Abstract
Rhodococcus equi is a facultative intracellular bacterial pathogen that causes pneumonia in foals and immunosuppressed humans. There are at least three virulence levels of R. equi and these pathogenicities are associated, in mice, with the presence of virulence plasmids. This study focused on cytokine secretion, in mice, in the course of a primary infection with sublethal doses of R. equi strains of different virulence levels (virulent, intermediately virulent and avirulent). Tumour necrosis factor (TNF) and interferon-γ (IFN-γ), but not interleukin-4 (IL-4) and interleukin-10 (IL-10), were induced endogenously in mice in relation to the multiplication and clearance of virulent and intermediately virulent strains of R. equi. These cytokines were not detected in mice infected with avirulent R. equi. Deaths occurred among mice treated with monoclonal antibodies (mAbs) against either TNF or IFN-γ prior to sublethal dose infection with virulent and intermediately virulent strains of R. equi, but not with avirulent R. equi. These results suggested that cytokine production depended largely on the virulence levels of R. equi: TNF and IFN-γ were required early during infection with virulent R. equi to limit replication and clearance of bacteria within the organs, but they were not necessary for limiting infection with avirulent R. equi.
INTRODUCTION
Rhodococcus equi is a facultative, intracellular, Gram-positive coccobacillus, which causes suppurative pneumonia and ulcerative enteritis in foals aged 1–3 months.1 Recently, R. equi has been recognized as an emerging opportunistic pathogen of immunocompromised hosts, such as human immunodeficiency virus (HIV)-infected patients, and the number of R. equi pneumonia case reports in patients with acquired immune deficiency syndrome (AIDS) has increased.2–4
The discovery of virulence-associated antigens and virulence plasmids has clarified some aspects of the virulence of R. equi.5–9 At least three virulence levels of R. equi have been identified: virulent R. equi, which has 15 000 to 17 000 MW antigens and a virulence plasmid of 85–90 kb, and causes suppurative pneumonia in foals (mouse LD50 = 106); R. equi strains of intermediate virulence, which have a 20 000 MW antigen and a virulence plasmid of 79–100 kb, and reside in the submaxillary lymph nodes of pigs (mouse LD50 =107); and avirulent R. equi, which has no virulence-associated antigens or plasmid (mouse LD50>108).10,11 Epidemiological studies have revealed that these virulent R. equi live separately in horses and pigs, and that avirulent R. equi is distributed widely in our environment.12 In humans, the majority of isolates from patients with AIDS were either virulent or of intermediate virulence,9 and most isolates from immunocompromised patients without AIDS were avirulent.13
Studies on R. equi infection in foal and mouse models have contributed some understanding on the aspects of pathogenesis and immunology.1,4,14,15 However, the relative contributions of cell-mediated immunity and humoral immunity to resistance against R. equi infections remain unclear and paradoxical.1,4,14 Passive transfer of hyperimmune equine plasma was demonstrated to have a protective effect in foals and to decrease the incidence and severity of R. equi pneumonia on farms where the infection was endemic;16,17 however, vaccination of mares and foals with R. equi virulence-associated protein did not protect foals.18,19 On the other hand, cell-mediated immunity has been shown to be crucial in host defence against R. equi in mouse models.14,15 Live virulent R. equi, rather than killed or avirulent organisms, elicited protective immunity to R. equi infection in mice, and studies using monoclonal antibodies (mAbs) and transgenic knockout mice indicated that CD4+ T cells participated in the clearance of R. equi.20–24
The virulence mechanism in R. equi is still unknown, but at least two important virulence determinants are involved in the pathogenesis of the disease: one is the virulence plasmid, which is required for R. equi to grow within host cells; and the other is the granulomagenic activity that is related to the lipids and nature of the cell wall of the species, which induces the characteristic pathological changes.25,26 The immune mechanism that mediates resistance during primary infection with R. equi strains, which have three different virulence levels, also remains unresolved.12 To determine which components of the immune system confer protection to primary R. equi infection in mice, we evaluated the host immune response to infection with various strains of R. equi in one strain of mice. In the present study, the role of cytokines in the development of infection in mice infected with three different virulence types of R. equi was examined. Our results showed that cytokine production depended largely on the virulence types of R. equi.
