Abstract
We report that cultured rat peritoneal cells spontaneously synthesize nitric oxide and this is associated with active suppression of mast cell secretory function. Addition of interleukin-4 (IL-4) or the nitric oxide synthase inhibitor N-monomethyl-l-arginine to peritoneal cells inhibited nitric oxide synthesis and enhanced anti-IgE-mediated mast cell degranulation, measured as serotonin release. Interferon-γ (IFN-γ) completely overcame the enhancement of serotonin release and suppression of nitrite production induced by IL-4. Over several experiments, with or without IL-4 and/or IFN-γ, serotonin release correlated inversely with nitrite production. On a cell-for-cell basis, non-mast cells produced ≈30 times more nitrite than mast cells in peritoneal cell populations, with or without IFN-γ stimulation. The nitric oxide donor S-nitrosoglutathione inhibited anti-IgE-induced serotonin release from purified mast cells, whereas 8-bromo-cyclic GMP, the guanylate cyclase inhibitor 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one, superoxide dismutase and the peroxynitrite scavenger uric acid, were without effect. We conclude that IL-4 and IFN-γ reciprocally regulate mast cell secretory responsiveness via control of nitric oxide synthesis by accessory cells; the nitric oxide effect on mast cells is direct but does not involve cyclic GMP or peroxynitrite.
INTRODUCTION
The degranulation of rodent peritoneal mast cells is reciprocally regulated by the T-lymphocyte-derived cytokines interferon-γ (IFN-γ) and interleukin-4 (IL-4). IFN-γ inhibits1–3 whereas IL-4 enhances2,4,5 IgE-dependent mast cell activation and associated mediator release. IFN-γ exerts suppressive actions on other functions of rodent peritoneal mast cells, for example clonal proliferation6 and tumour necrosis factor-α (TNF-α)-mediated cytotoxicity.7 These effects on degranulation and cytotoxicity are shared by IFN-α/β.7–9 In contrast to IFN-γ, IL-4 generally exerts enhancing effects on mast cell function. For example, IL-4 enhances not only degranulation2,4,5 but also clonal proliferation of mouse peritoneal mast cells;10 it up-regulates cytokine gene expression by the human HMC-1 mast cell line,11 and IgE receptor expression on mouse peritoneal5 and human cord blood-derived mast cells.12 Thus, in general, IFN-γ exerts inhibitory effects whereas IL-4 exerts positive influences on mast cell functions.
Recently we reported that the inhibitory effect of IFN-γ on degranulation of mast cells in mouse peritoneal cell populations is via induction of nitric oxide.3 Several lines of evidence pointed to this conclusion: the IFN-γ activity was blocked by the competitive nitric oxide synthase (NOS) inhibitor N-monomethyl-l-arginine (l-NMMA) or by depletion of the NOS substrate l-arginine from the extracellular medium, and the IFN-γ effect was mimicked by nitric oxide donors. The cytokine was considerably more potent in mixed peritoneal cells compared with purified mast cells, suggesting that non-mast cells are the primary source of the nitric oxide, and this was confirmed by direct measurement of nitrite production.3 In view of the above, the aim of the present investigation was to determine whether the reciprocal effects of IL-4 and IFN-γ on mast cell degranulation could be accounted for by reciprocal regulation of nitric oxide synthesis. We also investigated whether nitric oxide exerts its effects on mast cells by activating guanylate cyclase to elevate cellular cyclic guanosine monophosphate (cGMP), or by interaction with superoxide to generate the cytotoxic radical peroxynitrite.
MATERIALS AND METHODS
Peritoneal cell and purified mast cell cultures
Cells were obtained by peritoneal lavage of male or female Brown Norway rats (200–300 g, Liverpool University outbred stock) with 50 ml of Hanks' balanced salt solution (HBSS, Gibco Life Technologies, Paisley, UK). The cells were washed twice in HBSS by centrifugation at 260 g for 5 min, and finally resuspended in Dulbecco's modified Eagle's medium (DMEM, Gibco, containing 5% fetal calf serum (FCS, Gibco), 4·0 mm l-glutamine and 50 μg/ml gentamicin (complete DMEM; cDMEM) to give 106 nucleated cells/ml comprising 5–10% mast cells as determined by metachromatic staining with 0·02% toluidine blue. In some experiments mast cells were purified to >98% by centrifugation through 7·5 ml of 65% isotonic Percoll solution (Sigma, Poole, UK) at 400 g for 15 min. The cells were cultured in quadruplicate as 0·5–1·0 ml volumes in conical sterile plastic tubes at 37° with 5% CO2 in air for 24 hr. Various compounds were added to the cultures as described in the Results and in the figure legends. Recombinant rat IL-4 was from Serotec (Oxford, UK) or Peprotech (London, UK) and IFN-γ was from Serotec. Concentrations of IL-4 (40 ng/ml) and IFN-γ (100 ng/ml) were optimized in pilot experiments. The l-NMMA, S-nitrosoglutathione (SNOG) and 8-bromo-cGMP were from Calbiochem-Novabiochem (Nottingham, UK); superoxide dismutase (SOD) and urate were from Sigma; 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one (ODQ) was from Alexis Corporation (Nottingham, UK).
Cell challenge
One hour prior to the termination of the cultures, 1 μCi/ml of 3H-labelled serotonin (5-[1,2–3H(N)]-hydroxytryptamine creatinine sulphate, specific activity 27 Ci/mmol; DuPont NEN, Dreiech, Germany), that is selectively incorporated into mast cell granules, was added to each culture. At the termination of the cultures, the cells were sedimented, the supernatant medium was retained for nitrite assay, and the cells were washed twice with 1 ml of wash medium (DMEM with 1% FCS) and resuspended in 1 ml of challenge medium (cDMEM buffered to pH 7·0 with 10·0 mm HEPES). The cells (100 μl) were added in duplicate to either 100 μl of challenge medium (as a control), 100 μl of 1/1000 rabbit anti-rat IgE (raised in house) or 100 μl of 0·05% Triton-X-100 (to lyse the cells). The cells were incubated for 20 min at 37° with 5% CO2 in air, after which they were centrifuged at 350 g for 2 min. Then, 100 μl of the supernatant fraction was removed and added to 2 ml of scintillation cocktail (Aqualuma Plus, Lumac LSC. BV., Groningen, the Netherlands). The samples were analysed for [3H] activity over 1 min on a liquid scintillation counter (Packard Tri-Carb 1500, Packard Instrument Co., Downers Grove, IL). Percentage specific release of [3H]serotonin from mast cells was assessed as [(a–b)/(c–b)]×100 where a = counts per minute (c.p.m.) for anti-IgE-treated cells, b = c.p.m. for control cells and c = c.p.m. for lysed cells.
