, Shevchenko A, Mann M, Lundblad V, Cech T R. Science. 1997;276:561–567. doi: 10.1126/science.276.5312.561. [DOI] [PubMed] [Google Scholar]
- 20.Thomas B J, Rothstein R. Cell. 1989;56:619–630. doi: 10.1016/0092-8674(89)90584-9. [DOI] [PubMed] [Google Scholar]
- 21.Kyrion G, Liu K, Liu C, Lustig A J. Genes Dev. 1993;7:1146–1159. doi: 10.1101/gad.7.7a.1146. [DOI] [PubMed] [Google Scholar]
- 22.Kennedy B K, Austriaco N R, Jr, Zhang J, Guarente L. Cell. 1995;80:485–496. doi: 10.1016/0092-8674(95)90499-9. [DOI] [PubMed] [Google Scholar]
- 23.Becker D M, Fikes J D, Guarente L. Proc Natl Acad Sci USA. 1991;88:1968–1972. doi: 10.1073/pnas.88.5.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Smeal T, Claus J, Kennedy B, Cole F, Guarente L. Cell. 1996;84:633–642. doi: 10.1016/s0092-8674(00)81038-7. [DOI] [PubMed] [Google Scholar]
- 25.Hardy C F J, Sussel L, Shore D. Genes Dev. 1992;6:801–814. doi: 10.1101/gad.6.5.801. [DOI] [PubMed] [Google Scholar]
- 26.Buck S W, Shore D. Genes Dev. 1995;9:370–384. doi: 10.1101/gad.9.3.370. [DOI] [PubMed] [Google Scholar]
- 27.Marcand S, Buck S W, Moretti P, Gilson E, Shore D. Genes Dev. 1996;10:1297–1305. doi: 10.1101/gad.10.11.1297. [DOI] [PubMed] [Google Scholar]
- 28.Gottschling D E, Aparicio O M, Billington B L, Zakian V A. Cell. 1990;63:751–762. doi: 10.1016/0092-8674(90)90141-z. [DOI] [PubMed] [Google Scholar]
- 29.D’Mello N P, Jazwinski S M. J Bacteriol. 1991;173:6709–6713. doi: 10.1128/jb.173.21.6709-6713.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]