MATERIALS AND METHODS
Experimental animals
Female ddY mice were obtained from S. L. C. Hamamatsu (Shizuoka, Japan) at 5 weeks of age and were housed under standard conditions.
Preparation of bacteria
R. equi ATCC 33701 (virulent), its plasmid-cured derivative, ATCC 33701P− (avirulent) and A5 (intermediately virulent) were used. Virulence of the strains has been reported elsewhere.6,9 The 50% lethal doses (LD50) of ATCC 33701, ATCC 33701P− and A5 were 2·6×106, >108 and 1·0×107, respectively. The strains were grown in Brain–Heart Infusion broth (BHI; Difco Laboratories, Detroit, MI). Cultures of these strains were incubated in a rotary shaker at 100 r.p.m. for 48 hr at 30°, and the cultures were stored as suspensions of cells in 20% glycerol at −80°. Aliquots (1 ml) were periodically thawed, and viable colony-forming units (CFU) were quantified by plating serial dilutions on nutrient agar plates. For inoculation of mice, bacterial cultures were thawed and washed with 0·01 m phosphate-buffered saline (PBS; pH 7·4) immediately before use and then diluted to a predetermined number in PBS; actual numbers of bacteria inoculated were confirmed by plate counts at the time of injection.
Determination of viable R. equi bacteria in the organs
The number of bacteria in the liver, spleen and lungs were estimated at various time intervals following intravenous inoculation. Mice were killed by cervical dislocation and their organs were removed aseptically and homogenized in 2 ml of sterile PBS, as described previously.26 Results were expressed as mean CFU±standard error (SE) per gram of organ for each group of three mice, and transformed by log10.
Preparation of organs for cytokine assays
The liver, spleen and lung homogenates used for interferon-γ (IFN-γ), tumour necrosis factor (TNF), interleukin (IL)-4 and IL-10 assays were prepared as described previously.27,28 Half of each organ, aseptically removed from mice as described previously, was suspended in RPMI-1640 medium (Gibco Laboratories, Grand Island, NY) containing 1% (wt/vol) 3 (cholamidopropyl) dimethylammonio-)-1-propanesulfonate (CHAPS; Wako Pure Chemical Co., Kyoto, Japan) and 10% of the homogenates were ground up using a Dounce grinder. After the homogenates were clarified by centrifugation at 2000 g for 20 min at 4°, the organ extracts were stored at −80° until required for the cytokine assay.
TNF assay
TNF was quantified by a double-sandwich enzyme-linked immunosorbent assay (ELISA), as described previously.27 Flat-bottom 96-well plate wells were coated with 200 ng of hamster antirecombinant mouse (rMu) TNF mAb (Genzyme Co., Boston, MA) in 0·1 m carbonate buffer (pH 9·6). Following washing three times with PBS containing 0·05% Tween 20, the plates were blocked with 0·1 m carbonate buffer containing 0·1% bovine serum albumin (BSA; Sigma Chemical Co., St. Louis, MO) and 2% goat serum (Vector Laboratories, Burlingame, CA). The wells were incubated with 100 μl of a sample, in duplicate, at 4° and then exposed to 5 μg rabbit polyclonal antirMuTNF immunoglobulin G (IgG) in PBS containing 0·1% BSA and 2% goat serum. The plate was developed by using peroxidase-labelled goat antirabbit IgG (Jackson Immunoresearch Laboratories, Inc., Avondale, PA) and o-phenylendiamine (Wako) substrate. Selected amounts of rMuTNF-α (Genzyme), diluted in 100 μl of PBS containing 0·1% BSA and 2% goat serum, were used to create a standard curve to accompany each experiment. The assay was determined to detect TNF concentrations as low as 25 pg/ml.
IFN-γ assay
The IFN-γ assay was carried out by using a double-sandwich ELISA, as described previously.27 Purified rat antimouse IFN-γ mAb, produced by hybridoma R4-6 A2 and rabbit antirMu IFN-γ serum, was used for the ELISA. All ELISAs were run with rMu IFN-γ, which was produced and purified by Genetech, Inc. (San Francisco, CA).
