Abstract
We have previously presented evidence of a highly organized and compartmentalized structure of the small intestinal lamina propria of the pig. In this study, we conducted a detailed analysis of the T-cell populations found at this site, and compared these T cells with cell populations found in other tissue sites and the periphery. We showed that the CD4+ and CD8+ T-cell populations found in the pig gut are of memory phenotype, defined by CD45 isoform expression, but show few signs of recent activation. They show a high degree of phenotypic and therefore presumably functional homogeneity. Both CD4- and CD8-positive cells show strong parallels in the patterns of surface molecule expression, suggesting similar pressures on differentiation. The unique combination of surface molecules found on lamina propria T cells is found only infrequently on cells in other lymphoid sites.
INTRODUCTION
The immunological tissue of the gut (GALT) has two major components: the so-called organized lymphoid tissue of the Peyer's patches (PP) and the diffuse lymphoid tissue of the remaining gut containing large numbers of T and other cells, both in the epithelial compartment and the lamina propria (LP). PP are generally accepted to be inductive sites, but LP T cells are thought to be predominantly of memory phenotype. Two major hypotheses for the role of LP T cells have been advanced: they may fulfil surveillance functions and provide recall responses to enteric pathogens, or they may be involved in the regulation of immune responses to harmless commensal bacteria or food-derived antigens. Functional evidence for both these roles has recently emerged.1,2
If LP T cells are classical memory cells providing defence against gut pathogens, several inconsistencies, both functional and phenotypic, need to be explained: systemic memory T cells respond vigorously to specific recall antigen, and secrete high levels of cytokines, whereas naive T cells require a greater degree of stimulation and respond less vigorously.3 T cells from the LP rarely show such antigen-specific proliferation. This is true for mucosally as well as peripherally administered antigens, including rectally administered ones, in spite of clear proliferative T-cell responses from other sites.4,5 It has also been reported that human CD4+ LP T cells respond with low interleukin (IL)-2 secretion and poor proliferative responses following ligation of the CD3/T-cell receptor (TCR) complex, but high IL-2 secretion and relatively greater proliferation has been reported following stimulation with concanavalin A (Con A) or via CD2,6 although proliferative responses are still at a reduced level relative to peripheral T cells. We have previously found that following polyclonal activation, pig LP T cells express the IL-2 receptor (IL-2R) with kinetics similar to those of peripheral T cells. However, although they express mRNA encoding IL-4 and secrete high amounts of this cytokine, message for IL-2 is only transiently expressed and IL-2 secretion is very low.7 Phenotypically, although human LP T cells have been reported to be predominantly of memory phenotype, the levels of CD29, elevated on peripheral memory T cells, are low.8 However, human LP T cells are thought to show evidence of activation in situ.4,9–11
The question arises whether cells in the LP are a memory T-cell subset, which can also be found in other peripheral or tissue sites, or whether they are a fundamentally different set of effector T cells, reflected by a fundamentally different phenotype, supporting the second hypothesis.
In this study, we have conducted a detailed phenotypic analysis of pig LP T cells, in comparison with T cells isolated from other sites, to approach this question. The pig has a number of advantages for studies specifically of this diffuse immunological tissue: the pig is a large, monogastric omnivore and therefore unlike humans, comparison of systemic and intestinal cells is possible in normal individuals. Unlike rodents, our method allows largely separate analysis of PP cells, LP cells and intraepithelial lymphocytes.
T cells from peripheral sites and solid lymphoid tissue are functionally and phenotypically heterogeneous in all species studied. Both CD4+ and CD8+ cells can be further subdivided by the expression of surface molecules, reflecting their developmental and activation status, such as the expression of CD45 isoforms,12,13 adhesion molecules, major histocompatibility complex (MHC) class II and the IL-2 receptor. Pig T cells show a further level of heterogeneity. There are a number of cell types that are only rarely seen in other species, such as large numbers of CD4/CD8 double positive as well as CD4/CD8 double-negative T cells.14,15 Furthermore, two surface molecules have been identified on pig T cells that are also associated with their activated/memory status. The surface molecule recognized by SwC1 is lost following activation,16 while that associated with SwC8 is found on T cells with blast morphology.17
MATERIALS AND METHODS
Cell isolation
Leucocytes were isolated from thymus (THY), spleen (SPL), peripheral blood (PBL), mesenteric lymph node (MLN), prefemoral lymph node (PFLN) and jejunal LP.
