Abstract
U937 cells provide a co-stimulatory signal for CD3-mediated T-cell activation which is independent of the CD28/CD80/CD86 interaction. This study set out to identify which molecules contribute to this co-stimulatory activity. Monoclonal antibodies (mAb) to the known accessory molecules CD11a, CD18, CD54 and CD45, all inhibited T-cell proliferation. Although CD11a/18 mAb inhibited U937/T-cell cluster formation as well as proliferation, CD45 enhanced the size of the clusters formed, suggesting that this was not the only mechanism of inhibition. The alternative co-stimulatory pathway provided by U937 cells preferentially stimulated a response in the CD18+ T-cell population, and this reflected the reduced sensitivity of CD8+ T cells to CD28-mediated activation. Monoclonal antibodies to three molecules, CD53, CD98 and CD147, also inhibited U937-dependent T-cell proliferation. The mAb to CD98 and CD147 were inhibitory when prepulsed on to the U937 cells, suggesting an effect mediated by these molecules on the antigen-presenting cell.
INTRODUCTION
The activation of T cells in response to antigen (Ag) requires at least two types of signals to be provided by an antigen-presenting cell (APC).1 The first of these is mediated via the interaction of the antigen-specific T-cell receptor (TCR) with peptide that is associated with major histocompatibility complex (MHC) molecules on the surface of the APC.2 As a result of this interaction, the TCR-associated CD3 complex mediates intracellular signals that are necessary but not sufficient for T-lymphocyte clonal proliferation.3,4 The second critical signal is mediated either by proteins on the T lymphocytes that interact with co-stimulatory molecules on the surface of the APC5,6 or by soluble cytokines produced by APC acting on the T cell. Unlike TCR-mediated signals, these co-stimulatory signals are not Ag specific. The type of co-stimulatory signal that is delivered is dependent on several factors, including not only the type and activation status of the APC,7 but also the type of T cell (CD4+ or CD8+), and whether the T cells are of naive or memory phenotype.8
Several cell surface co-stimulatory interactions have been implicated in T-lymphocyte activation. The most thoroughly investigated are those between CD80 (B7.1)/CD86 (B7.2) on the APC, and CD28/CTLA-45,6,9–11 on the T cells, between CD70 and CD27,12,13 between CD2 and CD58, CD59, or CD48,14–16 and between the β2 integrins, especially α1/β2 (lymphocyte function-associated antigen-1; LFA-1; CD11a/CD18) and the intracellular adhesion molecules (ICAM).17,18 However, there have been several suggestions that there may well be other additional co-stimulatory molecules. An example of this is the previous study in which Johnson & Jenkins et al. demonstrated that the monoblastoid U937 cell can act as an accessory cell providing co-stimulatory signals for T-cell activation induced by antibody against CD3.19 This co-stimulatory activity was independent of the CD80/CD86–CD28 pathway, as U937 cells do not express CD80, and express very low levels of CD86.20,21 Antibodies against CD11a/CD18 were not able to substitute for U937 cells in this model, suggesting that an ICAM –β2 integrin interaction also did not provide the essential second signal.
In this study we have re-examined the U937-dependent activation of T cells, with the objective of identifying the novel candidate co-stimulatory molecules that have been proposed. In addition, we have also re-examined the requirement for the β2–integrin–ICAM interaction in CD80- and CD86- independent T-cell activation, and the relationship between the co-stimulatory signals and the phenotype of the activated T cells. The results have significant implications both for our understanding of T-cell activation, and in terms of the immune response to tumours and auto-antigens.
MATERIALS AND METHODS
Cell line
The human monoblastoid U937 cell line was used as the accessory cell in a T-cell co-stimulation assay. U937 cells were grown in RPMI-1640 complete medium (RPMI-1640 supplemented with 10% fetal calf serum, 2 mm l-glutamine, 100 U penicillin/streptomycin, and 20 μm β-mercaptoethanol). Cells used in the assay were irradiated using an X-ray generator (4000 rads in 20 min). The post-irradiated viable cells were separated from non-viable cells over Ficoll–Hypaque (Sigma, Poole, UK; density 1·077).
T cells
Human tonsils (from routine tonsillectomies, Royal National Throat Nose and Ear Hospital, London, UK) were used as a source of T cells. Tonsils were cut into small pieces with a scalpel, digested with collagenase (Sigma) (1 mg/ml) for 90 min at 37° and pushed through a nylon mesh (Cadisch, London, UK.) (125-μm pore size) to separate lymphomedullary cells from the surrounding connective tissue. The remaining cells were separated by centrifugation through a five-step iso-osmolar Percoll gradient (Pharmacia, Uppsala, Sweden) at 600 g for 30 min. The high density cell populations (50–70% Percoll) were further depleted of monocytes by the removal of adherent cells, and depleted of B cells by immunomagnetic beads (anti-mouse IgG Dynabeads, Dynal, Norway) using CD19 (BU12) and MHC class II (L243) (both gifts of D. Hardie, University of Birmingham, Birmingham, UK) as primary reagents. To purify further the T cells into CD8+ and CD4+ sub-populations, either a CD4 monoclonal antibody (mAb; QS4120) or a CD8 mAb (UCHT4) (both gifts of Dr Diana Wallace, ICRF Human Tumour Immunology Unit, London, UK) was added before the immunomagnetic bead depletion step.
Monoclonal antibodies
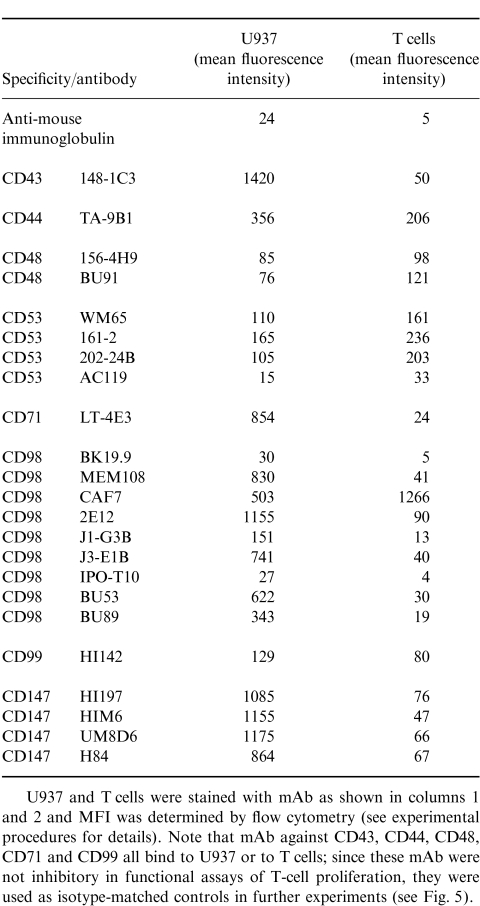
The initial mouse mAb used were from the 6th Human Leucocyte Differentiation Antigen Workshop, at the concentration suggested by the workshop organizers. In subsequent assays additional reagents were obtained and used as described. All mAb used are listed in Table 1.
Table 1.
