Abstract
Mice injected with Rhodococcus aurantiacus by the intravenous (i.v.) route show neurological disorders, hemiparesis, vertical headshake and turn-round gait after day 7 postinfection (p.i.). Neurological symptoms caused by i.v. inoculation of R. aurantiacus were relieved by treatment with levodopa (l-dopa). R. aurantiacus was isolated from the brain and was found to be completely eliminated at day 7 p.i. Focal encephalitis was mainly observed in the brain stem, and T cells could be isolated from the brain after day 7 p.i. Administration of both an anti-CD4 monoclonal antibody (mAb) and an anti-CD8 mAb suppressed neurological symptoms. These results suggest that R. aurantiacus induces movement disorders in mice, and that the symptoms are mediated by T cells infiltrating the brain, rather than directly by the bacterium.
INTRODUCTION
Infectious diseases cause movement disorders when the inflammation occurs in the central nervous system (CNS). In particular, it is reported that symptoms of Parkinson's disease sometimes appear after infection of the CNS. This is called postencephalitis parkisonism. Nocardia induces encephalitis in the mouse, and often causes movement disorders. This complication is improved by levodopa (l-dopa).1 Kohbata et al. reported that a movement disorder was observed after the elimination of Nocardia.1 This suggests that the neurological symptoms are caused by inflammation induced by the bacterium, rather than by a direct destructive mechanism of the bacterium.
We also observed movement disorders in mice infected with Rhodococcus aurantiacus. R. aurantiacus is closely related to the genera Nocardia.2 Intravenous injection of R. aurantiacus into mice causes a generalized granulomatous disease resembling sarcoidosis.3 Granuloma formation dependent on T cells and the production of cytokines was observed in the lung, liver, lymph nodes and spleen in the infected mice.2 The infected mice did not show apparent symptoms of infectious diseases, and bacterial proliferation was not detected. Therefore, we speculate that the bacteria invading the brain through intravenous (i.v.) infection cause inflammation and induce a movement disorder.
The cause of movement disorders like Parkinson's disease is unknown. At present, the therapy for such movement disorders is mainly supplementation with l-dopa in response to the decrease in the number of dopaminergic neurons. However, it is possible that some movement disorders are caused by infectious and/or inflammatory diseases in the brain, and these symptoms might be treatable by anti-inflammatory therapy.
We therefore analysed the mechanism of the movement disorder induced by R. aurantiacus infection in mice.
MATERIALS AND METHODS
Mice
Five-week-old female ddY mice (obtained from SLC, Hamamatsu, Shizuoka, Japan) were used in this study.
Administration of L-dopa
The infected mice were administered (i.v.) 2·5 mg/kg/day l-dopa in 0·2 ml of saline from day 3 postinfection (p.i.) to day 30 p.i. The control mice were treated with 0·2 ml of saline.
Determination of numbers of bacteria in the brain
The brain was suspended in saline and 10% (wt/vol) organ homogenates were prepared with a Dounce grinder. The number of viable R. aurantiacus in the infected mouse brain was counted by plating serial 10-fold dilutions of organ homogenates in saline on nutrient agar plates (Nissui Pharmaceutical Co. Ltd, Tokyo, Japan). Colonies on the plates were routinely counted 42–48 hr later.
Histology
Brains and livers were evaluated histologically on day 20 p.i. using formalin-fixed paraffin sections stained with haematoxylin and eosin. The size of each granuloma in the liver was measured using an ocular micrometer. The mean granuloma size was determined from the size of granulomas in 10 randomly selected optical fields of each section.
Isolation of mononuclear cells (MNC) from brain tissue and subsequent flow cytometric analysis
The method of isolation of MNC from brain tissue was as reported previously.4 After the infected mice were killed by perfusion of phosphate-buffered saline (PBS) through the heart, the brain was removed. The brain tissues of five mice were dissociated by passage through 100-mesh stainless steel screens and resuspended in RPMI-1640 medium. Dissociated tissues were centrifuged for 10 min at 200 g and resuspended in 4 ml of 70% Percoll (Pharmacia, Santa Ana, CA) in PBS at 24°. Fractions from the gradients were collected by puncturing a hole in the bottom of the tube.
For flow cytometric analysis (Becton-Dickinson, San Jose, CA) of the distribution of the CD4+ and CD8+ cells, MNC were stained using a phycoerythrin (PE)-conjugated anti-L3T4 monoclonal antibody (mAb) (Becton-Dickinson) and a fluorescein isothiocyanate (FITC)-conjugated anti-Lyt-2 mAb (Becton-Dickinson).