Peripheral blood was collected with the addition of 1 mm EDTA and isolated by density-gradient centrifugation.
LP leucocytes were isolated from the ileum by a method described previously.18 Briefly, an inverted, inflated gut sac was incubated in 1 mm EDTA in calcium- and magnesium-free Hanks' balanced salt solution (HBSS) at 37° on a shaking platform for a total period of approximately 3 hr. The tissue was then digested in RPMI Dutch Modification (Life Technologies Ltd, Paisley, UK) containing 100 U/ml collagenase (Collagenase Grade V; Sigma, Poole, Dorset, UK) at 37° for 1 hr. Tissue fragments from lymph nodes and spleen were similarly digested. This was followed by isolation of leucocytes on a 40%/75% Percoll gradient. Thymocytes were isolated by mechanical disruption.
Monoclonal antibodies (mAbs)
Antibodies to CD2 (clone MSA4), CD4 (clone 10-2H2) and CD8 (clone 76-2-11) were donated by Dr J. K. Lunney (USDA, Beltsville, MD); to CD3 (clone FY 1H2) by Dr H. Yang (Pirbright, Woking, UK); to CD18 (clone PNK-I) by Dr Y. B. Kim (Finch University of Health, North Chicago, IL); to CD29 (clone UCP 1D2) by Dr F. Zuckermann (University of Illinois, Urbana, IL) and to CD44 (clone BAG40 A) by Dr W. Davis (Washington State University, Pullman, WA). We thank all donors for the use of their antibodies. The anti-CD25 (clone K231.3B2), CD45 (clone K252.1E4), CD45RA (clone MIL13), CD45RC (clone MIL5), SwC1 (clone K263.3D7), SwC8 (clone MIL3) and anti-MHC class II DQ (clone K274.3G8) antibodies were prepared by the authors.
Flow cytometry
Leucocytes (1×106) were suspended in 50 μl of phosphate-buffered saline (PBS) containing 0·2% sodium azide (PBSA) and incubated with 50 ml each of optimized concentrations of mAbs for 1 hr at 4°. Cells were washed in PBSA and incubated with 50 μl each of pretitrated isotype-specific affinity-purified goat antimouse antisera, biotinylated or conjugated to fluorescein isothiocyanate (FITC) and phycoerythrin (PE), respectively (Southern Biotechnology, Birmingham, AL). For two-colour flow cytometry, only PE and FITC conjugates were used; for three-colour flow cytometry, the third colour was visualized using the appropriate isotype-specific biotinylated antiserum followed by a third step consisting of the addition of 1 μl of streptavidin–Tricolor (Caltag Laboratories, Burlingame, CA). Cells were washed in PBSA, and fluorescence was quantified using an EPICS Flow Cytometer (Coulter Electronics, Luton, Bedfordshire, UK). Isotype-matched pure mouse immunoglobulins were used to determine non-specific fluorescence of controls. Cells were electronically gated to exclude contaminating erythrocytes, small debris (FALS) and granulocytes (RALS). For three-colour flow cytometry, electronic gating based on co-expression of CD45RC, CD4 and/or CD8 was used to identify T cells of memory phenotype. The two-colour flow cytometric studies were repeated for six animals; the three-colour studies were conducted for three animals.
Animals
Samples were taken from slaughter-weight healthy pigs of White Landrace breed.