List of monoclonal antibodies (mAb) that were used in the analysis of the U937/CD3 T-cell activation in this study
Phenotypic analysis
Both U937 and isolated T cells were phenotyped using mouse mAb (Table 1), and a (FITC) fluorescence isothiocyanate-conjugated rabbit anti-mouse IgG secondary antibody (Dako, Dakopatts, High Wycombe UK). Cells were examined by fluorescence-activated cell sorter (FACS; Coulter, Luton, UK), and the results were analysed using Hewlett Packard software.
U937/CD3 T-cell proliferation assay
Irradiated U937 cells, T cells, and CD3 mAb (Harlan-Sera Lab, Loughborough UK); were plated in flat-bottomed 96-well plates (Nunc, Paisley, UK). Mouse mAb were added in triplicate wells. After 48 hr the proliferation of the T cells was assessed by measuring uptake of radiolabelled thymidine ([3H]TdR) (ICN Biomedical, High Wycombe UK) over 16 hr. The assay was harvested on to filters and counted on a scintillation counter (Wallac, Turku, Finland).
Preincubation of U937 cells with inhibitory antibodies
To examine the role of any inhibitory mAb further, 2×105 irradiated U937 were pre-plated in a U-bottomed 96-well plate, mAb were added and the cells were incubated for 1 hr at 37°. The cells were then washed in complete media twice to remove excess mAb. Cells numbers were counted and 104 irradiated/mAb-coated U937 cells were included in the same assay system as before. As an internal control proliferation of the irradiated, mAb-coated U937 cells was assessed by tritiated thymidine incorporation.
Cell viability studies
For cell viability studies the assay was set up in the identical way as the co-stimulation assay, but in duplicate. Cells were harvested at 48 hr without isotope and viability was assessed by trypan blue dye exclusion (Gibco, Paisley, UK). Viable cells were counted on a haemocytometer. Any mAb which caused a decrease in cell number compared to the original number of cells added to each well was considered to have a potential cytotoxic effect.
RESULTS
Monoblastoid U937 cells provide a co-stimulatory signal to resting tonsillar T cells, in a U937/CD3 T-cell proliferation assay
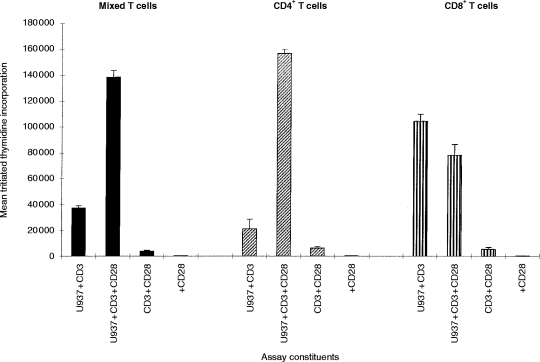
The ability of U937 cells to co-stimulate a proliferative response in resting (high-density) tonsillar T cells is shown in Fig. 1. The response was dependent on the numbers of both U937 (Fig. 1a) and T cells (Fig. 1b), although T-cell proliferation decreased at high concentrations of T cells in the assay, probably due to overcrowding in the well. T-cell proliferation was also dependent on the concentration of CD3 antibody used (data not shown). On the basis of these initial studies, further experiments were carried out using 104 U937 and 2×105 T cells/well, and a saturating concentration of 0·1 μg/ml CD3 antibody. U937 cells in the absence of CD3 antibody did not induce a response. In this study the CD3 mAb was given in soluble form, rather than bound to the culture well. Under these conditions, T-cell activation could be observed visually. There was gradual formation of large clusters of cells, containing many U937 and T cells (Fig. 1d). In the absence of CD3 mAb, clusters were also sometimes observed, but they were much smaller and included a much smaller percentage of the cells (Fig. 1c).
Figure 1.
U937 cells co-stimulate T cells in a CD3-dependent proliferation assay. (a) Increasing numbers of irradiated U937 cells were co-cultured with 2×105 purified tonsillar T cells, in the presence of 0·1 μg/ml CD3 mAb (UCHT1). (b) Increasing numbers of T cells were co-cultured with 104 irradiated U937 cells, in the presence of 0·1 μg/ml CD3 mAb (UCHT1). T-cell proliferation in both (a) and (b) was assessed by [3H]thymidine incorporation between 48 and 64 hr of culture. T cells and U937 together without CD3 mAb, and T cells with CD3 mAb but no U937 cells did not elicit a T-cell response. 5×104 irradiated U937 cells alone gave thymidine incorporation values of 2000 c.p.m. The values shown are the mean [3H]thymidine incorporation of triplicate wells±SD from one experiment. Similar results have been found in at least two other experiments. (c) and (d) Typical U937 – T-cell clusters were seen at ×40 magnification, approximately 48 hr after the start of the assay. (c) 2×105 purified tonsillar T cells, and 104 irradiated U937 cells. (d) As for (c) but with CD3 mAb (0·1 μg/ml).
CD11a, CD18 and CD54 antibodies inhibit the T-cell response in the U937/CD3 T-cell proliferation assay
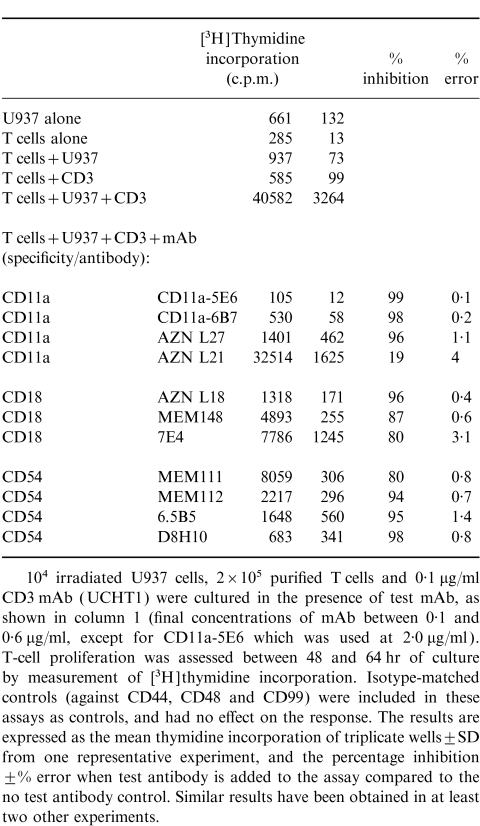
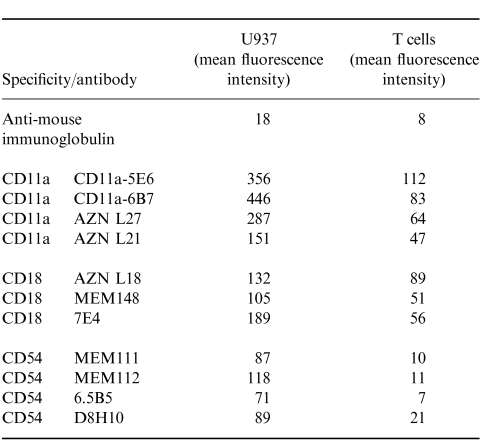
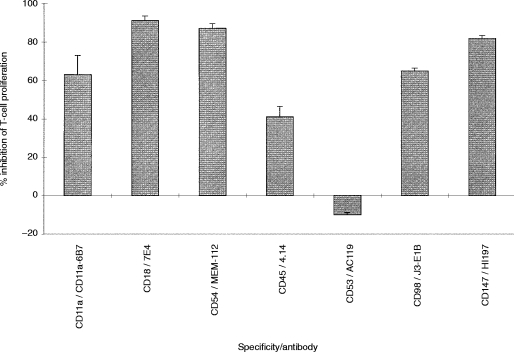
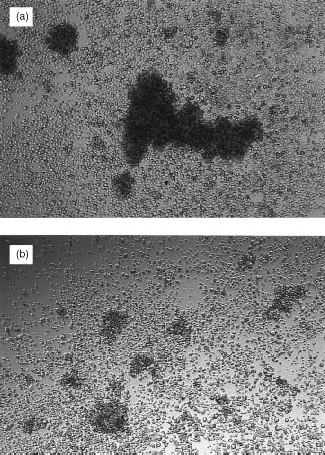
In a previous study19 antibodies to CD11a and CD18 did not inhibit U937-induced CD3-dependent T-cell activation. However, in this study (Table 1 and Table 2), a panel of antibodies to different epitopes on CD11a, CD18 and CD54 blocked U937-dependent T-cell activation. All mAb to CD11a and CD18 except one (AZN L21) blocked proliferation by 80% or more. Some antibodies to CD54 also blocked proliferation by more than 90%, suggesting that alternative CD11a CD18 ligands (e.g. ICAM-2 or 3) are not effective substitutes for CD54. In agreement with this finding, mAb to CD50 (ICAM-3) had only a very small effect on T-cell activation (data not shown). Antibodies to both CD11a and CD18 (Fig. 2, cf Fig. 1d) and CD54 (data not shown) also blocked the formation of T-cell clusters. The inhibition caused by all of the CD11a CD18 and CD54 mAb tested was dose dependent and was not due to cytotoxicity (as measured by trypan blue exclusion) (data not shown). Flow cytometry showed high levels of CD11a on U937, and much lower levels on purified T cells (Table 3). CD18 was present on both U937 and T cells, albeit at much lower concentrations. In contrast, CD54 was expressed only on U937, and not on the surface of T cells.