IL-4 and IL-10 assays
IL-4 and IL-10 levels were determined using ELISAs, as described previously.28 Flat-bottom 96-well plates were coated with 200 ng rat antimouse-IL-4 mAb (11B11; PharMingen, San Diego, CA) or rat antimouse-IL-10 mAb (JES5-2 A5; PharMingen) in 0·05 m carbonate buffer (pH 9·6). The plates were blocked with PBS containing 2·5% BSA. The wells were then incubated with 100 μl of a sample, in duplicate, overnight at 4° and were then exposed to 50 ng of biotinylated rat anti-IL-4 mAb (BVD6-24G2; PharMingen) or biotinylated rat anti-IL-10 mAb (SXC-1; PharMingen) in PBS containing 1% BSA. The plate was developed with avidin-biotinylated peroxidase complex (Vector Laboratories) and o-phenylendiamine (Wako) substrate. Selected amounts of rMuIL-4 (Genzyme) or rMuIL-10 (Genzyme), diluted in 100 μl of PBS containing 1% BSA, were used to create a standard curve to accompany each experiment. The assay was sensitive enough to detect concentrations of IL-4 and IL-10 below 15·7 pg/ml and 78·13 pg/ml, respectively.
In vivo depletion of endogenous IFN-γ and TNF
Hybridoma cells secreting mAbs against mouse IFN-γ (R4-6 A2; rat IgG1) and mouse TNF (MP6-XT22·11; rabbit IgG1) were used to study in vivo depletion of endogenous IFN-γ and TNF. mAbs found in the ascites fluid were partially purified by (NH4)SO4 precipitation. The mice were given a single intravenous injection of 1 mg rat antimouse IFN-γ mAb or rat antimouse TNF mAb (in 0·2 ml of pyrogen-free saline) 30 min before infection with R. equi, as described previously.27,28 Normal rat globulin was injected as a control.
RESULTS
Kinetics of bacterial growth in mice infected with sublethal doses of R. equi
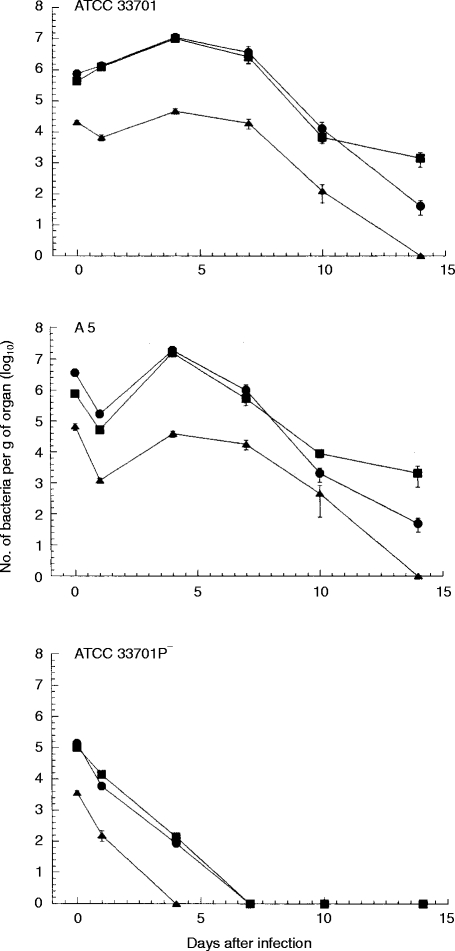
After the mice were infected intravenously with 4·0×105 of ATCC 33701 (virulent), 1·0×106 of A5 (intermediately virulent) or 3·3×105 of ATCC 33701P−(avirulent), the courses of infection were compared in the liver, spleen and lungs. Two distinct patterns emerged (Fig. 1). Strains ATCC 33701 and A5 demonstrated viable counts in the liver and spleen that decreased slightly 1 day after infection, followed by five to 10-fold increases over the next 3 days. Thereafter, there was a slow decline in viable counts. In the lungs, the viable counts decreased more rapidly, and without multiplication, than they did in the liver and spleen. By contrast, there was no apparent growth of bacteria in the liver, spleen and lungs of mice infected with ATCC 33701P−.
Figure 1.
The course of R. equi infection in mice infected intravenously with a sublethal dose of virulent strain ATCC 33701, intermediate virulent strain A5 or avirulent strain ATCC 33701P− was followed against time in the spleen (closed circle), liver (closed square) and lungs (closed triangle). Data shown are the mean number of bacterial counts±SEM (n = 3).