In vivo depletion of T cells
Hybridoma cells secreting mAbs directed against CD4 (L3T4) (GK1.5, rat immunoglobulin G2b (IgG2b), purchased from the American Type Culture Collection (ATCC), Rockville, MD)5 and CD8 (Lyt2) (53-6.72, rat IgG2a, purchased from the ATCC)6 were injected intraperitoneally (i.p.) into pristane-primed CD1 nu/nu mice. Ascitic fluids containing the mAbs were collected from the mice and the mAbs were partially purified by 50% (NH4)2SO4 precipitation followed by exhaustive dialysis against PBS.
Mice were given a single i.v. injection of anti-CD4mAb and/or anti-CD8 mAb (400 μg in 0·2 ml pyrogen-free saline) on day 3 p.i. As controls, normal rat globulin (NRG), rat IgG2a and IgG2b (Bio Pure, Bubendarf, Switzerland) were injected.
Statistical analysis
In the experiment on the inhibition of neurological symptoms by administration of l-dopa, anti-CD4 mAb and anti-CD8 mAb, the Wilcoxon and Cox–Mantel tests were used to analyse statistical significance of results.
RESULTS
Neurological symptoms in the mice infected with R. aurantiacus and the effect of L-dopa
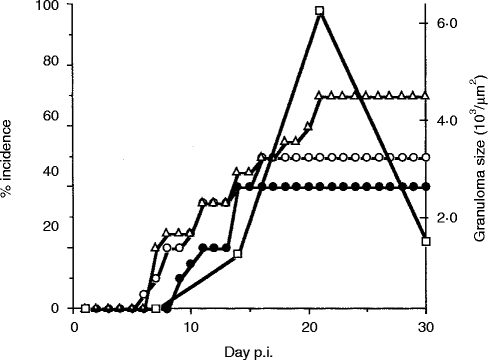
The mice infected with R. aurantiacus showed hemiparesis and vertical headshake after day 7 p.i. Hemiparesis was observed in 70%, vertical headshake in 50% and turn-round gait in 65% [AC4] of the infected mice (Fig. 1). The non-paralytic mice did not show any other neurological symptoms. Granulomas in the liver grew to maximum size by 3 weeks p.i. (Fig. 1).
Figure 1.
Movement disorders and granuloma formation in the liver during the course of R. aurantiacus infection. Each group used for the observation of movement disorders comprised 20 mice. Each group used for the measurement of granuloma formation comprised 20 mice, which were killed at the indicated time-points. Open circles, vertical headshake; closed circles, turn-round gate; open triangles, hemiparesis; open squares, granuloma formation in the liver. Both the peak of the incidence of movement disorders and granuloma formation were detected at 3 weeks p.i.
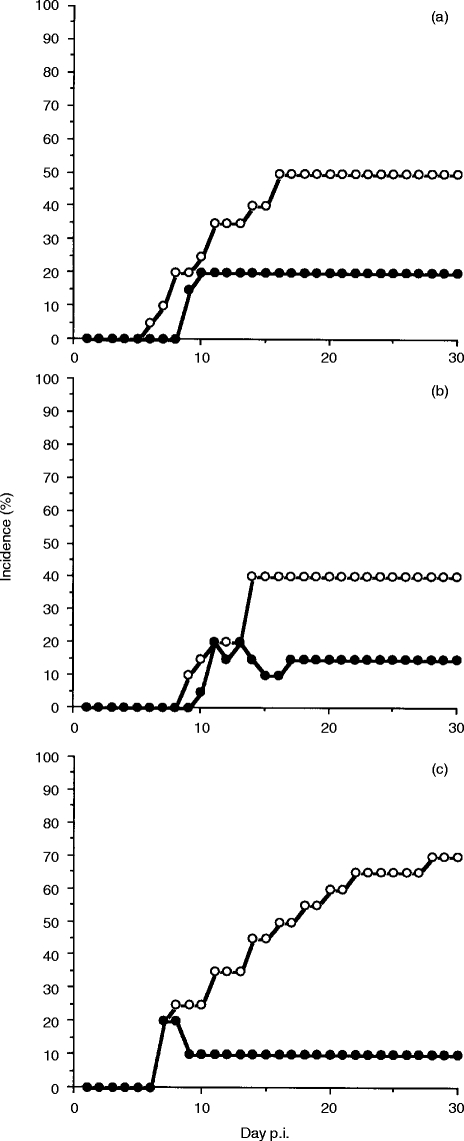
l-dopa administration significantly relieved the neurological symptoms of the infected mice (Fig. 2). Fifty per cent of the control mice showed vertical headshake compared with 20% of the l-dopa-treated mice (Fig. 2a, P <0·01), 40% of the control mice showed turn-round gate compared with 15% of the l-dopa-treated mice (Fig. 2b, P <0·01) and 70% of the control mice showed hemiparesis compared with 10% of the l-dopa-treated mice (Fig. 2c, P <0·01).