Table 2.
CD11a, CD18 and CD54 antibodies inhibit the T-cell response
Figure 2.
The CD18 antibody 7E4 inhibits the formation of U937 – T-cell clusters. CD18 mAb 7E4 (1/400 dilution) was added to 104 irradiated U937 cells, 2×105 purified tonsillar T cells, and 0·1 μg/ml of CD3 mAb (UCHT1) and cluster formation was assessed visually at ×40 magnification. Similar results were obtained with other CD11a, CD54 and other CD18 mAb.
Table 3.
CD11a, CD18 and CD54 antibodies inhibit the T-cell response. Mean fluorescence intensity (MFI) of U937 or T cells stained with mAb to CD11a, CD18 and CD54, analysed by flow cytometry
CD45 antibodies also inhibit T-cell responses, and cause the formation of abnormal U937/T cell clusters
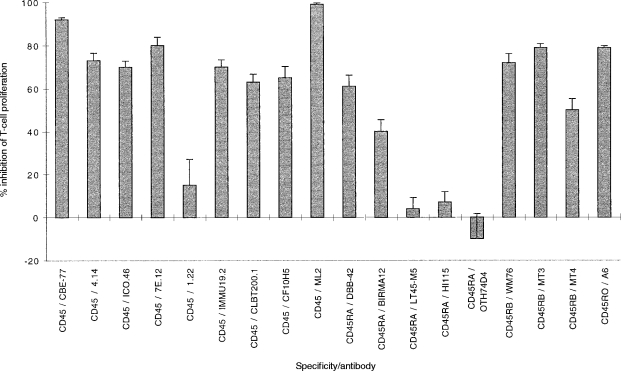
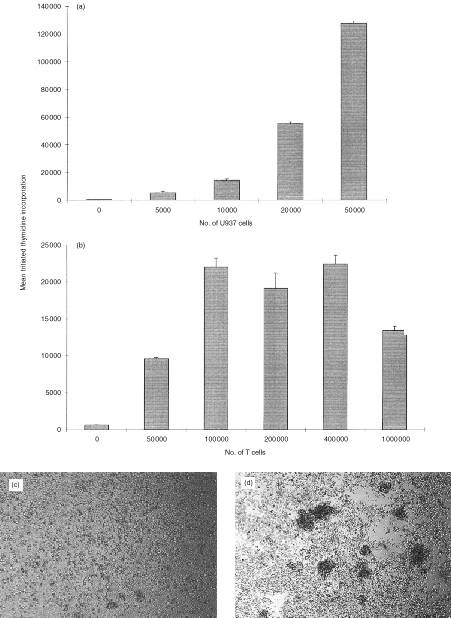
U937 cells express high levels of the leucocyte common antigen, CD45, but the function of this protein phosphatase in U937 (or indeed in other APC) is not clear. An effect of CD45 mAb on the ability of U937 cells to form clusters with T cells has been reported previously.21 Therefore a panel of CD45 mAb (Table 1) was tested for functional activity in the same assay, and the results of a representative experiment are shown in Fig. 3. Of 18 CD45 mAb, 14 inhibited T-cell proliferation: eight of nine ‘pan-CD45’ mAb, two of five CD45RA mAb, all three CD45RB mAb and one of one CD45RO mAb. In repeated experiments all these mAb were titrated into the assay to confirm that this inhibition was dose dependent, and that the lack thereof was not due to mAb concentration (data not shown). For example, the inhibitory CD45 mAb, IMMU19.2 and ML-2, and the non-inhibitory mAb 1.22 were all used at a concentration range from 0·5 μg/ml to 5 μg/ml; and likewise the inhibitory CD45RA mAb, BIRMA12 and DBB42 were effective at concentrations of 0·05 μg/ml and 0·25 μg/ml respectively, whereas the HI115 mAb had no effect even at a concentration of 10 μg/ml. All antibodies except one, CD45 (ICO46), had no cytotoxic effects on the assay. For this mAb, the per cent cell viability was 20%, suggesting that inhibition was due to killing. In expression analysis with the CD45 mAb, there was no correlation between quantitative expression [as judged by mean fluorescence intensity (MFI)] and the inhibitory capability of a particular mAb (data not shown). Paradoxically, CD45 mAb, whilst inhibiting T-cell proliferation caused the formation of larger U937–T-cell clusters which were identified readily by morphology (Fig. 4a), when compared to Fig. 1(d). The inhibitory CD45 mAb also caused an increase in U937–T-cell-clustering in the absence of CD3 mAb (Fig. 4b, cf. Fig. 1c). Although large U937–T-cell clusters were observed in wells with CD45 mAb and without CD3, no proliferation was detected in these wells (data not shown).
Figure 3.
CD45 antibodies inhibit the T-cell response. A panel of CD45 mAb were added to 104 irradiated U937 cells, 2×105 purified T cells and 0·1 μg/ml CD3 mAb (UCHT1). T-cell proliferation was assessed between 48 and 64 hr of culture by measurement of [3H]thymidine incorporation. The inhibitory response is shown as the mean percentage inhibition of proliferation in triplicate wells ±% error, when test antibody is added to the assay compared to proliferation in the absence of test antibody. Similar results have been obtained in at least two other experiments.