IFN-γ, TNF, IL-4 and IL-10 production in mice infected with sublethal doses of R. equi
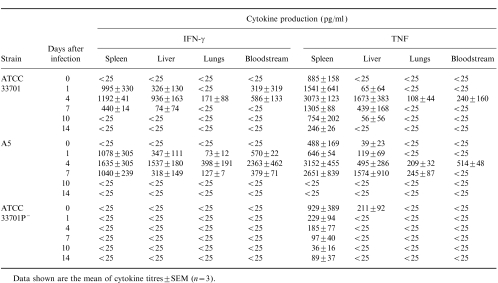
The titres of IFN-γ, TNF, IL-4 and IL-10 from pooled sera and extracts from the spleens, livers and lungs of mice were monitored at various time-points after infection with the three different strains of R. equi. The titres of IFN-γ and TNF in the spleens and livers of mice infected with virulent R. equi increased to day 4 after infection, in accordance with the number of bacteria in those organs, and then decreased (Table 1). IFN-γ and TNF were detected also in both the bloodstream and lungs, but with titres lower than those of the liver and spleen (Table 1). By contrast, minimal levels of IFN-γ and TNF were detected in the bloodstream and lungs of the mice infected with avirulent R. equi. In the spleens and livers, TNF appeared several hours after infection and the titre decreased gradually thereafter.
Table 1.
Kinetics of production of interferon-γ (IFN-γ) and tumour necrosis factor (TNF) in the spleen, liver, lungs and bloodstream of mice infected intravenously with a sublethal dose of virulent strain ATCC 33701, intermediate virulent strain A5 or avirulent strain ATCC 33701P−
Neither IL-4 nor IL-10 were detected in the mice infected with any strain of R. equi used in this study (data not shown).
Effect of in vivo administration of mAbs against IFN-γ and TNF in mice infected with a sublethal dose of R. equi ATCC 33701 or A5
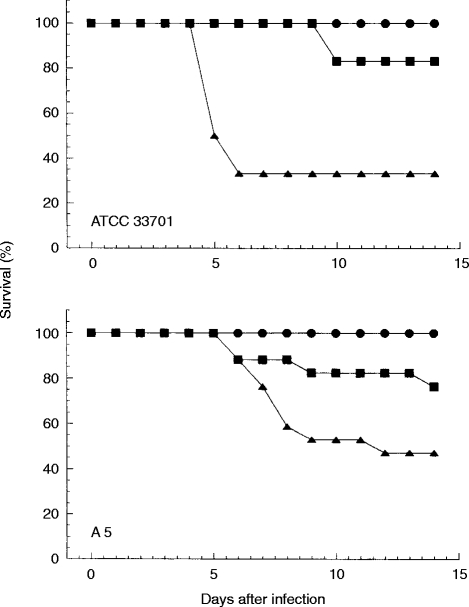
To determine the roles of IFN-γ and TNF in host defence against virulent R. equi, mice were injected with 1 mg anti-IFN-γ mAb, anti-TNF mAb or normal rat globulin 30 min before infection with a sublethal dose of virulent R. equi (2·3×105 of ATCC 33701 and 4·0×105 of A5). Sixty-seven per cent or 50% of the mice that received anti-TNF mAb died following a sublethal dose infection with ATCC 33701 or A5, respectively (Fig. 2). On the other hand, 17% or 23% of the mice that received anti-IFN-γ mAb, died following a sublethal dose infection with ATCC 33701 or A5, respectively. No normal rat globulin-treated mice died until 14 days postinoculation.
Figure 2.
Effect of IFN-γ and TNF neutralization on the survival of R. equi-infected mice. Mice were injected with 1 mg anti-IFN-γ mAb (closed square), anti-TNF mAb (closed triangle) or normal rat globulin (closed circle) 30 min before infection with a sublethal dose of virulent R. equi.