Figure 2.
Movement disorder rate during the course of R. aurantiacus infection in mice treated with l-dopa. Each group comprised 20 mice. (a) Vertical headshake; (b) turn-round gate; and (c) hemiparesis. Open circles, control animals; closed circles, l-dopa-treated animals. Administration of l-dopa significantly inhibited 60% of vertical headshake (P <0·01), 63% of turn-round gate (P <0·01) and 85% of hemiparesis (P <0·01).
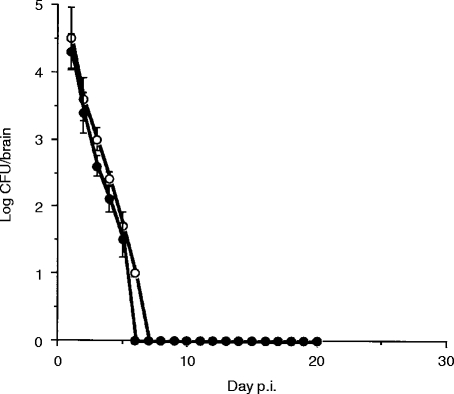
Proliferation and elimination of live bacteria in the infected mouse brain
We examined the proliferation and elimination of R. aurantiacus in the infected mouse brain. The bacteria were isolated from the brains of all infected mice. The number of the bacteria in the brain was highest at day 1 p.i. and fell to an undetectable level within 1 week (Fig 3, open circles). Proliferation of the bacteria was not detected. The number of the bacteria in the brain was not changed by l-dopa administration (Fig. 3, closed circles).
Figure 3.
Kinetics of bacterial numbers in the brain. Open circles, control animals; closed circles; l-dopa-treated animals. Each value is expressed as the average of 10 mice and standard deviation bars are shown. Bacteria were not detected after day 7 p.i. Administration of l-dopa did not affect elimination of the bacteria.
Histological findings in the infected mouse brain
To investigate pathological changes in the brains of the infected mice, we performed a histological study. The infected mice showed generalized focal encephalitis (Fig. 4). The lesions were located in the brain stem (Fig. 4a), putamen (Fig. 4b), hippocampus (Fig. 4c) and cerebellum (Fig. 4d). Lesions of the spinal cord were not detected and neither were Lewy-like bodies, apparent neuronal destruction and gliosis, nor meningitis, abcesses and granuloma. l-dopa administration did not show any anti-inflammatory effect histologically (data not shown).
Figure 4.
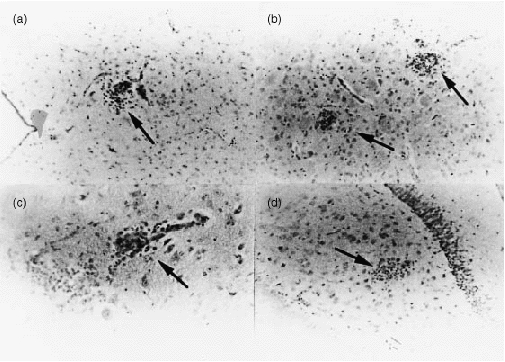
Histological examination of the infected mouse brain on day 14 p.i. The infected mice showed focal encephalitis. The lesions were located in the brain stem (a) (arrow, ×200), putamen (b) (arrow, ×200), substantia nigra (c) (×400) and cerebellum (d) (arrow, ×400).