RESULTS
CD3, CD4 and CD8 expression
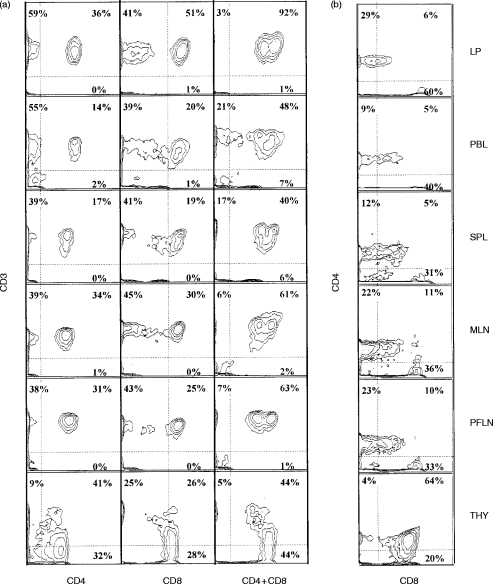
With the exception of the thymus, all cells expressing CD4 or high levels of CD8 co-expressed CD3 and were therefore, by definition, T cells (Fig. 1a). Co-expression studies or gating were therefore conducted on cell subsets that expressed either CD4 or high levels of CD8. The ratio of CD4+ and CD8high cells was slightly skewed towards CD8+ cells in LP; the mean CD4+:CD8high ratio in LP was 0·71:1 and in other peripheral tissues 1·01:1. The two unusual pig T-cell subsets, CD3+/CD4+/CD8+ and CD3+/CD4−/CD8− were also compared among LP and other tissues. We observed variable but significant numbers of CD3+/CD4−/CD8− in most peripheral tissues (21%±14) and also in thymus, but consistently low numbers in the LP (7%±4) (Fig. 1a). The CD4+/CD8+ cell population (Fig. 1b) was seen as a distinctive subset with intermediate levels of CD8 expression in all peripheral tissues (32%±0·3). In the LP, a smaller percentage of CD4+ cells (17%±8) co-expressed CD8 and at comparatively low levels. Additionally, a CD3− cellular subset expressing CD8 at low intensity was found, which was most numerous in peripheral blood and splenocytes, but was not present in LP. These cells have been reported to be natural killer (NK) cells.19
Figure 1.
(a) CD3 expression on CD4- and CD8-positive T cells. (b) CD8 and CD4 co-expression. Representative sample of six animals.
Levels of other surface molecules, such as CD45, CD3 and CD2 in LP, were comparable to the levels observed in other tissues (data not shown).
CD45 isoforms
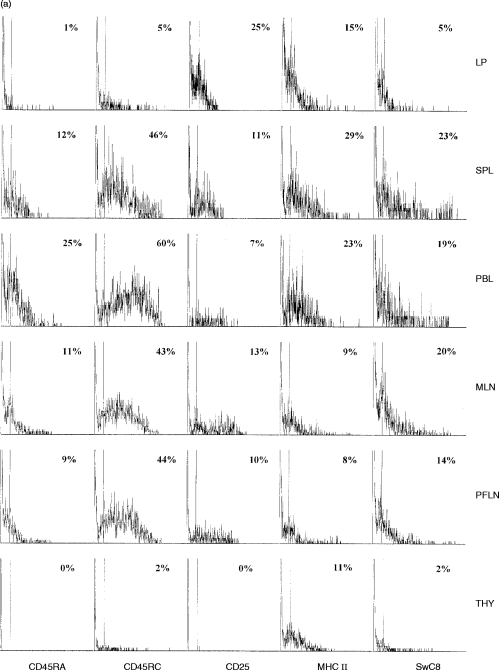
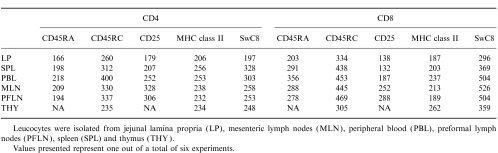
T cells from the periphery and other lymphoid tissue showed a highly heterogeneous expression of these surface molecules. A small percentage of LP T cells showed co-expression of these surface molecules; however, the intensity of this expression was consistently lower than on T cells from other sites (Fig. 2(a), 2(b) and Table 1).
Figure 2.
(a) CD45RA, CD45RC, CD25, MHC class II and SwC8 expression on CD4-positive T cells. Representative sample of six animals. (b) CD45RA, CD45RC, CD25, MHC class II and SwC8 expression on CD8-positive T cells. Representative sample of six animals.