Effect of in vivo administration of mAbs against IFN-γ and TNF on bacterial growth in mice infected with R. equi ATCC 33701, A5 or ATCC 33701P−
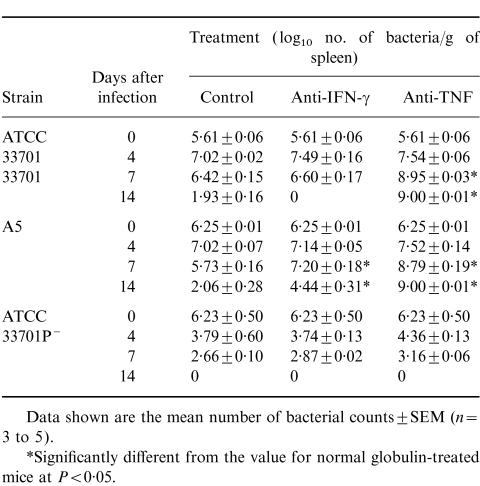
To confirm the effect of in vivo administration of mAbs against IFN-γ and TNF on host defence in mice, mice treated with 1 mg anti-IFN-γ mAb or anti-TNF mAb were infected with a sublethal dose of ATCC 33701, A5 or ATCC 33701P−, and the number of bacteria in the livers, spleens and lungs were determined on days 0, 4, 7 and 14 after infection. Suppression of the elimination of bacteria from these organs was observed in anti-IFN-γ and anti-TNF mAb-treated mice infected with R. equi strains of virulence and intermediate virulence, but not in normal rat globulin-treated mice, on days 7 and 14 (Table 2). Anti-TNF mAb treatment more effectively suppressed elimination of bacteria in mice than did anti-IFN-γ mAb.
Table 2.
Course of Rhodococcus equi infection in anti-interferon-γ (anti-IFN-γ) monoclonal antibody (mAb)-treated, anti-tumour necrosis factor (anti-TNF) mAb-treated or normal rat globulin-treated mice infected intravenously with a sublethal dose of virulent strain ATCC 33701 intermediate virulent strain A5 or avirulent strain ATCC 33701P− followed against time in the spleen
DISCUSSION
The present study focused on cytokine secretion, in mice, during the course of primary infection with R. equi strains of virulence, intermediate virulence and avirulence. TNF and IFN-γ, but not IL-4 and IL-10, were induced endogenously in mice in association with the multiplication and clearance of inoculated bacteria, and cytokine production depended largely on the virulence level of R. equi. Results obtained by neutralization of TNF and IFN-γin vivo by mAb clearly indicated the essential role of cytokines in the control and survival of virulent R. equi infection, but they did not affect avirulent R. equi. These data suggested that TNF and IFN-γ were secreted in response to microbial stimuli, and that TNF was required early in infection to limit replication of bacteria within the organs. This study also showed that the absence of the virulence plasmid prevented ATCC 33701 from increasing its systemic infection in cytokine-depleted mice.
Depletion of TNF in vivo by anti-TNF mAb led to a lethal course of virulent R. equi infection in mice given a sublethal dose, in contrast with the results of studies by Nordmann et al.20 They observed only inhibition of the ability of euthymic Swiss mice to clear an intravenous challenge at 7 days postinoculation by anti-TNF and/or anti-IFN-γ antibodies.20 This discrepancy might be explained by a difference in the virulence of R. equi used in the two studies because no effects of TNF neutralization were observed in the mice infected with avirulent R. equi in this study.
The number of bacteria in the organs of mice inoculated intravenously with virulent R. equi and treated with mAb to IFN-γ did not show significant reduction by day 4 (Table 2), and rose significantly at day 7 and 14. The effect of IFN-γ neutralization by mAb on the course of pulmonary R. equi infection was examined by Kanaly et al.22 who showed that BALB/c mice treated with mAb failed to clear pulmonary R. equi infection, resulting in the development of pulmonary granulomas. These and our data suggest that secretion of IFN-γ is essential for immune clearance of R. equi from mice.
Macrophage activation is a key component of the protective immune response to R. equi infection because the key event in the pathogenesis of R. equi infection in mice is considered to be the ability of virulent R. equi to survive and multiply in macrophages.29 A previous study showed that the pathogenesis of R. equi infection involves at least two important virulence determinants, both of which play critical roles in the disease: one is the virulence plasmid, which is required for R. equi to grow within host cells; and the other is the granulomagenic activity that is related to the lipid and cell wall contents of the species, which induces the characteristic pathological changes.26 The present study clearly showed a relationship between virulence of R. equi strains and their capacity to induce TNF secretion. More recently, Giguere & Prescott30 evaluated intracellular survival and measured cytokine induction by mouse macrophages infected with a virulent R. equi strain, 103+, its avirulent plasmid-cured derivative, 103−, and heat-killed 103+. The number of bacteria in the macrophages infected with 103−and heat-killed 103+ decreased progressively, whereas the 103+ numbers remained constant over 48 hr. However, their study found no difference in IL-12 and TNF production between virulent and avirulent R. equi.30 This difference from might reflect the in vitro nature of Giguere & Prescott's work.