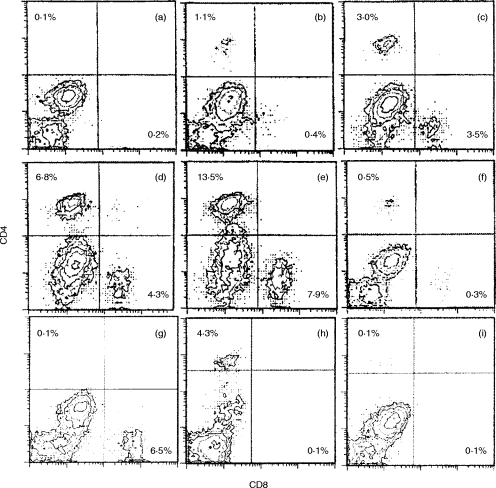
Flow cytometric analysis (FACS) of T cells infiltrating the infected mouse brain
R. aurantiacus elicits T-cell-mediated inflammation and granuloma formation. We therefore examined whether T cells infiltrated the brains of the infected mice. CD4+ cells and CD8+ cells were not detected in the normal mouse brain (Fig. 5a). In the infected mice, CD4+ cells and CD8+ cells infiltrated the brain from day 5 p.i. to day 30 p.i. (Fig. 5b, 5c, 5d, 5e) and they had disappeared by day 60 p.i.
Figure 5.
FACS analysis of mononuclear cells infiltrating the brain. These cells were stained with PE-conjugated anti-CD4 (GK1.5) and FITC-conjugated anti-CD8 (Lyt-2). Cells separated from the brains of five mice were analysed in each experiment. (a) Normal mice; (b) day 3 p.i.; (c) day 7 p.i.; (d) day 14 p.i.; (e) day 20 p.i.; (f) day 60 p.i.; (g) anti-CD4 mAb-treated mice on day 14 p.i.; (h) anti-CD8 mAb-treated mice on day 14 p.i.; and (i) anti-CD4 and anti-CD8 mAb-treated mice on day 14 p.i. The percentage of CD4-positive cells is shown in the upper left quadrant and at the lower right, that of CD8-positive cells. The number of T cells infiltrating the brain peaked on day 20 p.i. T cells in the brain had almost disappeared by day 60 p.i.
Administration of anti-CD4 mAb and anti-CD8 mAb inhibited the neurological symptoms of the infected mice
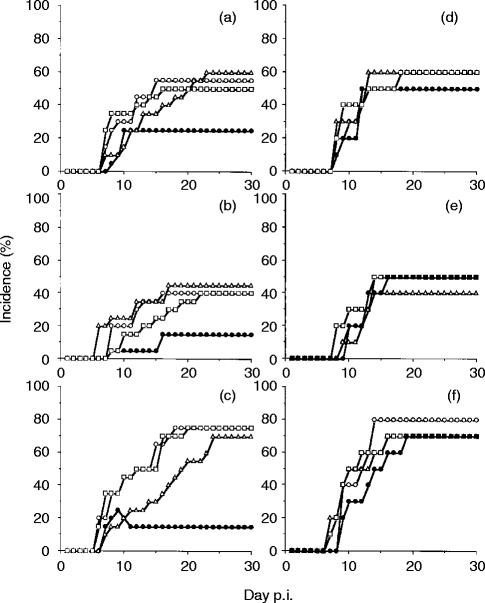
To define the role of T cells in the movement disorders of the infected mice, we administered anti-CD4 mAb and anti-CD8 mAb. The administration of both anti-CD4 and anti-CD8 mAbs, which depleted the T cells, reduced the neurological symptoms (Fig. 6, closed circles). Anti-CD4 and/or anti-CD8 mAb treatment inhibited infiltration of CD4+ cells and/or CD8+ cells on day 14 p.i. (Fig. 5g, 5hr, 5i). Fifty-five per cent of the control mice showed vertical headshake compared with 25% of anti-CD4 and anti-CD8 mAb-treated mice (Fig. 6a, P <0·01). Forty per cent of the control mice showed turn-round gate compared with 15% of anti-CD4 and anti-CD8 mAb-treated mice (Fig. 6b, P <0·05). Seventy-five per cent of the control mice showed hemiparesis compared with 20% of the mice treated with both mAbs (Fig. 6c, P <0·01). Neither anti-CD4 mAb (Fig. 6, open squares) nor anti-CD8 mAb (Fig. 6, open triangles) when used alone suppressed the symptoms.
Figure 6.
(a–c) Movement disorder rate during the course of R. aurantiacus infection in mice treated with NRG (open circles, control), anti-CD4 (open squares), anti-CD8 (open triangles) and both mAbs (closed circles). (a) Vertical headshake; (b) turn-round gate; and (c) hemiparesis. Each group comprised 20 mice. Only simultaneous administration of anti-CD4 and anti-CD8 mAb significantly inhibited the appearance of movement disorders: (a), P <0·01; (b), P <0·05; and (c) P <0·01. (d–f) Infected mice treated with NRG (open circles), rat IgG2b (open squares), rat IgG2a (open triangles) and both rat IgG2a and IgG2b (closed cicles). (d) Vertical headshake; (e) turn-round gate; and (f) hemiparesis. Each group comprised 10 mice. Treatment with isotypic immunoglobulin did not affect the appearance of movement disorders.