Figure 4.
The CD45 antibody 4.14 causes the formation of large U937–T-cell clusters. CD45 mAb 4.14 (1/400 dilution) was added to 104 irradiated U937 cells, 2×105 purified T cells and 0·1 μg/ml CD3 mAb (UCHT1) (a). The cells were cultured for approximately 48 hr and then photographed. In (b) the culture conditions were the same but no CD3 mAb was added. Other inhibitory CD45 mAb (see Fig. 3) had the same effect of increasing U937 – T-cell clustering, and this increase was independent of CD45 isotype.
Inhibition of T-cell proliferation by mAb to other cell surface molecules
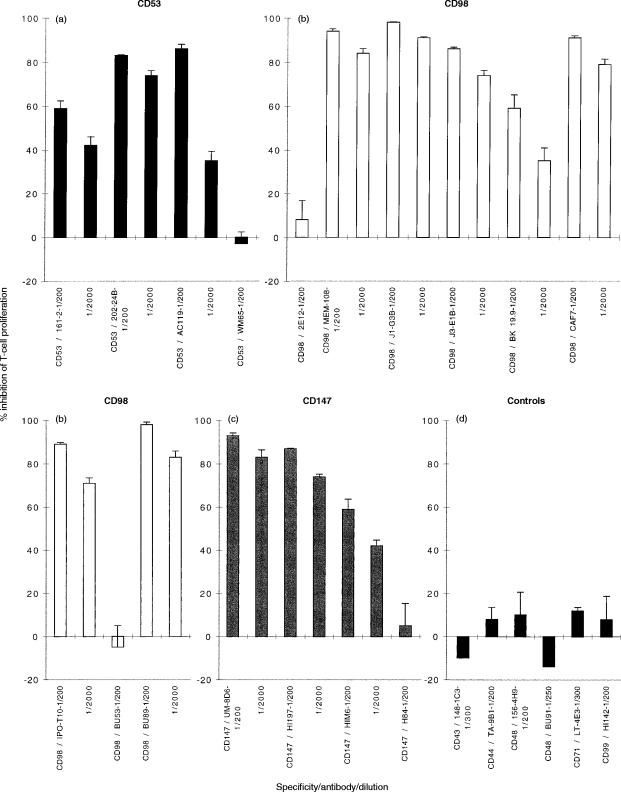
A panel of mAb to molecules that were known to be expressed on U937 cells, and had not previously been implicated in the activation of T-cell responses was tested for inhibitory activity. A preliminary screening (data not shown) identified inhibitory activity for mAb against CD53, CD98 and CD147. On the basis of this preliminary screen, a more detailed analysis of the role of the surface molecules recognised by these mAb was undertaken. Figure 5 shows the inhibitory activity of four CD53 mAbs, eight CD98 mAbs, and four CD147 mAbs, listed in table 1. The inhibitory activity was heterogeneous within each group. Two CD53 mAb (202-24B, shown at the final highest concentration used, 0·2 μg/ml; and AC119, shown at the final highest concentration of 0·25 μg/ml) were potent inhibitors (>75%) of T-cell proliferation, one mAb (161-2, shown at the final highest concentration of 0·25 μg/ml) inhibited less well (∼50%), whilst one mAb consistently failed to inhibit. Out of nine CD98 mAb tested, six [MEM-108, J1-G3B, J3-E1B, CAF7, IPO-T10 and BU89, shown at concentrations ranging from 0·025 (MEM108) to 32 (J3-E1B) μg/ml] were potent inhibitors (>75%) of T-cell proliferation; one (BK19.9, shown at a final highest concentration of 1·6 μg/ml caused moderate inhibition (∼60%), and two (2E12, highest concentration tested 1·2 μg/ml and BU53, highest concentration tested 0·8 μg/ml) had no effect. Out of four CD147 mAb tested, two (UM8D6, and HI197, both shown at final highest concentration tested of 0·9 μg/ml) were potent inhibitors (>75%) of T-cell proliferation, one (HIM6, shown at final highest concentration tested of 1.1 μg/ml) inhibited less well (∼50%), and one mAb had no effect. None of the mAb caused significant cytotoxicity as measured by trypan blue exclusion at the end of the culture period (data not shown). By comparison, a selection of other mAb of various isotype, and all sharing binding to U937 or T cells (see Table 4) did not give any inhibitory activity (Fig. 5).
Figure 5.
(a) CD53, (b) CD98 and (c) CD147 inhibit the T-cell response whereas (d) controls do not. Test mAb (final volume 1 μl mAb:200 μl) were added to cultures of 104 irradiated U937 cells, 2×105 purified T cells and 0·1 μg/ml CD3 mAb (UCHT1). Proliferation was assessed between 48 and 64 hr of culture by measurement of [3H]thymidine incorporation. The response is shown as the mean percentage inhibition of T-cell proliferation in triplicate wells ±% error, when test mAb is added to the assay compared to the proliferation in the absence of test mAb. The proliferation in the absence of test mAb was 49 876 c.p.m. Similar results have been obtained in at least two other experiments.
Table 4.
Levels of expression of cell surface antigens on both U937 and T cells
Table 3 shows the expression of these cell surface antigens, as measured by flow cytometry on both U937 and T cells. All the antigens tested were present on both T cells and U937, albeit at very different levels. Interestingly, the ratio of U937 to T-cell staining varied between different antibodies to CD98, suggesting that these antibodies may recognize CD98 isotypes, or epitopes selectively expressed by one or other cell type. In summary there was no simple relationship between quantitative levels of expression and degree of inhibition of T-cell proliferation, except for the mAb H84 (CD147) which showed the lowest levels of expression of any of the CD147 mAb.
Preincubation of U937 cells with mAb inhibits T-cell responses
To investigate whether the mAb that we had identified as inhibitors were having their effect via the co-stimulatory ability of U937 cells, or were acting via a direct effect on T cells, the U937 cells were incubated with a selection of mAb tested in Fig. 5, excess mAb was washed off, and the U937 cells were co-cultured with T cells and CD3 mAb as before. As shown in Fig. 6, the majority of the mAb tested retained their inhibitory activity when bound selectively to the U937 cells, although the percentage inhibition observed was lower than when the mAb was present continuously throughout the assay. The exception was the CD53 mAb tested, which showed no inhibitory activity when bound to U937 cells.
Figure 6.
Inhibitory activity of antibodies when bound selectively to U937 cells. Monoclonal antibodies were incubated with U937 cells for 1 hr at 37°. The mAb concentrations for these experiments were double those used for the assays outlined previously, i.e. the same protein concentration of mAb was used as before, but the dilution was 1:100 rather than 1:200. Excess mAb was removed by repeated washing, the cells were irradiated, and then 104 of U937/mAb complex were co-cultured with 2×105 purified T cells and 0·1 μg/ml CD3 mAb (UCHT1). Proliferation was assessed between 48 and 64 hr of culture by measurement of [3H]thymidine incorporation. The results are expressed as the mean percentage inhibition of T-cell proliferation in triplicate wells ±% error, when test mAb was added to the U937 cells compared to the proliferation in the absence of test mAb. Similar results have been obtained in at least two other experiments.