The release of cytokines is an important part of the immune response against an infection, and several studies have described the importance of TNF and IFN-γ in the immune response against R. equi.20,22,23 Indeed, in vivo neutralization of TNF and IFN-γ exacerbates R. equi infection.20,22 The present study clearly showed that the induction of TNF and IFN-γ in serum and organs of mice infected with virulent R. equi corresponded with the proliferation of R. equi in the organs; i.e. virulent R. equi induced higher cytokine secretion than did avirulent R. equi. The ability of macrophages to produce TNF appears to be a critical step in the activation of the first line of defence against foreign organisms, and it is not surprising that several pathogens have evolved virulence mechanisms that interfere with the host's cytokine responses. Interference with TNF-α has been reported for several bacteria, such as Yersinia, brucellae and Mycobacterium avium.31–33 Mice infected with wild-type Y. pestis produce much less TNF-α than mice infected with Y. pestis cured of a virulence plasmid (pYV), indicating that a factor suppressing TNF-α synthesis is encoded by the pYV plasmid.34 Therefore, virulent R. equi is quite different from those pathogens that induce suppression of TNF release.
A study by Nordmann et al.20 showed that in vivo TNF and IFN-γ serum levels in R. equi-infected mice remained below the level of detectability in mice infected with an AIDS isolate of R. equi. The strain used in Nordmann's study was not virulent because the LD50 of the strain demonstrated >108 bacteria in BALB/c mice by the intravenous route, suggesting that it was avirulent. This contention is supported by the results of our study in which low or no levels of TNF and IFN-γ were detected in the serum of mice infected with avirulent R. equi.
T helper 1 (Th1) cells are defined by their ability to produce IFN-γ and IL-2, while Th2 cells produce IL-4, IL-5 and IL-10.35,36 In mice and humans, many infectious agents produce preferential Th1 (type 1) or Th2 (type 2) immune responses.37 The relative balance of type 1 versus type 2 responses determines the outcome, including the ability of infected hosts to control a number of intracellular pathogens.23,28,37 This effect is mediated by the respective lymphokines produced.35,36 In this study, in vivo administration of anti-IFN-γ or anti-TNF mAb accelerated the death of mice inoculated with a sublethal dose of virulent R. equi, and no IL-4 or IL-10 could be detected in mice with virulent and avirulent R. equi infections. These results suggest that immune clearance of R. equi appears to be a Th1-like response and that IFN-γ is a primary mediator.4,22,23
In conclusion, TNF and IFN-γ were found to be critical cytokines that offered protection against primary infection with a sublethal dose of R. equi strains of virulence and intermediate virulence, but they did not suppress avirulent R. equi infection.
Acknowledgments
This study was supported by a Grant-in-Aid for Scientific Research from the Equine Research Institute, Japan Racing Association, and by a Grant-in-Aid for Scientific Research (10660306) from the Ministry of Education, Science, Sports and Culture, Japan.