DISCUSSION
Encephalitis caused by micro-organisms sometimes induces a movement disorder, resembling parkisonism, called postencephalitic parkisonism, although its frequency is low. However, encephalitis caused by specific agents, for example Economo encephalitis7 and Nocardia infection,1 frequently induces movement disorders, Encephalitis induced by R. aurantiacus infection also frequently causes movement disorders (Fig. 1). These movement disorders can be relieved by l-dopa (Fig. 2) and the lesions also exist in the nigro-striatal pathway (Fig. 4). The mechanisms that elicit movement disorders in these infectious diseases have not been reported. In R. aurantiacus infection, the appearance of movement disorders was observed after the elimination of the bacteria from the brain (Fig. 1 and Fig. 3) and coincided with granuloma formation, mediated by T cells in the liver (Fig. 1). Moreover, movement disorders were induced after T cells infiltrated the brain (Fig. 1 and Fig. 5), and depletion of both CD4+ cells and CD8+ cells relieved the symptoms (Fig. 6). These results suggest that encephalitis caused by R. aurantiacus is also a T-cell-mediated inflammatory disease rather than an infectious disease. We previously reported that depletion of CD8+ cells suppressed granuloma formation by R. aurantiacus, and depletion of CD4+ cells inhibited granuloma regression without a change of bacterial growth.8 The infected mice did not show symptoms of infection, for example, wasting. Therefore, infection with R. aurantiacus in the other organs is also a T-cell-mediated inflammatory disease. T cells stimulated by R. aurantiacus could infiltrate the brain and cause damage to the CNS, eliciting neurological symptoms.
T-cell-mediated inflammatory diseases exist in the CNS, for instance, multiple sclerosis9 and human T-cell lymphocytotrophic virus-1 (HTLV-1)-associated myelopathy.10 In Parkinson's disease, it is reported that the number of CD45RO+ T cells increase in the cerebrospinal fluid.11 However, it is not reported that T-cell-mediated inflammation directly causes movement disorders resembling parkisonism. On the other hand, infectious diseases in the brain sometimes cause movement disorders. The lesions in some types of infectious encephalitis could be distributed like those in Parkinson's disease, and lesions of infectious disease are always accompanied by inflammation. The inflammatory reaction in infection causes harm to the infected tissue. How do T cells infiltrating the brain induce movement disorders? First, the destructive mechanism of T cells acts via neuronal cells. T cells infiltrating the brain in R. aurantiacus infection will specifically react with the bacteria, but not with neuronal cells. Moreover, neurons do not directly react with T cells because of their low expression of major histocompatibility complex (MHC) molecules.12 Therefore, T cells would not directly destroy the neuronal cells. The bystander effect of T cells harms the neuronal cells. The destructive mechanism of cytokines produced by T cells is probably important in such cases. In the present study, only anti-CD4 and anti-CD8 mAb together suppressed neurological symptoms. We speculated that common cytokines produced by both CD4+ cells and CD8+ cells could induce movement disorders. For instance, tumour necrosis factor (TNF) is able to provoke cell death and is detectable in the spleen, liver and lungs of mice infected with R. aurantiacus (M. Asano, M. Kohanawa, T. Minagawa, unpublished data). It is reported that TNF can be produced by both CD4+ cells and CD8+ cells,13 and can destroy neurons in Alzheimer disease.14 Therefore, TNF produced by T cells infiltrating the brain would destroy neurons. Second, it is possible that injury of glial cells by T cells indirectly harms neurons because glial cells produce a neurotropic factor, which is necessary to support functions of neurons. Third, possibly a factor produced by T cells, other than cytokines, induces movement disorders. It is reported that T cells produce neuropeptides15 and neurotropic factors.16 These factors might influence the activity of the neurons, although whether T cells produce such a factor remains uncertain.