Nitrite assay
The nitrite content of cell culture supernatant fractions was determined by the Griess method.13 Samples (150 μl) were added in duplicate to 96-well microtitre plates, alongside duplicate sodium nitrite standards in the range 0–100 μm. Then, 50 μl of Griess reagent (1% sulphanilamide and 0·1%N-(1-naphthyl) ethylenediamine dihydrochloride in 45% ethanoic acid) was added to each well and the samples were incubated for 10 min at room temperature. Absorbances at 570 nm were read on an automatic plate reader (MR 600, Dynatech Instruments Inc., Torrance, CA). The values of nitrite concentration in the culture samples were obtained from the standard curve.
Flow cytometry
To measure cell surface levels of IgE, purified (> 98%) peritoneal mast cells were cultured in 0·5 ml volumes containing 2·5×105–5·0×105 cells. After 24 hr in the presence of various concentrations of the nitric oxide donor SNOG, the cells were washed twice in phosphate-buffered saline containing 0·5 mg/ml bovine serum albumin and 5 mm ethylenediamine tetraacetic acid (PBS–BSA) and resuspended in 50 μl of ice-cold PBS–BSA containing 1/50 rabbit anti-rat IgE or control normal rabbit serum for 30 min at 0° in the dark. The cells were washed three times in PBS–BSA before addition of sheep anti-rabbit IgG:fluorescein isothiocyanate (FITC) conjugate (1/50, Serotec) for 30 min at 0° in the dark. The cells were washed again and analysed by flow cytometry. All cell samples were analysed on a Becton Dickinson FACScan flow cytometer and the data were analysed using winMDI (version 2·5) software. The fluorescence threshold was set to give <10% false positives. Mean and peak fluorescence were measured in relative units.
Statistics
All cell culture experiments were performed in quadruplicate and all experiments were performed four times. The data was analysed by analysis of variance (anova) and group comparisons were analysed by Bonferroni-corrected Student's t-test.
RESULTS
Effects of IFN-γ and/or l-NMMA on anti-IgE-induced mast cell serotonin release and peritoneal cell nitrite production
Rat peritoneal cells (containing 5–10% mast cells) were cultured in cDMEM in the presence or absence of the nitric oxide synthase inhibitor l-NMMA (100 μm) and/or IFN-γ (100 ng/ml) for 24 hr prior to challenge with anti-IgE antibody. Cells cultured without either IFN-γ or l-NMMA released low levels (<10%) of serotonin upon challenge (Fig. 1a) and this was associated with high levels (>25 μm) of total culture nitrite (Fig. 1b). Addition of l-NMMA alone reduced nitrite levels to 25–30% of control values and this was associated with a three- to fourfold increase in anti-IgE-induced serotonin release (Fig. 1). Thus, spontaneous production of nitric oxide by peritoneal cells is responsible for active suppression of mast cell responsiveness. Addition of IFN-γ to the cultures increased nitrite production significantly (by 50%) but this was not associated with any further inhibition of serotonin release. Similar results to those shown in Fig. 1 were obtained with peritoneal cells from Wistar rats (data not shown).
Figure 1.
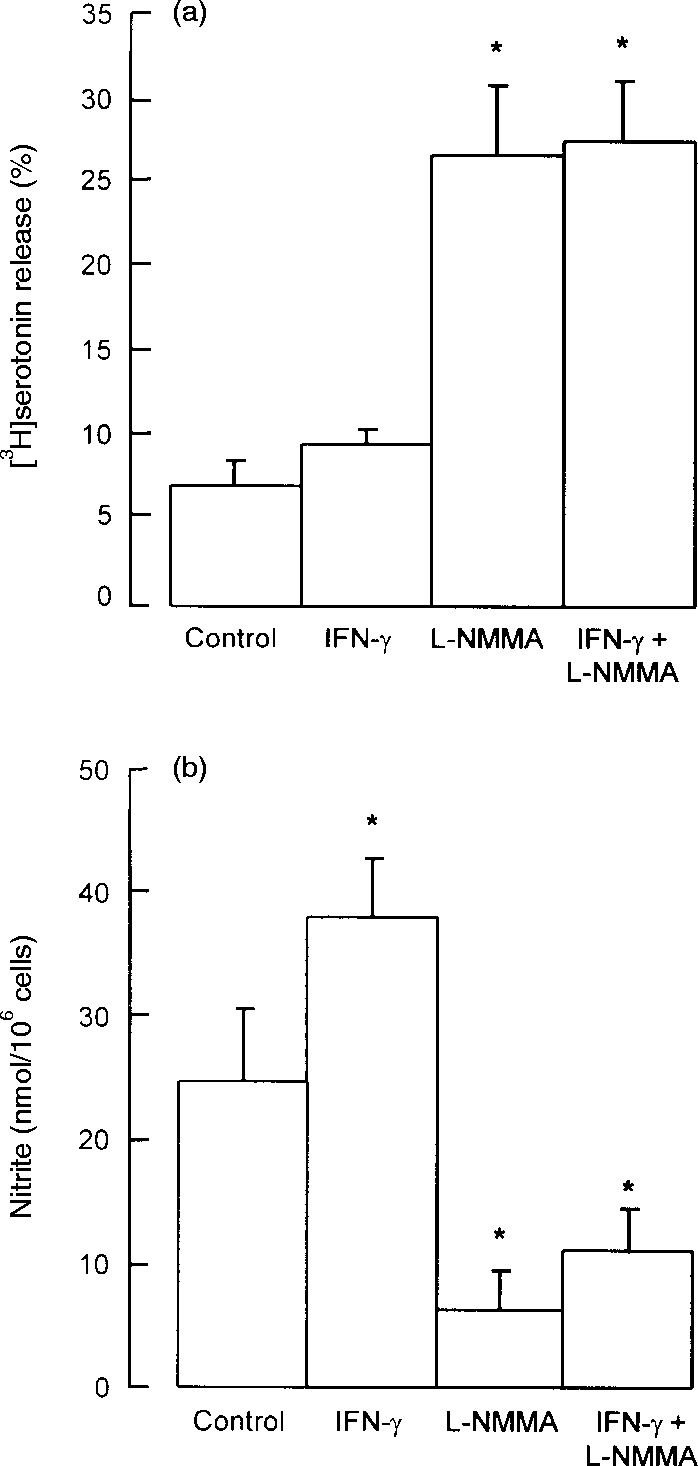
Effects of IFN-γ and l-NMMA on (a) mast cell serotonin release and (b) peritoneal cell nitrite production. Peritoneal cells were cultured for 24 hr with IFN-γ (100 ng/ml) or l-NMMA (0·1 mm), both compounds in combination, or with neither compound (control cells). At the end of the culture period supernatant medium was removed for measurement of nitrite, and the cells were washed and challenged with anti-IgE for measurement of serotonin release. Results are means±SEM for four experiments. *P <0·001 compared to control cells.