REFERENCES
- 1.Prescott JF. Rhodococcus equi: an animal and human pathogen. Clin Mircobiol Rev. 1991;4:20. doi: 10.1128/cmr.4.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Samies JH, Hatrhaway BN, Echols RM, Veazey JM, Pilon VA. Lung abscess due to Corynebacterium equi: report of the first case in a patient with aquired immune deficiency syndrome. Am J Med. 1986;80:685. doi: 10.1016/0002-9343(86)90825-9. [DOI] [PubMed] [Google Scholar]
- 3.Drancourt M, Bonnet E, Gallais H, Peloux Y, Raoult D. Rhodococcus equi infection in patients with AIDS. J Infect. 1992;24:123. doi: 10.1016/0163-4453(92)92746-6. [DOI] [PubMed] [Google Scholar]
- 4.Mosser DM, Hondalus MK. Rhodococcus equi: an emerging opportunistic pathogen. Trends Microbiol. 1996;4:29. doi: 10.1016/0966-842x(96)81502-2. [DOI] [PubMed] [Google Scholar]
- 5.Takai S, Koike K, Ohbushi S, Izumi C, Tsubaki S. Identification of 15- to 17-kilodalton antigens associated with virulent Rhodococcus equi. J Clin Mircobiol. 1991;29:439. doi: 10.1128/jcm.29.3.439-443.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Takai S, Sekizaki T, Ozawa T, Sugawara T, Watanabe Y, Tsubaki S. Association between a large plasmid and 15- to 17-kilodalton antigens in virulent Rhodococcus equi. Infect Immunol. 1991;59:4056. doi: 10.1128/iai.59.11.4056-4060.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tkachuk-Saad O, Prescott J. Rhodococcus equi plasmids: isolation and partial characterization. J Clin Microbiol. 1991;29:2696. doi: 10.1128/jcm.29.12.2696-2700.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Takai S, Watanabe Y, Ikeda T, et al. Virulence-associated plasmids in Rhodococcus equi. J Clin Microbiol. 1993;31:1726. doi: 10.1128/jcm.31.7.1726-1729.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Takai S, Imai Y, Fukumaga N, et al. Identification of virulence-associated antigens and plasmids in Rhodococcus equi from patients with AIDS. Vet Microbiol. 1995;172:1306. doi: 10.1093/infdis/172.5.1306. [DOI] [PubMed] [Google Scholar]
- 10.Wada R, Kamada M, Anzai T, et al. Pathogenicity and virulence of Rhodococcus equi in foals following intratracheal challenge. Vet Microbiol. 1997;56:301. doi: 10.1016/s0378-1135(97)00098-9. [DOI] [PubMed] [Google Scholar]
- 11.Takai S, Fukunaga N, Ochiai S, et al. Identification of intermediately virulent Rhodococcus equi isolates from pigs. J Clin Microbiol. 1996;34:1034. doi: 10.1128/jcm.34.4.1034-1037.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Takai S. Epidemiology of Rhodococcus equi infections: a review. Vet Microbiol. 1997;56:167. doi: 10.1016/s0378-1135(97)00085-0. [DOI] [PubMed] [Google Scholar]
- 13.Takai S, Sasaki Y, Ikeda T, Uchida T, Tsubaki S, Sekizaki T. Virulence of Rhodococcus equi isolates from patients with and without AIDS. J Clin Mircobiol. 1994;32:457. doi: 10.1128/jcm.32.2.457-460.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hines SA, Kanaly ST, Byrne BA, Palmer GH. Immunity to Rhodococcus equi. Vet Microbiol. 1997;56:177. doi: 10.1016/s0378-1135(97)00086-2. [DOI] [PubMed] [Google Scholar]
- 15.Hondalus MK. Pathogenesis and virulence of Rhodococcus equi. Vet Microbiol. 1997;56:257. doi: 10.1016/s0378-1135(97)00094-1. [DOI] [PubMed] [Google Scholar]
- 16.Madigan JE, Hietala S, Muller N. Protection against naturally acquired Rhodococcus equi pneumonia in foals by administration of hyperimmune plasma. J Reprod Fertil Suppl. 1991;44:571. [PubMed] [Google Scholar]
- 17.Martens RJ, Martens JG, Fiske RA, Hietala SK. Rhodococcus equi pneumonia: protective effects of immune plasma in experimentally infected foals. Equine Vet. 1989;21:249. doi: 10.1111/j.2042-3306.1989.tb02161.x. [DOI] [PubMed] [Google Scholar]
- 18.Becu T, Polledo G, Gaskin JM. Immunoprophylaxis of Rhodococcus equi pneumonia in foals. Vet Microbiol. 1997;56:193. doi: 10.1016/s0378-1135(97)00088-6. [DOI] [PubMed] [Google Scholar]
- 19.Martens RJ, Martens JG, Fiske RA. Failure of passive immunization by colostrum from immunized mares to protect foals against Rhodococcus equi. Equine Vet J Suppl. 1991;12:19. [Google Scholar]
- 20.Nordmann P, Ronco E, Genounou M. Involvement of interferon-gamma and tumor necrosis factor-alpha in host defense against Rhodococcus equi. J Infect Dis. 1993;167:1456. doi: 10.1093/infdis/167.6.1456. [DOI] [PubMed] [Google Scholar]