Inhibitory mAb inhibited both CD4+ and CD8+ T-cell-responses
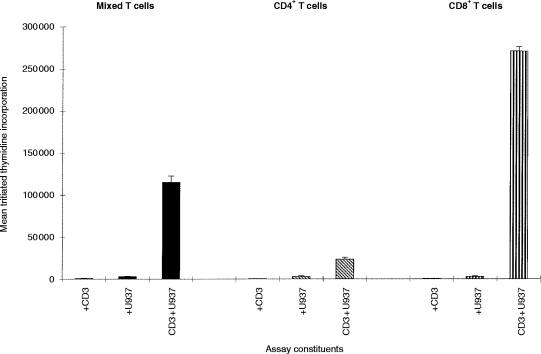
In order to test whether the inhibitory activities identified above were selective for CD4 versus CD8 T cells, tonsillar T cells were sub-purified into CD4+ and CD8+ fractions by immunodepletion. The purity of these fractions was confirmed by flow cytometry; 99% of the CD8-depleted cells were CD4+, and conversely 98% of the CD4-depleted cells were CD8+ (data not shown). As shown in Fig. 7, proliferation of the CD8+ cells was consistently greater (for an equal cell number) than that seen with either the CD4+ cells, or the mixed starting T-cells from the same tonsil. The relative increase was variable from tonsil to tonsil, with a range of between three- and 10-fold difference detectable between CD4+ and CD8+ T cells from the same individual. Although U937 cells selectively stimulated CD8+ cells in this assay, there was little difference in the capability of the mAb tested to inhibit either the CD4+ or CD8+ T-cell populations (Table 5).
Figure 7.
U937 cells co-stimulate predominantly CD8+ T cells in the CD3-dependent proliferation assay. U937cells (irradiated, 104) were in the presence of co-cultured with 2×105 T cells, purified CD4+ T cells, or purified CD8+ T cells (prepared as outlined in the Materials and Methods) and 0·1 μg/ml CD3 mAb (UCHT1). Proliferation was assessed between 48 and 64 hr of culture by [3H]thymidine incorporation between 48 and 64 hr of culture. The results are expressed as the mean percentage inhibition of T-cell proliferation in triplicate wells ±% error. Similar results have been obtained in at least two other experiments.
Table 5.
The inhibitory antibodies are equally effective with both CD4+ and CD8+ T cells
CD28 mAb stimulates proliferation of CD4+ T cells, but not of CD8+ T cells
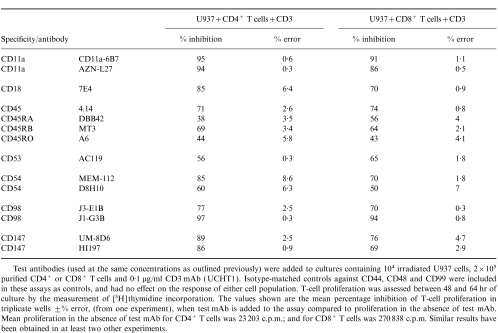
Since the molecules we had identified as being involved in T-cell activation in this model were apparently required for both CD4 and CD8 T-cell activation, we tested whether the selective failure of U937 to stimulate CD4 cells might be due to the absence of a CD28 ligand on these cells. Previous studies20 had reported that CD80 was absent on U937 cells, while CD86 was present at very low levels. We were unable to detect either CD80 or CD86 on U937 by flow cytometry (data not shown). In order to by-pass the requirement for CD80 and/or CD86, an agonistic CD28 mAb, 15E9, was added to the U937/CD3− T-cell proliferation assay (Fig. 8). The addition of CD28 mAb increased CD4+ T-cell proliferation eightfold, and increased the proliferation of unseparated T cells by 4·5-fold. However, the addition of CD28 antibody did not affect proliferation of CD8+ T cells. In the presence of CD3 mAb, CD28 mAb, or U937 cells alone, no proliferation of any T-cell subset was found. The addition of both CD3 and CD28, in the absence of U937, caused only a low level of T-cell proliferation, suggesting that CD28 signalling per se is insufficient to trigger the resting T cells in this model. The difference in sensitivity to CD28 antibody between CD4+ and CD8+ T-cell proliferation in response to CD28 antibody was not due to differences in CD28 expression, as flow cytometry demonstrated that CD28 was expressed equally on all three T-cell populations (data not shown).
Figure 8.
An agonistic CD28 antibody co-stimulates CD4+ T cells, but has no effect on CD8+ T cells. The CD28 mAb 15E9 (1/20) was added to 104 irradiated U937 cells, either 2×105 purified T cells, or 2×105 purified CD4+ or 2×105 purified CD8+ T cells, and 0·1 μg/ml CD3 mAb (UCHT1). T-cell proliferation was assessed between 48 and 64 hr of culture by measurement of [3H]thymidine incorporation. The results are expressed as the mean percentage inhibition of T-cell proliferation in triplicate wells ±% error. Similar results have been found in at least two other experiments.
DISCUSSION
The part played by accessory co-stimulatory molecules in the induction of adaptive immune responses has been explored extensively,5,11,16–18,22,23 and it has been suggested that the nature and expression pattern of these molecules may help to determine outcome. For example, engagement of MHC molecules in the absence of co-stimulation can act as a tolerizing rather than a priming event.24,25 This observation is already being exploited in the clinic, both in attempts to render tumour cells immunogenic and thus activate and harness a host anti-tumour response,26 and in attempts to interfere with auto-immune T-cell activation.27,28
Several different in vitro model systems have been used to define the role of these co-stimulatory molecules, ranging from primary cultures of APC and T cells to single epitope transfectant analysis. A previous study in our laboratory looked at the early role of clustering as a co-stimulatory event using adherent cells differentiated from the monoblastoid cell line, U937, as model APC.21 This cell line, without differentiation, has also been used in T-cell activation studies, where a CD3 antibody simulates the role of antigen.19 The conclusion of the latter study was that there are additional co-stimulatory mechanisms for T-cell activation, which have not as yet been identified.
In this study, we have examined this U937/CD3 T-cell model in further detail. Our assay used high-density tonsillar T cells rather than peripheral blood T cells, and soluble CD3 antibody rather than CD3 bound to the plastic surface, but is otherwise similar to the previous study.19 In our model CD11a CD18–CD54 (LFA-1–ICAM-1 complex) clearly did play a role, as evidenced by the inhibitory effects of several mAb (Table 2). The only exception, one CD11a mAb which was not inhibitory, AZNL21, also does not block other CD11a-mediated assay systems29 despite the fact that it does bind to CD11a transfectants. Thus this mAb may identify an epitope that is not implicated in the receptor–ligand association(s) of this molecule. A possible explanation for the discrepancy between our study and the lack of inhibitory activity seen with CD18 mAb in previous studies was that in the latter19 CD3 was immobilized on the bottom of the tissue culture well. When T cells respond to CD3 mAb orientated in such a two-dimensional fashion across the bottom of the well, adhesion molecules may not be as important as when three-dimensional clusters are formed between T cells and U937 cells with multiple contact sites. The inability of agonist mAb to CD18 to substitute for U937 cells19 does, however, suggest that activation via this ligand is not by itself sufficient. If this is correct, then adhesion between U937 and T cells may be a prerequisite for additional secondary co-stimulatory interactions to work.