Effects of IL-4 and/or l-NMMA on anti-IgE-induced mast cell serotonin release and peritoneal cell nitrite production
Addition of IL-4 (40 ng/ml) to 24 hr peritoneal cell cultures led to a 2·2-fold increase in anti-IgE-induced serotonin release compared with controls, whereas l-NMMA (100 μm) enhanced the response 3·3-fold (Fig. 2a). IL-4 and l-NMMA in combination at these concentrations produced a small, though not significantly greater, enhancement compared to either compound alone (Fig. 2a). IL-4 or l-NMMA alone at the above concentrations significantly decreased nitrite levels to 56% and 26%, respectively, of control values. In combination IL-4 and l-NMMA further reduced nitrite production to 10% of control values (Fig. 2b). When the concentration of l-NMMA was raised to 500 μm to completely block NOS activity, IL-4 produced no further enhancement of anti-IgE-induced serotonin release beyond that produced by l-NMMA alone (data not shown).
Figure 2.
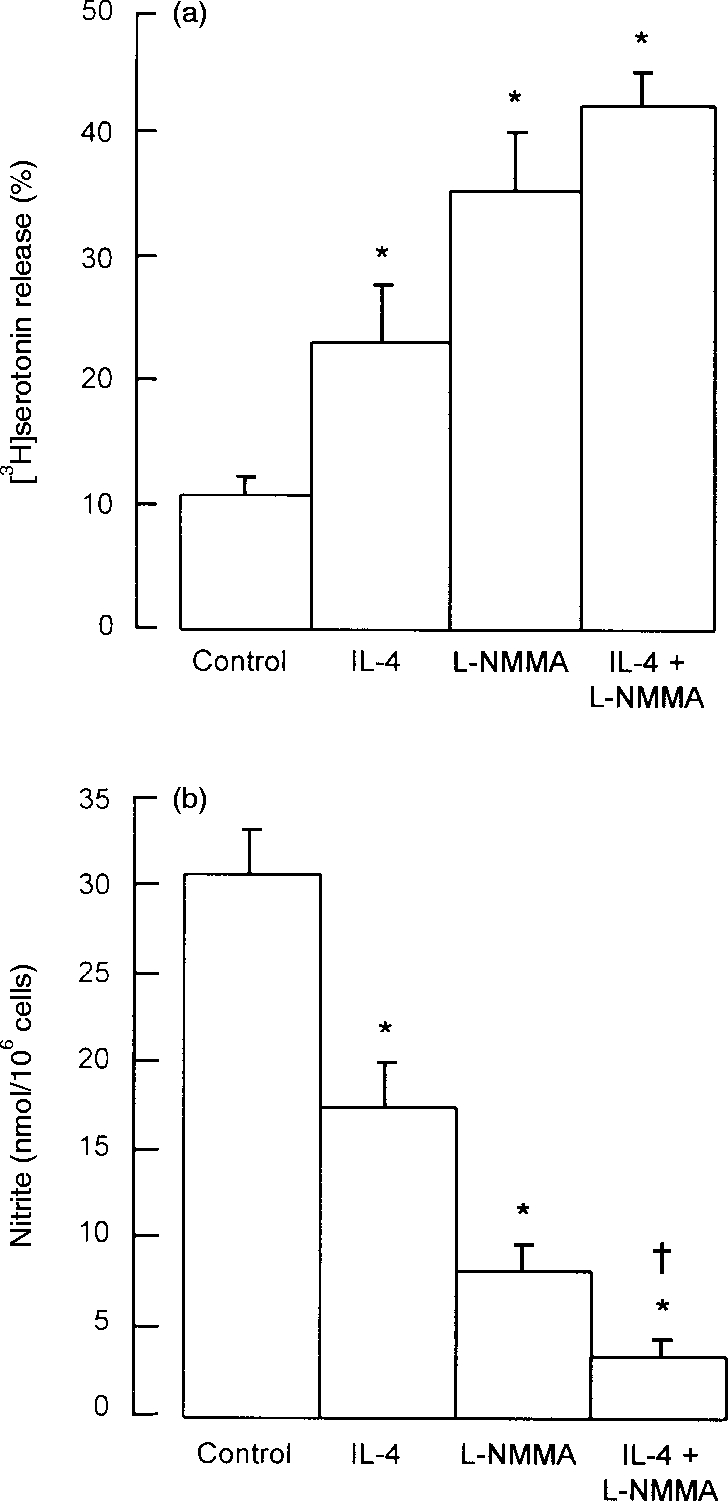
Effects of IL-4 and l-NMMA on (a) mast cell serotonin release and (b) peritoneal cell nitrite production. Peritoneal cells were cultured for 24 hr with IL-4 (40 ng/ml) or l-NMMA (0·1 mm), both compounds in combination, or with neither compound (control cells). At the end of the culture period, supernatant medium was removed for measurement of nitrite, and the cells were washed and challenged with anti-IgE for measurement of serotonin release. Results are means±SEM for four experiments. *P <0·001 compared to control cells; †P <0·05 compared to cells treated with l-NMMA alone.
Interactions of IL-4 and IFN-γ on anti-IgE-induced mast cell serotonin release and peritoneal cell nitrite production
Peritoneal cells were cultured for 24 hr with or without IL-4 (40 ng/ml) and/or IFN-γ (100 ng/ml). At the termination of culture an aliquot of supernatant medium was removed for nitrite assay and the cells were washed and challenged with anti-IgE. Anti-IgE-induced serotonin release was low (<5%) and nitrite production was high (>20 μm) for non-cytokine-treated cells (Fig. 3) as previously. IL-4 alone increased anti-IgE-induced serotonin release 3·2-fold and at the same time reduced nitrite production to half of control levels. IFN-γ alone did not suppress significantly the already low levels of mast cell degranulation, but completely overcame the enhancing effects of IL-4 on stimulated serotonin release (Fig. 3a). In the same cell populations, IFN-γ increased nitrite production to 2·6-fold control (no cytokine) levels in the absence of IL-4 and 2·4-fold in the presence of IL-4. Thus, the inhibitory effect of IL-4 on nitric oxide synthesis was completely overcome by IFN-γ, in parallel to the reversal by IFN-γ of the IL-4-dependent enhancement of mast cell degranulation (Fig. 3).
Figure 3.