- 21.Kanaly ST, Hines SA, Palmer GH. Failure of pulmonary clearance of Rhodococcus equi infection in CD4+ T-lymphocyte-deficient transgenic mice. Infect Immun. 1993;61:4929. doi: 10.1128/iai.61.11.4929-4932.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kanaly ST, Hines SA, Palmer GH. Cytokine modulation alters pulmonary clearance of Rhodococcus equi and development of granulomatous pneumonia. Infect Immun. 1995;63:3037. doi: 10.1128/iai.63.8.3037-3041.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kanaly ST, Hines SA, Palmer GH. Transfer of a CD4+ Th1 cell line to nude mice effects clearance of Rhodococcus equi from the lung. Infect Immun. 1996;64:1126. doi: 10.1128/iai.64.4.1126-1132.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nordmann P, Onco E, Nauciel C. Role of T-lymphocyte subsets in Rhodococcus equi infection. Infect Immun. 1992;60:2748. doi: 10.1128/iai.60.7.2748-2752.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Madarame H, Takai S, Matsumoto C, et al. Virulent and avirulent Rhodococcus equi infection in T-cell deficient athymic nude mice: pathologic, bacteriologic and immunologic responses. FEMS Immunol Med Microbiol. 1997;17:251. doi: 10.1111/j.1574-695X.1997.tb01019.x. [DOI] [PubMed] [Google Scholar]
- 26.Takai S, Madarame H, Matsumoto C, et al. Pathogenesis of Rhodococcus equi infection in mice: roles of virulence plasmids and granulomagenic activity of bacteria. FEMS Immunol Med Microbiol. 1995;11:181. doi: 10.1111/j.1574-695X.1995.tb00115.x. [DOI] [PubMed] [Google Scholar]
- 27.Nakane A, Numata A, Minagawa T. Endogenous tumor necrosis factor, interleukin-6 and gamma interferon levels during Listeria monocytogenes infection in mice. Infect Immun. 1992;60:523. doi: 10.1128/iai.60.2.523-528.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nakane A, Nishikawa S, Sasaki S, et al. Endogenous interleukin-4 but not interleukin-10 is involved in suppression of host resistance against Listeria monocytogenes infection in gamma interferon-depleted mice. Infect Immun. 1996;64:1252. doi: 10.1128/iai.64.4.1252-1258.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hondalus MK, Mosser DM. Survival and replication of Rhodococcus equi in macrophages. Infect Immun. 1994;62:4167. doi: 10.1128/iai.62.10.4167-4175.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Giguere S, Prescott JF. Cytokine induction in murine macrophages infected with virulent and avirulent Rhodococcus equi. Infect Immun. 1998;66:1848. doi: 10.1128/iai.66.5.1848-1854.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Boland A, Cornelis GR. Role of YopP in suppression of tumor necrosis factor alpha release by macrophages during Yersinia infection. Infect Immun. 1998;66:1878. doi: 10.1128/iai.66.5.1878-1884.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Caron E, Peyrard T, Kohler S, Cabane S, Liautard P, Dornand J. Live Brucella spp. fail to induce tumor necrosis factor alpha excretion upon infection of U937-derived phagocytes. Infect Immun. 1994;62:5267. doi: 10.1128/iai.62.12.5267-5274.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sarmento AM, Appelberg R. Relationship between virulence of Mycobacterium avium strains and induction of tumor necrosis factor alpha production in infected mice and in in vitro-cultured mouse macrophages. Infect Immun. 1995;63:3759. doi: 10.1128/iai.63.10.3759-3764.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nakajima R, Brubaker RR. Association between virulence of Yersinia pestis and suppression of gamma interferon and tumor necrosis factor alpha. Infect Immun. 1993;61:23. doi: 10.1128/iai.61.1.23-31.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mosmann TR, Coffman RL. Th1 and Th2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol. 1989;7:145. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- 36.Mosmann TR, Dad S. The expanding universe of T-cell subsets: Th1, Th2 and more. Immunol Today. 1996;17:138. doi: 10.1016/0167-5699(96)80606-2. [DOI] [PubMed] [Google Scholar]
- 37.Daugelat S, Kaufmann SH. Role of Th1 and Th2 cells in bacterial infections. Chem Immunol. 1996;63:66. [PubMed] [Google Scholar]