REFERENCES
- 1.Kohbata S, Beaman BL. l-dopa-responsive movement disorder caused by Nocardia asteroides localized in the brains of mice. Infect Immun. 1991;59:181. doi: 10.1128/iai.59.1.181-191.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Asano M, Nakane A, Kohanawa M, Minagawa T. Sequential involvement of NK cells and CD8+ T cells in granuloma formation of R. aurantiacus-infected mice. Microbiol Immunol. 1995;39:499. doi: 10.1111/j.1348-0421.1995.tb02234.x. [DOI] [PubMed] [Google Scholar]
- 3.Asano M, Nakane A, Minagawa T. Endogenous gamma interferon is essential in granuloma formation induced by glycolipid- containing mycolic acid in mice. Infect Immun. 1993;61:2872. doi: 10.1128/iai.61.7.2872-2878.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clatch RJ, Miller SD, Metzner R, Dal Canto MC, Lipton HL. Monocytes/macrophages isolated from the mouse central nervous system contain infectious Theiler's murine encephalomyelitis virus (TMEV) Virology. 1990;176:244. doi: 10.1016/0042-6822(90)90249-q. [DOI] [PubMed] [Google Scholar]
- 5.Dialynas DP, Quan ZS, Wall KA, et al. Characterization of the murine T cell surface molecule, designated L3T4, to the human Leu-3/T4 molecule. J Immunol. 1983;131:2445. [PubMed] [Google Scholar]
- 6.Ledbetter JA, Herzenberg LA. Xenogenic monoclonal antibodies to mouse lymphoid differentiation antigens. Immunol Rev. 1979;47:63. doi: 10.1111/j.1600-065x.1979.tb00289.x. [DOI] [PubMed] [Google Scholar]
- 7.Cheyette SR, Cummings JL. Encephalitis lethargica: lessons for contemporary neuropsychiatry. J Neuropsychiatr Clin Neurosci. 1995;7:125. doi: 10.1176/jnp.7.2.125. [DOI] [PubMed] [Google Scholar]
- 8.Asano M, Kohanawa M, Minagawa T, Nakane A. Reciprocal action of interferon-γ and interleukin-4 promotes granulomatous inflammation induced by Rhodococcus aurantiacus in mice. Immunology. 1996;88:394. doi: 10.1046/j.1365-2567.1996.d01-660.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hafler DA, Weiner HL. MS: a CNS and systemic autoimmune disease. Immunol Today. 1989;10:104. doi: 10.1016/0167-5699(89)90236-3. [DOI] [PubMed] [Google Scholar]
- 10.Elovaara I, Koenig S, Brewah AY, Woods RM, Lehky T, Jacobson S. High human T cell lymphotropic virus type 1 (HTLV-1) -specific precursor cytotoxic T lymphocyte frequencies in patients with HTLV-1-associated neurological disease. J Exp Med. 1993;177:1567. doi: 10.1084/jem.177.6.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fiszer U, Mix E, Fredrikson S, Kostulas V, Link H. Parkinson's disease and immunological abnormalities: increase of HLA-DR expression on monocytes in cerebrospinal fluid and of CD45RO+ T cells in peripheral blood. Acta Neurol Scand. 1994;90:160. doi: 10.1111/j.1600-0404.1994.tb02699.x. [DOI] [PubMed] [Google Scholar]
- 12.Joly E, Oldstone MB. Neuronal cells are deficient in loading peptides onto MHC class I molecules. Neuron. 1992;8:1185. doi: 10.1016/0896-6273(92)90138-4. [DOI] [PubMed] [Google Scholar]
- 13.Andersson U, Adolf G, Dohlsten M, Moller G, Sjogren HO. Characterization of individual tumor necrosis factor alpha- and beta-producing cells after polyclonal T cell activation. J Immunol Methods. 1989;123:233. doi: 10.1016/0022-1759(89)90227-5. [DOI] [PubMed] [Google Scholar]
- 14.Meda L, Cassatella MA, Szendrei GI, et al. Activation of microglial cells by beta-amyloid protein and interferon-gamma. Nature. 1995;374:647. doi: 10.1038/374647a0. [DOI] [PubMed] [Google Scholar]
- 15.Kogner P, Ericsson A, Barbany G, Persson H, Theodorsson E, Bjork O. Neuropeptide Y (NPY) synthesis in lymphoblasts and increased plasma NPY in pediatric B-cell precursor leukemia. Blood. 1992;80:1324. [PubMed] [Google Scholar]
- 16.Ehrhard PB, Erb P, Graumann U, Otten U. Expression of nerve growth factor and nerve growth factor receptor tyrosine kinase Trk in activated CD4-positive T-cell clones. Proc Natl Acad Sci USA. 1993;90:10984. doi: 10.1073/pnas.90.23.10984. [DOI] [PMC free article] [PubMed] [Google Scholar]