Table 1.
MFI (mean fluorescence intensity) of second surface molecule on CD4- and CD8-positive cells
Activation status
A similar number of peripheral, lymphoid and LP T cells expressed MHC class II (Fig. 2(a), 2(b) and Table 1). The number of LP CD4+ cells expressing the CD25 surface molecule was higher than in other tissues; however, the intensity of this expression was relatively low in comparison to levels found on activated cells in the peripheral and lymphoid populations (Fig. 2(a), 2(b) and Table 1). The expression of SwC1, a marker for resting T cells, was variable on T cells from peripheral sites, but consistently high on LP T cells (Fig. 3).The new pig cluster determinant, SwC8, found to be expressed at high intensity on T cells of blast cell phenotype,6 was present on a distinct subset of lymph node and peripheral cells (2(a), Fig. 2(b) and Table 1), but was largely absent from LP T cells.
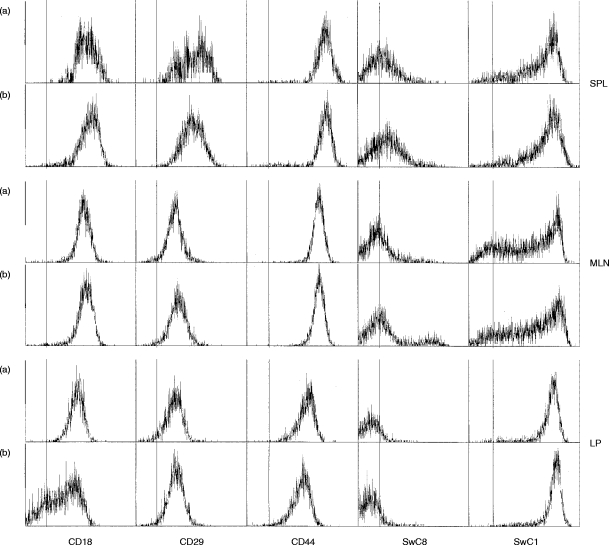
Figure 3.
Expression of CD18, CD29, CD44, SwC1 and SwC8, gated on CD45RC-negative, CD4-positive (a) and CD45RC-negative, CD8-positive (b) memory T cells. Representative sample of three animals.
Adhesion molecules
A comparison of cell adhesion molecules on memory T cells (gated for lack of expression of CD45RC) from spleen, MLN and LP showed that LP CD45RC-negative T cells showed consistently lower levels of all three adhesion molecules in comparison with the other two tissues (Fig. 3). Approximately 50% of LP CD8+ cells did not express any CD18 and LP CD4+ cells expressed CD18 at slightly lower levels than comparable cells from spleen and MLN. LP T cells expressed CD29 at levels comparable to those found on MLN cells, whereas splenic T cells showed biphasic and comparatively higher levels of CD29. CD44 expression was uniform for both CD4+ and CD8+ cells within each tissue, but differed between tissues, with LP T cells expressing the lowest and splenic T cells the highest levels.
DISCUSSION
Recent studies in humans and rodents have begun to demonstrate that the intestinal LP is not a simple effector site.
As reported for other species, our results demonstrate that pig T cells isolated from the periphery and various lymphoid organs are a heterogeneous mixture of cells, indicative of dynamic cell populations in various states of activation, age and functional specialization. In contrast, we found that the LP T-cell phenotype was much more restricted. This homogeneity was accompanied by a high degree of spatial organization, as shown in previous immunohistochemical studies.20,21 We observed the two major T-cell subsets, CD3+/CD4+ and CD3+/CD8+ cells, in a ratio comparable to that found in other sites; this agrees with findings in other species.22 These two major subsets were highly homogeneous and showed closely parallel phenotypes. T-cell subsets, uniquely present in pigs and reported by other workers, such as the CD3+/CD4+/CD8+ and the CD3+/CD4−/CD8− cells,14,23 were reduced in numbers.