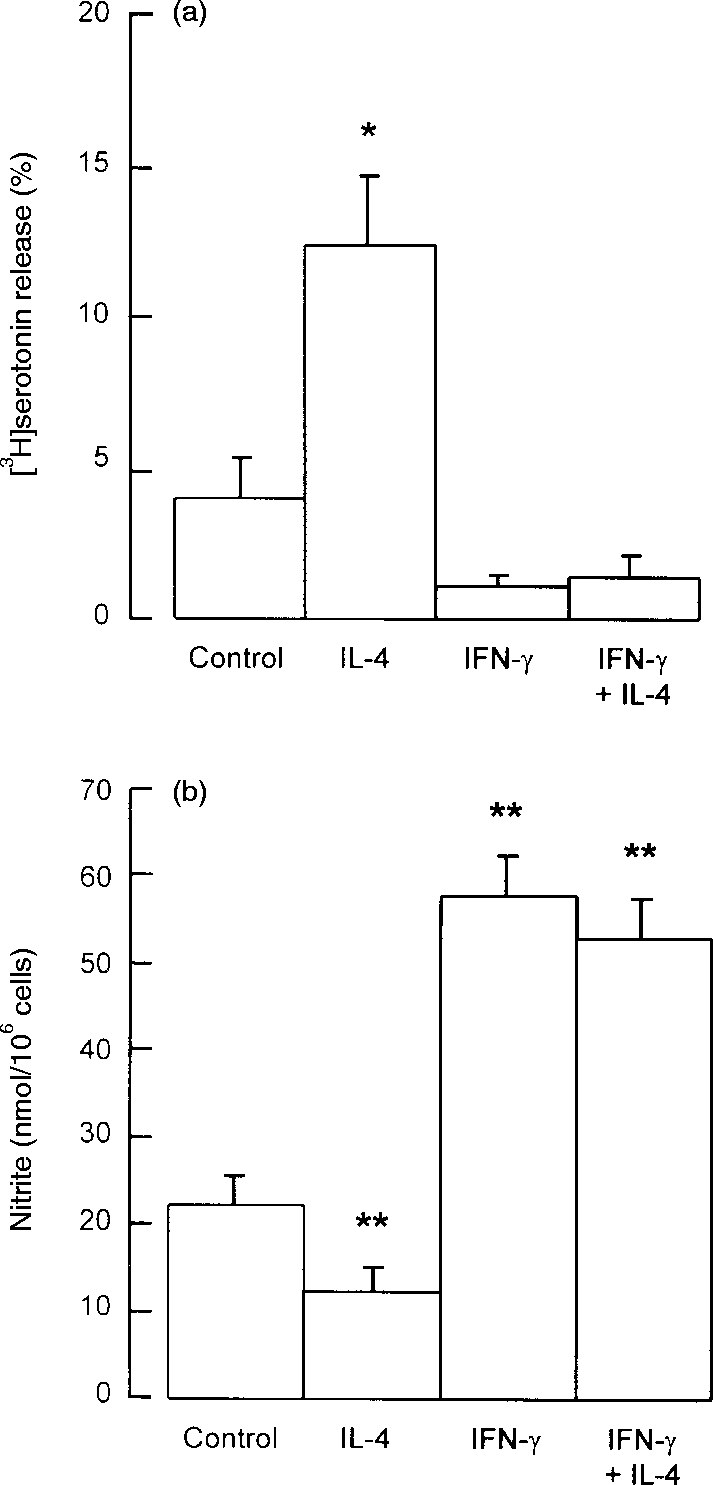
Effects of IFN-γ and IL-4 on (a) mast cell serotonin release and (b) peritoneal cell nitrite production. Peritoneal cells were cultured for 24 hr with IFN-γ (100 ng/ml) or IL-4 (40 ng/ml), both compounds in combination, or with neither compound (control cells). At the end of the culture period supernatant medium was removed for measurement of nitrite, and the cells were washed and challenged with anti-IgE for measurement of serotonin release. Results are means±SEM for four experiments. *P <0·01; **P <0·001 compared to control cells.
The data from Fig. 3 (obtained from four experimental conditions across four independent experiments) were analysed by linear regression to investigate the relationship between serotonin release and nitrite production. A value for the correlation coefficient, r2 of −0·793 was obtained giving a P-value of 0·0003, showing that serotonin release was significantly inversely related to levels of nitrite production under various conditions of culture, either with or without added cytokines.
Cell source of mast cell inhibitory nitric oxide
Peritoneal cells were separated through Percoll into purified (>98%) mast cells and mast cell-depleted (<2% mast cells) interface cells, and cultured for 24 hr with or without IFN-γ. Purified mast cells generated barely detectable nitrite whereas interface cells (<2% mast cells) generated high levels of nitrite (>40 μm) and this was increased in the presence of IFN-γ (Fig. 4). On a cell-for-cell basis, cells in the mast cell-depleted fraction produced 30 times more nitrite than purified mast cells. Considering that the purified mast cells contained 1–2% non-mast cells, these could have accounted in full for the low levels of nitrite released. Furthermore, the NOS inhibitor l-NMMA (100 μm) was without effect on serotonin release from purified mast cells (control anti-IgE-induced release was 33·4±4·4% and with l-NMMA was 36·1±4·7%; means±SEM for four experiments each performed in quadruplicate). This shows that purified mast cells do not produce sufficient nitric oxide to inhibit their own secretory responses. Also, anti-IgE-induced serotonin release from purified mast cells was considerably greater (up to eightfold) than that for unfractionated peritoneal cells (compare above data with that in Figs 1, 2 and 3), showing that removal of non-mast cells eliminates the source of nitric oxide and allows the mast cells to respond fully.
Figure 4.
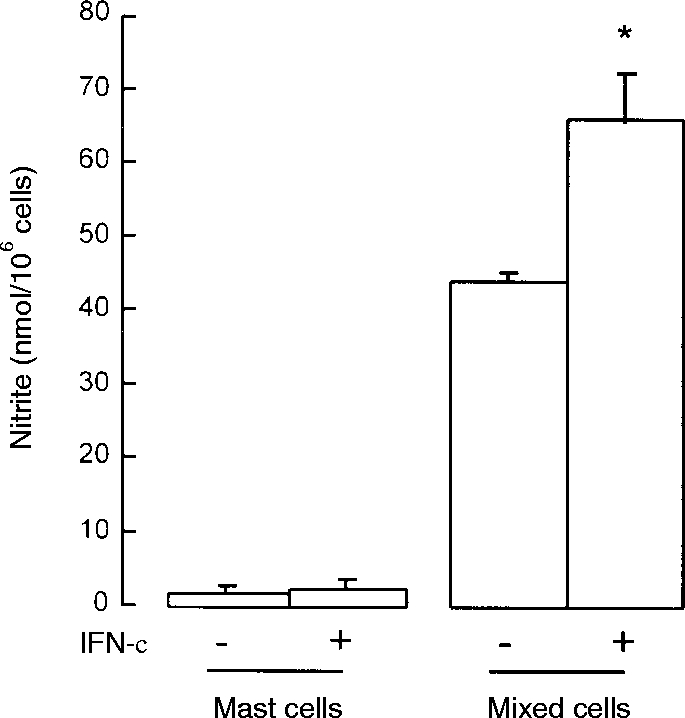
Nitrite production by purified (>98%) mast cells and mast cell-depleted (<2%) peritoneal cells separated on Percoll gradients. The cells were cultured for 24 hr with (+) or without (–) 100 ng/ml IFN-γ. Results are means±SEM for four experiments. *P <0·01 compared to non-IFN-γ-treated cells.