Another known co-stimulatory pathway that has been explored extensively in many model systems is the APC CD80 and/or CD86 interaction with CD28 on T cells. Since U937 cells do not express either CD80 or CD86, then an agonistic CD28 mAb should synergize with a CD3 mAb, and this was demonstrated in our study. This suggests that study of co-stimulation in the absence of the CD80 and CD86–CD28 surface molecule combination may be necessary to reveal some of the more subtle interactions which we have identified here. Furthermore, it is interesting that the CD28 pathway did not function equally for all T cells. The predominant response stimulated by U937 cells is of CD8+ rather than of CD4+ T cells, consistent with the expression of class I but not class II MHC on the U937 surface; when CD28 was added then the reaction induced was in the CD4+ component, implying that there are differential co-stimulation as well as MHC requirements for the two forms of T cell.30,31
A major aim of the present study was to identify which novel co-stimulatory molecules were implicated in U937-dependent T-cell activation using mAb-mediated inhibition as a starting point. By direct observation, one of the most striking effects seen was with a well-established cell surface marker, CD45 and its isoforms. CD45 mAb caused inhibition of proliferation but enhanced clustering, for both CD4+ and CD8+ T cells. CD45-mediated inhibition of CD3–TCR complex activation has been documented previously,32 and it has been suggested that CD45 ligation blocks the intracellular calcium flux normally associated with TCR signalling.33 Interfering with CD45 also blocks the dephosphorylation of the regulatory C-terminal domains of the CD4 and CD8-associated p56lck tyrosine kinase34,35 and/or of the TCR-associated p59fyn tyrosine kinase,35,36 and hence inhibit the classic intracellular cascade in the T cell. In the U937/CD3 T-cell model, however, CD45 activity is mediated, at least in part, by U937, since CD45 mAb selectively pre-bound to U937 before addition of T cells also enhanced clustering and inhibited proliferation (Fig. 6). The effect of CD45 on clustering, which (as in our previous study) was independent of the presence of CD3, and was therefore not linked to TCR signal transduction, implies that CD45 is involved in the adhesion arm of co-stimulation, perhaps through amplification of the CD11a/CD18–CD54 pathway.
An inhibitory effect on T-cell proliferation was seen with three of the four CD53 mAb (Fig. 5). This molecule, a member of the tetraspans superfamily of surface molecules, is known to be induced on T cells by TCR engagement during repertoire selection,37 to trigger nitric oxide release via a protein kinase C-dependent pathway;38 and to be associated with a tyrosine phosphatase activity.39 In human tonsillar B cells CD53 can form part of a multicomponent class II MHC complex.40 In the present study CD53 mAb-mediated inhibition was not seen in pre-pulsing experiments (Fig. 6), implying that the CD53 role is likely to be mediated at the T-cell level rather than via U937.
Two molecules newly identified here as being implicated in co-stimulation of both CD4+ and CD8+ T cells in this model were CD98 and CD147 (Figs 5 and 6). CD98 is a type II transmembrane glycoprotein which is important in cell survival during haemopoietic cell development,41 in the formation of human immunodeficiency virus-induced multinucleate giant cells,42,43 and has been shown to mediate a calcium influx when bound by Galectin 3.44 Although CD98 co-precipitates with CD3 in extracts derived from a human thymoma cell line,45 which would suggest a role at the T-cell level, pre-pulsing and T-cell sub-population experiments suggest that the effect is via the APC (Fig. 6). Further evidence that CD98 has a co-stimulatory role is that it is expressed at much higher levels on U937 and on dendritic cells46 than on resting T cells, and that it also may affect T-cell activation by these cells (Woodhead et al., submitted for publication). Detailed phenotypic analysis showed that one CD98 mAb stained T cells more than U937 cells, and there were also different patterns of expression between U937 and dendritic cells with the same CD98 mAb, therefore raising the possibility that different isoforms of CD98 exist. These results are consistent with the recently reported role for CD98 as a controlling mechanism for integrin-mediated adhesion,47 and in co-stimulation in xenoactivation.48
CD147, also known as neurothelin or basigin, is a member of the immunoglobulin gene superfamily49 which was first identified because of its role in the development and maintenance of the blood–brain barrier50 and which is known to be involved in actin co-localization and polarization of endothelial cells.51 CD147, is however, widely distributed, including expression on mitogen-activated T cells,52 and CD147 knockout mice have an increased mitogenic response in mixed leucocyte reactions.53 As for CD98, pre-pulsing and T-cell subset experiments suggest that CD147 on the U937 rather than on the T cell is important in co-stimulation.
It is not as yet clear from these studies how the inhibitory effects of the antibodies on U937 function are mediated: whether by blocking signalling into the APC, and hence production of a key mediator; or by altering a critical pattern of surface expression of known co-stimulatory molecules; or by blocking direct receptor–ligand interaction with a molecule on the T-cell surface.
CD98 and CD147 are both molecules that would be present at sites of inflammation: CD98 as an activation antigen, and CD147 at sites of endothelial outgrowth. Their contribution may therefore be to play a parallel role in the induction of innate and adaptive immunity. These questions are currently being investigated further in our laboratories.
In conclusion, therefore, we have used the U937/CD3 T-cell proliferation model, and have shown that classic accessory molecules (CD11a, CD18, CD54 and CD45) are important for co-stimulation; and that novel molecules, in particular CD98 and CD147, are also implicated in this process, most probably acting at the level of the APC rather than the T cell. Delivery of either of these molecules to tumours may be a way to enhance their immunogenicity, and hence potentiate immunotherapeutic intervention in cancer. Conversely, the ability to block these alternative co-stimulatory pathways may provide another strategy to modulate the pathological immune reactions associated with autoimmunity.
Acknowledgments
This work was supported by a UK MRC PhD studentship (T.J.S.), a grant from the Sir Jules Thorn Charitable Trust (V.E.W.), a Shanks fellowship (P.S.H.), a Wellcome Trust vacation studentship (H.A.) and a grant from the UK Multiple Sclerosis Society (M.G.). The kind gift of monoclonal antibodies from Drs A.J. van Agthoven, G.Aversa, D. A. Fox, D. L. Hardie, A. J. Henniker, V. Horejsi, W. Knapp, Y. van Kooyk, K. Sagawa, D. C. Shen, K. M. Skubitz, D. C. Shen, H. Taskov, R. Vilella, and the organizers of the relevant mAb panels for the 6th HLDA is gratefully acknowledged.