The increased number of CD25+ LP T cells, reported in other species, has generally been interpreted to be indicative of an increased activational status of these cells.4,9,10,22,24 However, although the number of CD25+ T cells in the pig was relatively high, in agreement with findings for humans, their mean fluorescence intensity was consistently low. This was not a result of the inability of LP T cells to express the IL-2R, as we have shown previously that pig LP T cells express CD25 in similar proportions and at similarly high intensity to splenocytes following ConA activation in vitro.7,18 In a recent study of CD4+ and CD8+ LP cells in healthy macaques, a significant proportion of LP T cells expressed CD25, but the intensity of this expression was also very low.25 Interestingly, exposure to viral infection, in this case simian immunodeficiency virus (SIV), resulted in a considerable increase in intensity of CD25. LP T cells in pigs and other species therefore appear to have a low surface density of CD25, indicating either cells that have not experienced maximal stimulation or cells of advanced memory phenotype, where CD25 has been largely lost again.14,26 In either case, these T cells do not appear to be at a stage of maximal stimulation. The expression of CD8 on peripheral CD4+ pig T cells is also thought to be indicative of activation.15 CD8 co-expression on LP CD4+ cells was present but also relatively low. MHC class II, another activation marker, was expressed on pig LP T cells at levels similar to that found in the periphery. In addition, expression of two cluster determinants, uniquely characterized in pigs, also suggests that these are not classical activated cells. Both SwC1 and SwC8 have been clustered in recent pig CD workshops.16,27 SwC1 is recognized by our antibody K263.3D7 and represents a surface molecule of 21 000 MW, which is widely expressed on resting T cells and monocytic and myeloid cells. SwC1 expression has been found to be down-regulated rapidly following activation,16 yet LP T cells show uniformly high expression. SwC8 is recognized by our antibody MIL3 and represents a surface molecule of 32 000 MW.17,28 SwC8 is expressed at high intensity by T-cell subsets from peripheral sites and lymph nodes with light scatter characteristics of blast cells, but expressing both high- and low-molecular-weight isoforms of CD45. Very few LP cells express SwC8.
In agreement with findings for other species, the lack of expression of the low-molecular-weight isoforms, CD45RA and CD45RC, on the majority of LP T cells, suggests that these cells are antigen experienced. A small percentage of cells express CD45RC, but again at comparatively low levels. However, other cell-surface markers elevated on classical memory T cells, such as the adhesion molecules CD18, CD29 and CD44, were relatively low. In fact, CD8+ cells from the LP contained a large subset of cells that were CD18 negative. Relatively low levels of CD29 have also been reported for human LP T cells.8 The evidence for the memory status of pig LP T cells is therefore contradictory. It has been suggested that CD45 isoforms are not reliable indicators of memory cells because human CD45RO+ cells can re-express CD45RA in vivo.29 Reversion of CD45RC− memory cells to CD45RC+ expression has also been reported in the rat, both in vivo and in vitro.30 However, loss of CD45RC is certainly driven by antigenic exposure and therefore by definition indicative of a ‘memory’ cell. Thus, although memory T cells may express CD45RC, there have been no reports of mature antigen-naïve T cells expressing CD45RO or lacking CD45RC. Additionally, developmental studies have shown that the appearance of T cells in the LP of the pig is driven entirely by antigen because the LP of newborn31 and gnotobiotic piglets32 is devoid of T cells.
This conflicting evidence highlights the difficulties in distinguishing molecules associated with memory rather than the activational status of a cell. As yet, the time course of acquisition and subsequent loss of such surface molecules in vivo is largely unknown. The combination of these activation/memory-associated molecules on the cell surface must presumably depend on the exact time point and degree of antigenic stimulation experienced by the cell, which in the case of LP cells appears to be different from the one of classical memory cells, but similar for the majority of both CD4+ and CD8+ cells.
We suggest that the overall evidence shows that pig LP T cells are antigen-driven and antigen-experienced cells, but with features that distinguish them from classical ‘memory’ cells. The overall evidence is compatible with T cells of advanced memory status, where some molecules, usually associated with activation/memory, have been lost again.