Effect of a nitric oxide donor on anti-IgE-induced mast cell serotonin release and surface IgE expression
To establish whether nitric oxide can inhibit mast cell degranulation directly, purified peritoneal mast cells (>98%) were cultured with the nitric oxide donor SNOG for 24 hr (pilot experiments had shown this incubation time was optimal). SNOG produced a concentration-dependent inhibition of anti-IgE-induced serotonin release with 76% inhibition reached at 1000 μm (Fig. 5a). Under identical experimental conditions SNOG did not influence cell surface expression of IgE (Fig. 5b). Thus, nitric oxide acts directly on mast cells to inhibit IgE-mediated secretion and its actions are on postreceptor coupling events rather than on expression of cell surface IgE.
Figure 5.
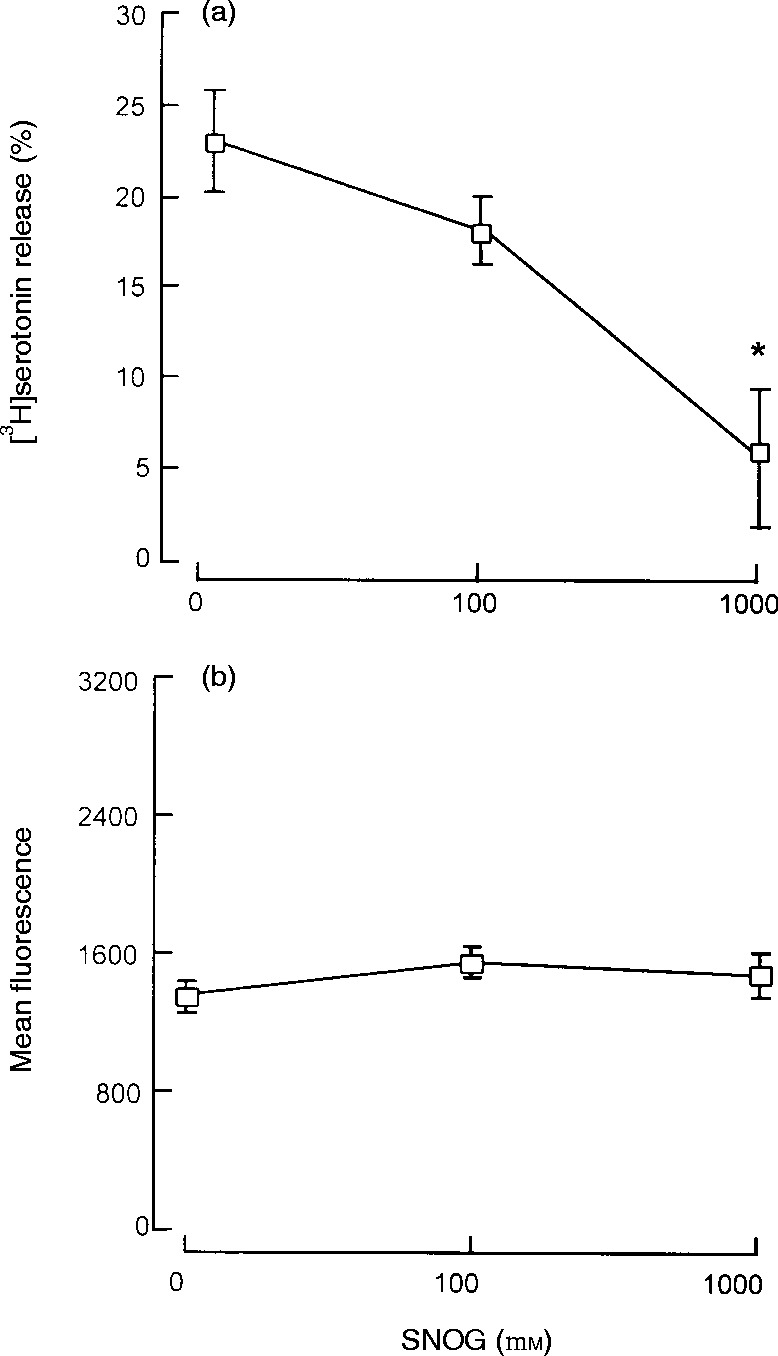
Effect of SNOG on mast cell degranulation and surface IgE expression. Purified (>98%) mast cells were cultured for 24 hr with SNOG before challenge with anti-IgE for measurement of serotonin release (a) or flow cytometric analysis of IgE expression expressed as mean fluorescence (b). Results are means±SEM for four experiments. *P <0·001 compared to control cells.
Possible involvement of cGMP in regulation of mast cell degranulation
To determine whether the mast cell regulatory effects of nitric oxide might be through cGMP, a physiological mediator of many nitric oxide effects, we adopted two approaches. In the first we tested a cell-permeable analogue of cGMP, 8-bromo-cGMP14 for direct inhibitory effects on mast cells. Second, we examined the selective guanylate cyclase inhibitor ODQ15,16 for enhancing effects on mast cells in unfractionated peritoneal cell populations that were actively generating nitric oxide.
Culture of purified peritoneal mast cells with 8-bromo-cGMP (0·5–1·0 mm) for 24 hr had no effect on anti-IgE induced serotonin release (for example, control release was 28·6±3·5% and with 1·0 mm of the cyclic nucleotide was 29·8±1·6%; means±SEM, n = 4). Similarly, culture of unfractionated peritoneal cells for 24 hr with ODQ (10·0 nm to 1·0 μm) had no effect on stimulated serotonin release (control release was 22·8±2·7% and with 1 μm ODQ was 19·3±2·7%; means±SEM, n = 4). These concentrations of ODQ were tested on an isolated aortic ring smooth muscle preparation and found to be potent in the suppression of relaxation induced by SNOG (data not shown).
Possible involvement of superoxide and peroxynitrite in nitric oxide-induced suppression of mast cell degranulation
Under conditions where superoxide is generated in the presence of nitric oxide, these two radicals can interact to generate the highly toxic peroxynitrite molecule that mediates certain of the cytotoxic effects of nitric oxide.17,18 Thus, superoxide also reduces nitrite production from nitric oxide. Although superoxide can be synthesized by macrophages, its synthesis is not thought to be under the reciprocal control of IFN-γ and IL-4. Nevertheless, we tested SOD, an enzyme that depletes superoxide19,20 and uric acid, that blocks the cytotoxicity of peroxynitrite17,18 for effects on mast cell degranulation. Unfractionated peritoneal cells were cultured for 24 hr with 100 ng/ml of IFN-γ either in the absence (controls) or presence of SOD (200 units/ml), uric acid (0·5 mm) or l-NMMA (100 μm), the latter as a positive control mast cell regulator. These concentrations of SOD and uric acid have been shown to be effective inhibitors in macrophage cell cultures generating superoxide.17–20 The results shown in Fig. 6 demonstrate that l-NMMA inhibited nitrite production and enhanced anti-IgE-induced serotonin release as expected but SOD and uric acid were without significant effect on either parameter.
Figure 6.