REFERENCES
- 1.Schwartz RH. Costimulation of T lymphocytes: the role of CD28, CTLA-4, and B7/BB1 in interleukin-2 production and immunotherapy. Cell. 1992;71:1065. doi: 10.1016/s0092-8674(05)80055-8. [DOI] [PubMed] [Google Scholar]
- 2.Schwartz RH. T-lymphocyte recognition of antigen in association with gene products of the major histocompatibility complex. Annu Rev Immunol. 1985;3:237. doi: 10.1146/annurev.iy.03.040185.001321. [DOI] [PubMed] [Google Scholar]
- 3.Barber EK, Dasgupta JD, Schlossman SF, Trevillyan JM, Rudd CE. The CD4 and CD8 antigens are coupled to a protein-tyrosine kinase (p56lck) that phosphorylates the CD3 complex. Proc Natl Acad Sci USA. 1989;86:3277. doi: 10.1073/pnas.86.9.3277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weiss A, Iwashima M, Irving B, et al. Molecular and genetic insights into T cell antigen receptor signal transduction. Adv Exp Med Biol. 1994;365:53. doi: 10.1007/978-1-4899-0987-9_6. [DOI] [PubMed] [Google Scholar]
- 5.Jenkins MK, Johnson JG. Molecules involved in T-cell costimulation. Curr Opin Immunol. 1993;5:361. doi: 10.1016/0952-7915(93)90054-v. [DOI] [PubMed] [Google Scholar]
- 6.Linsley PS, Brady W, Grosmaire L, Aruffo A, Damle NK, Ledbetter JA. Binding of the B cell activation antigen B7 to CD28 costimulates T cell proliferation and interleukin 2 mRNA accumulation. J Exp Med. 1991;173:721. doi: 10.1084/jem.173.3.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thomas R, Davis LS, Lipsky PE. Comparative accessory cell function of human peripheral blood dendritic cells and monocytes. J Immunol. 1993;151:6840. [PubMed] [Google Scholar]
- 8.Byrne JA, Butler JL, Cooper MD. Differential activation requirements for virgin and memory T cells. J Immunol. 1988;141:3249. [PubMed] [Google Scholar]
- 9.Linsley PS, Brady W, Urnes M, Grosmaire LS, Damle NK, Ledbetter, JA CTLA-4 is a second receptor for the B cell activation antigen B7. J Exp Med. 1991;174:561. doi: 10.1084/jem.174.3.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Norton SD, Zuckerman L, Urdahl KB, Shefner R, Miller J, Jenkins MK. The CD28 ligand, B7, enhances IL-2 production by providing a costimulatory signal to T cells. J Immunol. 1992;149:1556. [PubMed] [Google Scholar]
- 11.Moudgil KD, Sercarz EE. Dominant determinants in hen eggwhite lysozyme correspond to the cryptic determinants within its self-homologue, mouse lysozyme: implications in shaping of the T cell repertoire and autoimmunity. J Exp Med. 1993;178:2131. doi: 10.1084/jem.178.6.2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brown GR, Meek K, Nishioka Y, Thiele DL. CD27-CD27 ligand/CD70 interactions enhance alloantigen-induced proliferation and cytolytic activity in CD8+ T lymphocytes. J Immunol. 1995;154:3686. [PubMed] [Google Scholar]
- 13.Hintzen RQ, Lens SM, Lammers K, Kuiper H, Beckmann MP, van Lier RA. Engagement of CD27 with its ligand CD70 provides a second signal for T cell activation. J Immunol. 1995;154:2612. [PubMed] [Google Scholar]
- 14.Liversidge J, Dawson R, Hoey S, McKay D, Grabowski P, Forrester JV. CD59 and CD48 expressed by rat retinal pigment epithelial cells are major ligands for the CD2-mediated alternative pathway of T cell activation. J Immunol. 1996;156:3696. [PubMed] [Google Scholar]
- 15.Menu E, Tsai BC, Bothwell AL, Sims PJ, Bierer BE. CD59 costimulation of T cell activation. CD58 dependence and requirement for glycosylation. J Immunol. 1994;153:2444. [PubMed] [Google Scholar]
- 16.van der Merwe PA, Barclay AN, Mason DW, et al. Human cell-adhesion molecule CD2 binds CD58 (LFA-3) with a very low affinity and an extremely fast dissociation rate but does not bind CD48 or CD59. Biochemistry. 1994;33:10149. doi: 10.1021/bi00199a043. [DOI] [PubMed] [Google Scholar]
- 17.Damle NK, Klussmann K, Aruffo A. Intercellular adhesion molecule-2, a second counter-receptor for CD11a/CD18 (leukocyte function-associated antigen-1), provides a costimulatory signal for T-cell receptor-initiated activation of human T cells. J Immunol. 1992;148:665. [PubMed] [Google Scholar]
- 18.van Seventer GA, Shimizu Y, Horgan KJ, Shaw S. The LFA-1 ligand ICAM-1 provides an important costimulatory signal for T cell receptor-mediated activation of resting T cells. J Immunol. 1990;144:4579. [PubMed] [Google Scholar]
- 19.Johnson JG, Jenkins MK. Monocytes provide a novel costimulatory signal to T cells that is not mediated by the CD28/B7 interaction. J Immunol. 1994;152:429. [PubMed] [Google Scholar]
- 20.Palmer EM, van Seventer GA. Human T helper cell differentiation is regulated by the combined action of cytokines and accessory cell-dependent costimulatory signals. J Immunol. 1997;158:2654. [PubMed] [Google Scholar]
- 21.King PD, Batchelor AH, Lawlor P, Katz DR. The role of CD44, CD45, CD45RO, CD46 and CD55 as potential anti-adhesion molecules involved in the binding of human tonsillar T cells to phorbol 12-myristate 13-acetate-differentiated U937 cells. Eur J Immunol. 1990;20:363. doi: 10.1002/eji.1830200220. [DOI] [PubMed] [Google Scholar]
- 22.Johnson JG, Jenkins MK. Accessory cell-derived signals required for T cell activation. Immunol Res. 1993;12:48. doi: 10.1007/BF02918368. [DOI] [PubMed] [Google Scholar]
- 23.Mondino A, Jenkins MK. Surface proteins involved in T cell costimulation. J Leukoc Biol. 1994;55:805. doi: 10.1002/jlb.55.6.805. [DOI] [PubMed] [Google Scholar]
- 24.Boussiotis VA, Freeman GJ, Gribben JG, Nadler LM. The critical role of CD28 signalling in the prevention of human T-cell anergy. Res Immunol. 1995;146:140. doi: 10.1016/0923-2494(96)80247-1. [DOI] [PubMed] [Google Scholar]
- 25.Boussiotis VA, Barber DL, Lee BJ, Gribben JG, Freeman GJ, Nadler LM. Differential association of protein tyrosine kinases with the T cell receptor is linked to the induction of anergy and its prevention by B7 family-mediated costimulation. J Exp Med. 1996;184:365. doi: 10.1084/jem.184.2.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen L. Manipulation of T cell response to tumors by targeting on costimulatory pathway. Leukemia. 1997;3:567. [PubMed] [Google Scholar]
- 27.Boussiotis VA, Gribben JG, Freeman GJ, Nadler LM. Blockade of the CD28 co-stimulatory pathway: a means to induce tolerance. Curr Opin Immunol. 1994;6:797. doi: 10.1016/0952-7915(94)90087-6. [DOI] [PubMed] [Google Scholar]