Functional studies of LP T cells support the conclusion of fundamental differences between LP and peripheral T cells in the pig: in vitro-activated pig LP T cells transcribe IL-4 in preference to IL-2,7,18 again consistent with advanced memory status.13 These cells occupy an environment of potentially high antigenic exposure, which contains many cells with high levels of MHC class II. We have previously reported high levels of MHC class II antigens on other cells in the pig intestinal LP,20,21,33 including on endothelial cells. Such non-professional antigen-presenting cells (APC) have the potential to anergize T-cell responses.34 The low levels of adhesion molecules, such as CD18, CD29 and CD44, may also be relevant in this respect. Such adhesion molecules are important in cell–cell interactions, especially between T cells and APC. The low levels of these molecules on LP T cells suggest a lack of important functional properties usually found in memory cells. The memory status of these cells, and their failure to secrete IL-2 after activation, raises questions as to their functional response following local antigenic stimulation. Highly differentiated cells become increasingly prone to apoptosis and are dependent on exogenous cytokines for survival.13 We suggest that this may also be true for pig LP T cells, rendering them suitable targets for immunoregulation.
There are clearly differences between species. Human LP T cells are capable of secreting IL-2 following stimulation via CD235,36 and show signs of activation other than CD25 expression, i.e. the very early activation antigen CD69.9 The population of cells in human and mouse is also more heterogeneous: greater numbers of CD45RA+ cells –≈10–20%24,35– may affect the response. However, it seems unlikely that the fundamental immunological function of the same organ varies between different species.
We suggest that, in spite of some genuine differences between species, there are sufficient unusual phenotypic and functional characteristics to question the role of LP T cells in local immune responses. This population may not be capable of responding to, or even be specific for, antigens of enteric pathogens, but may represent a population undergoing local regulation or negative selection.
REFERENCES
- 1.Khoo UI, Proctor IE, Macpherson AJS. CD4+ T cell down-regulation in human intestinal mucosa. J Immunol. 1997;158:3626. [PubMed] [Google Scholar]
- 2.Molberg O, Nilsen EM, Sollid LM, et al. CD4(+) T cells with specific reactivity against astrovirus isolated from normal human small intestine. Gastroenterology. 1998;114:115. doi: 10.1016/s0016-5085(98)70639-0. [DOI] [PubMed] [Google Scholar]
- 3.Pilling D, Akbar AN, Bacon PA, Salmon M. CD4(+) CD45RA(+) T cells from adults respond to recall antigens after CD28 ligation. Int Immunol. 1996;8:1737. doi: 10.1093/intimm/8.11.1737. [DOI] [PubMed] [Google Scholar]
- 4.Zeitz M, Quinn TC, Graeff AS, James SP. Mucosal T cells provide helper function but do not proliferate when stimulated by specific antigen in Lymphogranuloma venereum proctitis in nonhuman primates. Gastroenterology. 1988;94:353. doi: 10.1016/0016-5085(88)90422-2. [DOI] [PubMed] [Google Scholar]
- 5.Vancott JL, Brim TA, Lunney JK, Saif LJ. Contribution of antibody-secreting cells induced in mucosal lymphoid-tissues of pigs inoculated with respiratory or enteric strains of coronavirus to immunity against enteric coronavirus challenge. J Immunol. 1994;152:3980. [PubMed] [Google Scholar]