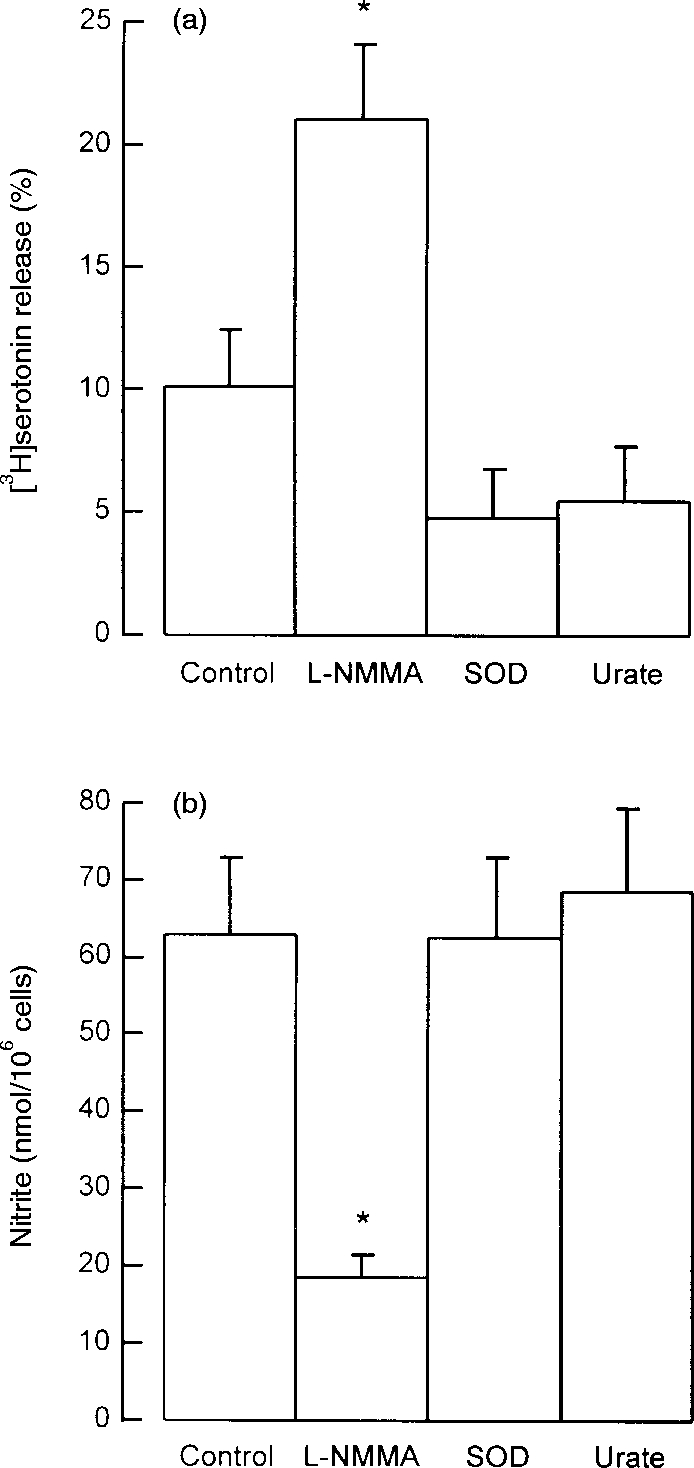
Effects of l-NMMA, SOD and urate on (a) mast cell serotonin release and (b) peritoneal cell nitrite production. Peritoneal cells were cultured for 24 hr in the absence (control) or presence of l-NMMA (0·1 mm), SOD (200 units/ml) or urate (0·5 mm). IFN-γ (100 ng/ml) was added to all cultures. Supernatant medium was removed for measurement of nitrite, and the cells were washed and challenged with anti-IgE for measurement of serotonin release. Results are means±SEM for four experiments. *P <0·001 compared to control cells.
DISCUSSION
We found that cultured rat peritoneal cells spontaneously generate high levels of nitrite, a marker of nitric oxide synthesis, without requirement for stimulation with IFN-γ, and mast cells from these cultures responded weakly to anti-IgE by releasing low levels of granule-associated serotonin. Addition of l-NMMA, a reversible inhibitor of NOS, to the cultures inhibited nitrite production and enhanced stimulated serotonin release (both by a factor of 3–4) showing that the spontaneous endogenous production of nitric oxide suppresses mast cell responsiveness. Although IFN-γ enhanced nitric oxide synthesis significantly, this was not sufficient to suppress mast cell degranulation further. IL-4, on the other hand, reduced nitric oxide synthesis and enhanced mast cell responses (both by a factor of ≈ 2), indicating that the mast cell enhancing activity of IL-4 is by inhibition of NOS. When nitric oxide synthesis was fully blocked by l-NMMA, IL-4 was without any further mast cell-enhancing effect, confirming that its effect on degranulation is by inhibition of nitric oxide synthesis.
Our observation that IFN-γ overcame the NOS inhibiting and mast cell enhancing effects of IL-4 reveals that although IFN-γ alone does not further inhibit mast cell responses in cell populations that are already producing nitric oxide at a high rate, it does induce nitric oxide synthesis and inhibits mast cell responses in populations in which nitric oxide synthesis (e.g. after IL-4 treatment) is at a low level. Therefore IL-4 and IFN-γ reciprocally regulate mast cell responses via control of NOS activity in rat peritoneal cells. The reciprocal control of mast cell activation by IFN-γ and IL-4 via nitric oxide synthesis suggests that IgE/mast cell-mediated allergic reactions may be regulated during the progression of the immune response in a direction depending on which subset of T helper lymphocytes (IFN-γ producing Th1 cells or IL-4 producing Th2 cells21) selectively develops.
Confirming a direct effect of nitric oxide on mast cells, we showed that the nitric oxide donor SNOG inhibited anti-IgE-induced serotonin release from purified rat peritoneal mast cells. Furthermore, SNOG did not influence mast cell surface expression of IgE, demonstrating that nitric oxide targets signalling events downstream of receptor cross-linkage.
As reported for purified peritoneal mast cells from the mouse,3 those from the rat do not produce detectable nitric oxide. Also, our observation that l-NMMA was without effect on degranulation of purified mast cells cultured with or without IFN-γ confirms that these cells do not produce sufficient nitric oxide, even if produced at low levels, to inhibit their own responsiveness. Monocyte/macrophages are the most abundant cell type in rodent peritoneal populations, and neutrophils and lymphocytes are also present at a few percent. All of these cell types produce nitric oxide,22–25 and at least in the case of monocyte/macrophages nitric oxide production is reciprocally regulated by IFN-γ and IL-4.26–29 Therefore it seems almost certain that the principal cell source of IFN-γ/IL-4-regulated nitric oxide production in the peritoneal cavity is the monocyte/macrophage.
Our finding that rat peritoneal mast cells themselves produce very little or no nitric oxide is at odds with previous studies using this cell type.30–33 We found that non-mast cells generate >30 times more nitrite than mast cells in both resting or IFN-γ-stimulated populations. Even in purified mast cell preparations the 1–2% contaminating non-mast cells could have contributed appreciably to the low levels of nitrite production observed. Certainly with non-mast cell contamination reaching 3%30,31 or even 15%,32,33 we believe reports that peritoneal mast cells produce significant amounts of nitric oxide should be re-evaluated. A more recent report has shown that mouse bone marrow-derived mast cells generate nitric oxide in response to IgE-dependent activation.34 This may represent a difference in terms of mast cell phenotype or method of activation (IFN-γ versus IgE/Ag).