- 28.Guinan EC, Gribben JG, Boussiotis VA, Freeman GJ, Nadler LM. Pivotal role of the B7:CD28 pathway in transplantation tolerance and tumor immunity. Blood. 1994;84:3261. [PubMed] [Google Scholar]
- 29.Hogg N. In Leucocyte Typing VI. New York and London: Garland Publishing, Inc.; 1997. CD11a Workshop Panel report; p. 343. [Google Scholar]
- 30.Costello R, Cerdan C, Pavon C, et al. The CD2 and CD28 adhesion molecules induce long term autocrine proliferation of CD4+ T cells. Eur J Immunol. 1993;23:608. doi: 10.1002/eji.1830230304. [DOI] [PubMed] [Google Scholar]
- 31.Ghiotto-Ragueneau M, Battifora M, Truneh A, Waterfield MD, Olive D. Comparison of CD28-B7.1 and B7.2 functional interaction in resting human T cells: phophatidylinositol 3-kinase association to CD28 and cytokine production. Eur J Immunol. 1996;26:34. doi: 10.1002/eji.1830260106. [DOI] [PubMed] [Google Scholar]
- 32.Kiener PA, Mittler RS. CD45-protein tyrosine phosphatase cross-linking inhibits T cell receptor CD3-mediated activation in human T cells. J Immunol. 1989;143:23. [PubMed] [Google Scholar]
- 33.Shivnan E, Clayton L, Allridge L, Keating KE, Gullberg M, Alexander DR. CD45 monoclonal antibodies inhibit TCR-mediated calcium signals, calmodulin-kinase IV/Gr activation, and oncoprotein 18 phosphorylation. J Immunol. 1996;157:101. [PubMed] [Google Scholar]
- 34.Guttinger M, Gassmann M, Amrein KE, Burn P. CD45 phosphotyrosine phosphatase and p56lck protein tyrosine kinase: a functional complex crucial in T cell signal transduction. Int Immunol. 1992;4:1325. doi: 10.1093/intimm/4.11.1325. [DOI] [PubMed] [Google Scholar]
- 35.Ledbetter JA, Deans JP, Aruffo A, et al. CD4, CD8 and the role of CD45 in T-cell activation. Curr Opin Immunol. 1993;5:334. doi: 10.1016/0952-7915(93)90050-3. [DOI] [PubMed] [Google Scholar]
- 36.Shiroo M, Goff L, Biffen M, Shivnan E, Alexander D. CD45 tyrosine phosphatase-activated p59fyn couples the T cell antigen receptor to pathways of diacylglycerol production, protein kinase C activation and calcium influx. EMBO J. 1992;11:4887. doi: 10.1002/j.1460-2075.1992.tb05595.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pieters RH, Bol M, Ariens T, et al. Selective inhibition of immature CD4−CD8+ thymocyte proliferation, but not differentiation, by the thymus atrophy-inducing compound di-n-butyltin dichloride. Immunology. 1994;81:261. [PMC free article] [PubMed] [Google Scholar]
- 38.Bosca L, Lazo PA. Induction of nitric oxide release by MRC OX-44 (anti-CD53) through a protein kinase C-dependent pathway in rat macrophages. J Exp Med. 1994;179:1119. doi: 10.1084/jem.179.4.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Carmo AM, Wright MD. Association of the transmembrane 4 superfamily molecule CD53 with a tyrosine phosphatase activity. Eur J Immunol. 1995;25:2090. doi: 10.1002/eji.1830250743. [DOI] [PubMed] [Google Scholar]
- 40.Angelisova P, Hilgert I, Horejsi V. Association of four antigens of the tetraspans family (CD37, CD53, TAPA-1, and R2/C33) with MHC class II glycoproteins. Immunogenetics. 1994;39:249. doi: 10.1007/BF00188787. [DOI] [PubMed] [Google Scholar]
- 41.Warren AP, Patel K, McConkey DJ, Palacios R. CD98: a type II transmembrane glycoprotein expressed from the beginning of primitive and definitive hematopoiesis may play a critical role in the development of hematopoietic cells. Blood. 1996;87:3676. [PubMed] [Google Scholar]
- 42.Ohgimoto S, Tabata N, Suga S, et al. Molecular characterization of fusion regulatory protein-1 (FRP-1) that induces multinucleated giant cell formation of monocytes and HIV gp160-mediated cell fusion. FRP-1 and 4F2/CD98 are identical molecules. J Immunol. 1995;155:3585. [PubMed] [Google Scholar]
- 43.Suga S, Tsurudome M, Ito M, et al. Human immunodeficiency virus type-1 envelope glycoprotein gp120 induces expression of fusion regulatory protein (FRP)-1/CD98 on CD4+ T cells: a possible regulatory mechanism of HIV-induced syncytium formation. Med Microbiol Immunol Berlin. 1997;185:237. doi: 10.1007/s004300050036. [DOI] [PubMed] [Google Scholar]
- 44.Dong S, Hughes RC. Galectin-3 stimulates uptake of extracellular Ca2+ in human Jurkat T-cells. FEBS Lett. 1996;395:165. doi: 10.1016/0014-5793(96)01031-9. [DOI] [PubMed] [Google Scholar]
- 45.Cerny J, Stockinger H, Horejsi V. Noncovalent associations of T lymphocyte surface proteins. Eur J Immunol. 1996;26:2335. doi: 10.1002/eji.1830261010. [DOI] [PubMed] [Google Scholar]
- 46.Woodhead VE, Binks MH, Chain BM, Katz DR. From sentinel to messenger: an extended phenotypic analysis of the monocyte to dendritic cell transition. Immunology. 1998;94:552. doi: 10.1046/j.1365-2567.1998.00547.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fenczik CA, Sethi T, Ramos JW, Hughes PE, Ginsberg MH. Complementation of dominant suppression implicates CD98 in integrin activation. Nature. 1997;390:81. doi: 10.1038/36349. [DOI] [PubMed] [Google Scholar]
- 48.Diaz LA, Friedman AW, He X, Kuick RD, Hanash SM, Fox DA. Monocyte-dependent regulation of T lymphocyte activation through CD98. Int Immunol. 1997;9:1221. doi: 10.1093/intimm/9.9.1221. [DOI] [PubMed] [Google Scholar]
- 49.Seulberger H, Unger CM, Risau W. HT7, Neurothelin, Basigin, gp42 and OX-47 – many names for one developmentally regulated immunoglobulin-like surface glycoprotein on blood-brain barrier endothelium, epithelial tissue barriers and neurons. Neurosci Lett. 1992;140:93. doi: 10.1016/0304-3940(92)90690-9. [DOI] [PubMed] [Google Scholar]
- 50.Schlosshauer B. Neurothelin: molecular characteristics and developmental regulation in the chick CNS. Development. 1991;113:129. doi: 10.1242/dev.113.1.129. [DOI] [PubMed] [Google Scholar]
- 51.Schlosshauer B, Bauch H, Frank R. Neurothelin: amino acid sequence, cell surface dynamics and actin colocalization. Eur J Cell Biol. 1995;68:159. [PubMed] [Google Scholar]
- 52.Kasinrerk W, Fiebiger E, Stefanova I, Baumruker T, Knapp W, Stockinger H. Human leukocyte activation antigen M6, a member of the Ig superfamily, is the species homologue of rat OX-47, mouse basigin, and chicken HT7 molecule. J Immunol. 1992;149:847. [PubMed] [Google Scholar]
- 53.Igakura T, Kadomatsu K, Taguchi O, et al. Roles of basigin, a member of the immunoglobulin superfamily, in behavior as to an irritating odor, lymphocyte response, and blood-brain barrier. Biochem Biophys Res Commun. 1996;224:33. doi: 10.1006/bbrc.1996.0980. [DOI] [PubMed] [Google Scholar]