- 6.Boirivant M, Fuss I, Fiocchi C, Klein JS, Strong SA, Strober W. Hypoproliferative human lamina propria T cells retain the capacity to secrete lymphokines when stimulated via the CD2/CD28 pathways. Proc Assoc Am Phys. 1996;108:55. [PubMed] [Google Scholar]
- 7.Bailey M, Plunkett F, Clarke A, Sturgess D, Haverson K, Stokes CR. Activation of T cells from the intestinal lamina propria of the pig. Scand J Immunol. 1998;48:177. doi: 10.1046/j.1365-3083.1998.00365.x. [DOI] [PubMed] [Google Scholar]
- 8.Zeitz M, Ullrich R, Schneider T, Schieferdecker HL, Riecken EO. Cell differentiation and proliferation in the gastrointestinal tract with respect to the local immune system. Ann NY Acad Sci. 1994;733:75. doi: 10.1111/j.1749-6632.1994.tb17258.x. [DOI] [PubMed] [Google Scholar]
- 9.De Maria R, Fais S, Silvestri M, et al. Continuous in vivo activation and transient hyporesponsiveness to TCR/CD3 triggering of human gut lamina propria lymphocytes. Eur J Immunol. 1993;23:3104. doi: 10.1002/eji.1830231209. [DOI] [PubMed] [Google Scholar]
- 10.Schieferdecker HL, Ullrich R, Weiss-Breckwoldt AN, et al. The HML-1 antigen of intestinal lymphocytes is an activation antigen. J Immunol. 1990;144:2541. [PubMed] [Google Scholar]
- 11.Zeitz M, Greene WC, Peffer NJ, James SP. Lymphocytes isolated fronm the intestinal lamina propria of normal nonhuman primates have increased expression of genes associated with T-cell activation. Gastroenterology. 1988;94:647. doi: 10.1016/0016-5085(88)90235-1. [DOI] [PubMed] [Google Scholar]
- 12.Akbar AN, Terry L, Timms A, Beverley PCL, Janossy G. Loss of CD45R and gain of UCHL1 reactivity is a feature of primed T cells. J Immunol. 1988;140:2171. [PubMed] [Google Scholar]
- 13.Salmon M, Pilling D, Borthwick NJ, et al. The progressive differentiation of primed T cells is associated with an increasing susceptibility to apoptosis. Eur J Immunol. 1994;24:892. doi: 10.1002/eji.1830240417. [DOI] [PubMed] [Google Scholar]
- 14.Yang H, Parkhouse ME. Phenotypic classification of porcine lymphocyte subpopulations in blood and lymphoid tissues. Immunology. 1996;89:76. doi: 10.1046/j.1365-2567.1996.d01-705.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pescovitz MD, Sakopolous AG, Gaddy JA, Husmann RJ, Zuckermann FA. Porcine peripheral blood CD4(+) CD8(+) dual-expressing T cells. Vet Immunol Immunopathol. 1994;43:53. doi: 10.1016/0165-2427(94)90120-1. [DOI] [PubMed] [Google Scholar]
- 16.Saalmüller A, Aasted B, Canals A, et al. Analysis of Mab reactive with the porcine SwC1. Vet Immunol Immunopathol. 1994;43:255. doi: 10.1016/0165-2427(94)90145-7. [DOI] [PubMed] [Google Scholar]
- 17.Haverson K, Stokes CR, Bailey M. A novel surface molecule on a subset of CD8-positive pig T cells. Immunology. 1997;92:29. [Google Scholar]
- 18.Bailey M, Hall L, Bland PW, Stokes CR. Production of cytokines by lymphocytes from spleen, mesenteric lymph-node and intestinal lamina propria of pigs. Immunology. 1994;82:577. [PMC free article] [PubMed] [Google Scholar]
- 19.Saalmüller A, Hirt W, Maurer S, Weiland E. Discrimination between 2 subsets of porcine CD8(+) cytolytic T lymphocytes by the expression of CD5 antigen. Immunology. 1994;81:578. [PMC free article] [PubMed] [Google Scholar]
- 20.Wilson AD, Haverson K, Southgate K, Bland PW, Stokes CR, Bailey M. Expression of major histocompatibility complex class-II antigens on normal porcine intestinal endothelium. Immunology. 1996;88:98. doi: 10.1046/j.1365-2567.1996.d01-640.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Haverson K, Stokes CR, Bailey M. Immunological structure and antigen-presenting cells in the intestinal lamina propria. Periodic Biolog. 1997;99:335. [Google Scholar]