Nitric oxide is unique among intercellular messengers in that it diffuses indiscriminately into neighbouring cells in which it activates either physiological or toxic processes by chemical interaction with nucleophilic groups, by virtue of its unpaired outer electron.22,23,25 The physiological processes mediated by nitric oxide, for example neurotransmission and vascular smooth muscle relaxation, are through interaction with the haem group on guanylate cyclase resulting in elevation of cGMP.25 On the other hand, the toxic processes mediated by the radical are thought to be through its interactions with other intracellular nucleophilic targets, for example by nitrosylation of thiol proteins or deamination of DNA.22,23,25 Furthermore, under conditions of high oxidative stress, when superoxide may be generated alongside nitric oxide, these two radicals interact to form the highly cytotoxic peroxynitrite.17,18 In the present study we found that purified mast cell responses were not influenced by the cell-permeable cGMP analogue 8-bromo-cGMP,14 and the guanylate cyclase inhibitor ODQ15,16 was ineffective at modifying mast cell degranulation, even though it was biologically active in suppressing nitric oxide effects in a vascular smooth muscle preparation. Further experiments showed that the responsiveness of mast cells in nitric oxide-producing peritoneal cell populations was not affected by superoxide dismutase,19,20 that breaks down superoxide, or the peroxynitrite blocker uric acid.17,18 We conclude that the suppression of mast cell responses is not dependent on superoxide or peroxynitrite production and that nitric oxide does not mediate its mast cell suppressive actions via cGMP. Consistent with our conclusion that superoxide is not involved, mouse macrophages stimulated with IFN-γ plus lipopolysaccharide only generate free oxygen radicals in l-arginine-depleted medium.18 Previous reports33,35 claimed that mast cell cGMP was elevated by nitric oxide. However, the first33 used peritoneal cells containing a high proportion of non-mast cells and used non-mast cell-selective activating agents, and neither study reported experiments with cell permeable cGMP analogues or guanylate cyclase inhibitors. The nitric oxide effect on mast cells appears to be non-toxic as evidenced by the fact that the cells appeared morphologically normal after extended culture in the presence of nitric oxide, they excluded trypan blue dye, and actively incorporated tritiated serotonin as efficiently as cells cultured in the absence of nitric oxide.
In conclusion, IFN-γ and IL-4 reciprocally regulate mast cell secretory responses and nitric oxide is the focal intermediate involved. Although the inhibitory effect of nitric oxide on mast cells appears to be non-toxic, the radical does not appear to act via cGMP. The nitric oxide may conjugate to nucleophilic groups on specific proteins or factors important to secretion-coupling events that are activated subsequent to IgE cross-linkage. The identity of these molecular targets remains to be elucidated.
Acknowledgments
This work was funded by the Medical Research Council. We thank David Briggs for technical assistance with the flow cytometry.
Abbreviations
- cDMEM
complete Dulbecco's modified Eagle's medium containing 5% fetal calf serum
- cGMP
cyclic guanosine monophosphate
- DMEM
Dulbecco's modified Eagle's medium
- FCS
fetal calf serum
- HBSS
Hanks' balanced salt solution
- IFN-γ
interferon-γ
- IL
interleukin
- l-NMMA
N-monomethyl-l-arginine
- NOS
nitric oxide synthase
- ODQ
1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one
- PBS
phosphate-buffered saline
- PBS–BSA
PBS containing 0·5 mg/ml bovine serum albumin
- SNOG
S-nitrosoglutathione
- SOD
superoxide dismutase
REFERENCES
- 1.Coleman JW, Buckley MG, Holliday MR, Morris AG, et al. Interferon-γ inhibits serotonin release from mouse peritoneal mast cells. Eur J Immunol. 1991;21:2559. doi: 10.1002/eji.1830211037. [DOI] [PubMed] [Google Scholar]
- 2.Holliday MR, Banks EMS, Dearman RJ, Kimber I, Coleman JW, et al. Interactions of IFN-γ with IL-3 and IL-4 in the regulation of serotonin and arachidonate release from mouse peritoneal mast cells. Immunology. 1994;82:70. [PMC free article] [PubMed] [Google Scholar]
- 3.Eastmond NC, Banks EMS, Coleman JW, et al. Nitric oxide inhibits IgE-mediated degranulation of mast cells and is the principal intermediate in IFN-γ-induced suppression of exocytosis. J Immunol. 1997;159:1444. [PubMed] [Google Scholar]
- 4.Coleman JW, Holliday MR, Kimber I, Zsebo KM, Galli SJ, et al. Regulation of mouse peritoneal mast cell secretory function by stem cell factor, IL-3 or IL-4. J Immunol. 1993;150:556. [PubMed] [Google Scholar]
- 5.Banks EMS, Coleman JW, et al. A comparative study of peritoneal mast cells from mutant IL-4 deficient and normal mice: evidence that IL-4 is not essential for mast cell development but enhances secretion via control of IgE binding and passive sensitization. Cytokine. 1995;8:190. doi: 10.1006/cyto.1996.0027. [DOI] [PubMed] [Google Scholar]
- 6.Takagi M, Koike K, Nakahata T, et al. Antiproliferative effect of IFN-γ on proliferation of mouse connective tissue-type mast cells. J Immunol. 1990;145:1880. [PubMed] [Google Scholar]
- 7.Bissonnette EY, Befus AD, et al. Inhibition of mast cell-mediated cytotoxicity by IFN-α/β and γ. J Immunol. 1990;145:3385. [PubMed] [Google Scholar]
- 8.Swieter M, Ghali WA, Rimmer C, Befus D, et al. Interferon-α/β inhibits IgE-dependent histamine release from rat peritoneal mast cells. Immunology. 1989;66:606. [PMC free article] [PubMed] [Google Scholar]
- 9.Bissonnette EY, Chin B, Befus AD, et al. Interferons differentially regulate histamine and TNF-α in rat intestinal mucosal mast cells. Immunology. 1995;86:12. [PMC free article] [PubMed] [Google Scholar]