- 22.James SP, Zeitz M. Human gastrointestinal mucosal T cells. In: Ogra PL, Mestecky J, Lamm ME, Strober W, Mcghee JR, Bienenstock J, editors. Handbook of Mucosal Immunology. San Diego, CA, USA: Academic Press, Inc.; 1994. pp. 275–286. [Google Scholar]
- 23.Saalmüller A, Reddehase MJ, Buhring HJ, Jonjic S. Simultaneous expression of CD4 and CD8 antigens by a substantial proportion of resting porcine T lymphocytes. Eur J Immun. 1987;17:1297. doi: 10.1002/eji.1830170912. [DOI] [PubMed] [Google Scholar]
- 24.Schreiber S, Macdermott RP, Raedler A, Pinnau R, Bertovich MJ, Nash GS. Increased activation of isolated intestinal lamina propria mononuclear cells in inflammatory bowel disease. Gastroenterology. 1991;101:1020. doi: 10.1016/0016-5085(91)90729-5. [DOI] [PubMed] [Google Scholar]
- 25.Veazey RS, Demaria M, Chalifoux LV, et al. Gastrointestinal tract as a major site of CD4+ T-cell depletion and viral replication in SIV infection. Science. 1998;280:427. doi: 10.1126/science.280.5362.427. [DOI] [PubMed] [Google Scholar]
- 26.Male D, Cooke A, Owen M, Trowsdale J, Champion B. Advanced Immunology. 3. London: Mosby; 1996. [Google Scholar]
- 27.Haverson K, Zuckermann F, Saalmüller A, Lipp J, Aasted B, Stokes CR. Summary of workshop findings for porcine adhesion molecule subgroup. Vet Immunol Immunopathol. 1998;60:351. doi: 10.1016/s0165-2427(97)00111-6. [DOI] [PubMed] [Google Scholar]
- 28.Haverson K, Bailey M, Higgins VR, Bland PW, Stokes CR. Characterization of monoclonal antibodies specific for monocytes, macrophages and granulocytes from porcine peripheral blood and mucosal tissues. J Immunol Methods. 1994;170:233. doi: 10.1016/0022-1759(94)90398-0. [DOI] [PubMed] [Google Scholar]
- 29.Michie CA, McLean A, Alcock C, Beverley PCL. Life-span of human lymphocyte subsets defined by CD45 isoforms. Nature. 1992;360:264. doi: 10.1038/360264a0. [DOI] [PubMed] [Google Scholar]
- 30.Bell EB, Sparshott SM. Interconversion of CD45R subsets of CD4 T cells in vivo. Nature. 1990;348:163. doi: 10.1038/348163a0. [DOI] [PubMed] [Google Scholar]
- 31.Vega-Lopez MA, Bailey M, Telemo E, Stokes CR. Effect of early weaning on the development of immune cells in the pig small intestine. Vet Immunol Immunopathol. 1995;44:319. doi: 10.1016/0165-2427(94)05309-g. [DOI] [PubMed] [Google Scholar]
- 32.Rothkötter HJ, Pabst R. Lymphocyte subsets in jejunal and ileal Peyer's patches in normal and gnotobiotic minipigs. Immunology. 1989;67:103. [PMC free article] [PubMed] [Google Scholar]
- 33.Stokes CR, Haverson K, Bailey M. Antigen presenting cells in the porcine gut. Vet Immunol Immunopathol. 1996;54:171. doi: 10.1016/s0165-2427(96)05704-2. [DOI] [PubMed] [Google Scholar]
- 34.Lombardi G, Arnold K, Uren J, et al. Antigen presentation by interferon-gamma-treated thyroid follicular cells inhibits interleukin-2 (IL-2) and supports IL-4 production by B7-dependent human T cells. Eur J Immunol. 1997;27:62. doi: 10.1002/eji.1830270110. [DOI] [PubMed] [Google Scholar]
- 35.Targan SR, Deem RL, Liu M, Wang S, Nel A. Definition of a lamina propria T-cell responsive state. J Immunol. 1995;154:664. [PubMed] [Google Scholar]
- 36.Boirivant M, Fuss I, Fiocchi C, Klein JS, Strong SA, Strober W. Hypoproliferative human lamina propria T cells retain the capacity to secrete lymphokines when stimulated via CD2/CD28 pathways. Proc Assoc Am Phys. 1996;108:55. [PubMed] [Google Scholar]