- 10.Hamaguchi Y, Kanakura Y, Fujita Y, et al. Interleukin 4 as an essential factor for in vitro clonal growth of murine connective tissue-type mast cells. J Exp Med. 1987;165:268. doi: 10.1084/jem.165.1.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Buckley MG, Williams CMM, Thompson J, Pryor P, Ray K, Butterfield JH, Coleman JW, et al. IL-4 enhances IL-3 and IL-8 expression in a human leukemic mast cell line. Immunology. 1995;84:410. [PMC free article] [PubMed] [Google Scholar]
- 12.Toru HRAC, Nonoyama S, Suzuki K, Tata J, Nakahata T, et al. Induction of the high-affinity IgE receptor (FcεRI) on human mast cells by IL-4. Int Immunol. 1996;8:1367. doi: 10.1093/intimm/8.9.1367. [DOI] [PubMed] [Google Scholar]
- 13.Green LC, Wagner DA, Glogowski J, Skipper PL, Wishnok JS, Tannenbaum SR, et al. Analysis of nitrate, nitrite and [15N]-labelled nitrate in biological fluids. Anal Biochem. 1982;126:131. doi: 10.1016/0003-2697(82)90118-x. [DOI] [PubMed] [Google Scholar]
- 14.Harbrecht BG, Wang SC, Simmons RL, Billear TR, et al. Cyclic GMP and guanylate cyclase mediate lipopolysaccharide-induced Kupffer cell tumor necrosis factor-α synthesis. J Leukoc Biol. 1995;57:297. doi: 10.1002/jlb.57.2.297. [DOI] [PubMed] [Google Scholar]
- 15.Garthwaite J, Southam E, Boulton CL, Nielsen EB, Schmidt K, Mayer B, et al. Potent and selective inhibition of nitric oxide-sensitive guanylyl cyclase by 1H- [1,2,4]oxadiazolo[4,3-a]quinoxalinone. Mol Pharmacol. 1995;48:184. [PubMed] [Google Scholar]
- 16.Moro MA, Russell RJ, Cellek S, et al. cGMP mediates the vascular and platelet actions of nitric oxide: confirmation using an inhibitor of the soluble guanylyl cyclase. Proc Natl Acad Sci USA. 1996;93:1480. doi: 10.1073/pnas.93.4.1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Radi R, Cosgrove TP, Beckman JS, Freeman BA, et al. Peroxynitrite-induced luminol chemiluminescence. Biochem J. 1993;290:51. doi: 10.1042/bj2900051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xia Y, Zweier JL, et al. Superoxide and peroxynitrite generation from inducible nitric oxide synthase in macrophages. Proc Natl Acad Sci USA. 1997;94:6954. doi: 10.1073/pnas.94.13.6954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Beckman JS, Chen J, Ischiropoulos H, Crow JP, et al. Oxidative chemistry of peroxynitrite. Meth Enzymol. 1994;233:229. doi: 10.1016/s0076-6879(94)33026-3. [DOI] [PubMed] [Google Scholar]
- 20.Beckman JS, Beckman TW, Chen J, Marshall PA, Freeman BA, et al. Apparent hydroxyl radical production by peroxynitrite – implications for endothelial injury from nitric oxide and superoxide. Proc Natl Acad Sci USA. 1990;87:1620. doi: 10.1073/pnas.87.4.1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mosmann TR, Coffman RL, et al. Heterogeneity of cytokine secretion patterns and function of helper T cells. Adv Immunol. 1989;46:111. doi: 10.1016/s0065-2776(08)60652-5. [DOI] [PubMed] [Google Scholar]
- 22.Moncada S, Palmer RMJ, Higgs EA, et al. Nitric oxide: its physiology, pathology and pharmacology. Pharm Rev. 1991;43:109. [PubMed] [Google Scholar]
- 23.Kröncke K-D, Fehsel K, Kolb-Bachofen V, et al. Inducible nitric oxide synthase and its product nitric oxide, a small molecule with complex biological activities. Biol Chem Hoppe-Seyler. 1995;376:327. doi: 10.1515/bchm3.1995.376.6.327. [DOI] [PubMed] [Google Scholar]
- 24.Barnes PJ, Liew FY, et al. Nitric oxide and asthmatic inflammation. Immunol Today. 1995;16:128. doi: 10.1016/0167-5699(95)80128-6. [DOI] [PubMed] [Google Scholar]
- 25.Lincoln J, Hoyle CHV, Burnstock G, et al. Nitric Oxide in Health and Disease. Cambridge University Press: Cambridge; 1997. [Google Scholar]
- 26.Stuehr DJ, Marletta MA, et al. Induction of nitrite/nitrate synthesis by BCG infection, lymphokines or interferon-γ. J Immunol. 1987;139:518. [PubMed] [Google Scholar]
- 27.Ding AH, Nathan CF, Stuehr DJ, et al. Release of reactive nitrogen intermediates and reactive oxygen intermediates from mouse peritoneal macrophages. Comparison of activating cytokines and evidence for independent production. J Immunol. 1988;141:2407. [PubMed] [Google Scholar]
- 28.Liew FYLIY, Severn A, Millot S, Schmidt J, Salter M, Moncada S, et al. A possible novel pathway of regulation by murine T-helper type-2 (Th2) of a Th1 cell-activity via the modulation of the induction of nitric oxide synthase on macrophages. Eur J Immunol. 1991;21:2489. doi: 10.1002/eji.1830211027. [DOI] [PubMed] [Google Scholar]
- 29.Bogdan C, Nathan C, et al. Modulation of macrophage function by transforming growth factor beta, interleukin-4 and interleukin-10. Ann N Y Acad Sci. 1993;685:713. doi: 10.1111/j.1749-6632.1993.tb35934.x. [DOI] [PubMed] [Google Scholar]
- 30.Bissonnette EY, Hogaboam CM, Wallace JL, Befus AD, et al. Potentiation of tumor necrosis factor-α-mediated cytotoxicity of mast cells by their production of nitric oxide. J Immunol. 1991;147:3060. [PubMed] [Google Scholar]
- 31.Hogaboam CM, Befus AD, Wallace JL, et al. Modulation of rat mast cell reactivity by IL-1β. Divergent effects on nitric oxide and platelet-activating factor release. J Immunol. 1993;151:3767. [PubMed] [Google Scholar]
- 32.Salvemini D, Masini E, Anggard E, Mannaioni PF, Vane J, et al. Synthesis of a nitric oxide-like factor from l-arginine by rat serosal mast cells: stimulation of guanylate cyclase and inhibition of platelet aggregation. Biochem Biophys Res Commun. 1990;169:596. doi: 10.1016/0006-291x(90)90372-t. [DOI] [PubMed] [Google Scholar]
- 33.Salvemini D, Masini E, Pistelli A, Mannaioni PF, Vane J, et al. Nitric oxide: a regulatory mediator of mast cell reactivity. J Cardiovas Pharmacol. 1991;17(Suppl. 3):S258. [Google Scholar]
- 34.Bidri M, Ktorza S, Vouldoukis I, et al. Nitric oxide pathway is induced by FcεRI and up-regulated by stem cell factor in mouse mast cells. Eur J Immunol. 1997;27:2907. doi: 10.1002/eji.1830271124. [DOI] [PubMed] [Google Scholar]
- 35.Bidri M, Béchere P-A, Le Goff L, et al. Involvement of cyclic nucleotides in the immunomodulatory effects of nitric oxide on murine mast cells. Biochem Biophys Res Comm. 1995;210:507. doi: 10.1006/bbrc.1995.1689. [DOI] [PubMed] [Google Scholar]


