Abstract
The mouse monoclonal antibody MR6 recognizes a 200 000 MW protein (gp200-MR6), which is expressed highly on human thymic cortical epithelial cells. The antigen is also expressed on some epithelial tumours and we have previously shown that MR6 inhibits the proliferation of the colon carcinoma cell lines HT29. However, the role of this molecule in the thymus is not known. In order to generate reagents that could be used in murine thymic functional studies we isolated antibodies specific to human gp200-MR6, using a phage display library expressing single-chain (sFv) antibodies. Three independent clones were isolated by panning with purified protein and their specificity was confirmed by immunohistochemistry, Western blotting and flow cytometry. In addition to human thymus, these phage antibodies also recognized the homologous antigen in mouse, pig and other species. Expressed as soluble sFv one of these clones inhibited the proliferation of HT29 cells and a mouse thymic epithelial cell line, suggesting that this antibody exhibits similar functional activity to MR6. In fetal thymic organ culture, thymocytes recovered from thymic lobes cultured in the presence of this sFv, were reduced in number fivefold compared with the control and the majority remained at the double-negative stage of development. These data indicate that gp200-MR6 plays an important role in thymocyte development. In addition, this is the first report to demonstrate that specific sFv can be used to study, and alter, thymic development. This work also highlights the advantage of phage antibody technology in selecting such reagents for functional assays.
INTRODUCTION
The monoclonal antibody (mAb) MR6 was raised against human thymic stromal cells; it shows strong labelling of the cortical epithelium1 and much weaker labelling of macrophages, lymphocytes and dendritic cells.2 Immunoelectron microscopy has revealed that this labelling is localized predominantly on the surface of epithelial cells.3 Biochemical analysis by either immunoprecipitation or Western blotting has shown that mAb MR6 recognizes a single glycoprotein chain of 200 000 MW (gp200-MR6).4
Our in vitro studies of the peripheral immune system using mAb MR6 have shown that it inhibits interleukin-4 (IL-4)-dependent immunoglobulin class switching to IgE in allergen-stimulated B cells, IL-4-induced proliferation of T-cell clones and expansion of the IL-4-dependent T helper type 2 (Th2) subset.5,6 In addition we have also examined the role of gp200-MR6 on epithelial cells using human colorectal carcinoma cell lines, HT29 and SW1222, and found that either mAb MR6 or IL-4 significantly reduced cell growth.7 Moreover, both these reagents enhanced the crypt-like glandular differentiation of SW122 in three-dimensional collagen gel culture.7 These data therefore suggest that gp200-MR6 may be functionally related to the IL-4 receptor and that ligation of gp200-MR6 at the cell surface has either an antagonistic or agonistic effect according to whether IL-4 is acting as either a growth or a maturation factor, respectively. However, the role of this molecule in the thymus is not known, this is primarily because there is a lack of suitable in vitro thymic assays in the human system in comparison to those that are used in the mouse.8
An alternative strategy to hybridoma technology for the production of mAb is the selection of antibody fragments, such as single-chain fragments (sFv), displayed on the surface of filamentous phage.9–13 The development of libraries expressing antibody variable regions on the surface of filamentous phage and the selection of these recombinant molecules, with a range of binding activities and specificities, offers a powerful approach of generating antibodies without immunization.9–13 This technique therefore offers the potential to isolate antibodies that recognize evolutionarily conserved protein determinants, which may have a functional effect on biological assays from different species; as shown recently in studies with the human hepatocyte growth factor/scatter factor (HGF/SF).14 We therefore reasoned that isolation of phage antibodies specific to human gp200-MR6, which recognize evolutionarily conserved determinants and are then shown to have functional activity, should provide the opportunity to study this molecule in the murine thymus.
In this paper we describe the isolation and characterization of phage antibodies against purified human gp200-MR6, and demonstrate their reactivity not only to human thymus, but also to the thymus from several other species. Moreover, we show that soluble sFv from one of these phage antibodies exhibits functional activity on human and mouse epithelial cell lines, by reducing cell proliferation, and also disrupts thymocyte development in mouse fetal thymic lobes in vitro. Thus gp200-MR6 is likely to play an important role in thymic development. Our data also illustrate the advantage of phage display technology to select antibodies to evolutionarily conserved epitopes, and demonstrate, for the first time, that specific sFv can be used to study, and manipulate, intrathymic function.
MATERIALS AND METHODS
Antigen
The human cortical antigen gp200-MR6 was affinity purified from human thymus tissue lysate as previously described.15 Human paediatric thymus samples were obtained from patients undergoing open heart surgery (Great Ormond Street Hospital, London). Briefly, protein A-purified mAb MR6 was cross-linked to agarose AminoLink® beads (Pierce and Warriner, Chester, UK) according to the manufacturer’s instructions. Thymic lysate was incubated at 4° with the column for 18 hr. The column was washed extensively with phosphate-buffered saline (PBS), the gp200-MR6 was eluted using 0·1 m glycine pH 3·0 and neutralized with 1 m Tris–HCl, pH 9·0. Protein was analysed by sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS–PAGE)16 on a 7·5% gel which was then immunoblotted.
Selection of phage antibody
A ‘single pot’ human semi-synthetic antibody phage display library as described by Nissim et al.10 (a kind gift of Dr G. Winter, Cambridge, UK) was used. The library was rescued with VC3M13 helper phage (Stratagene, Cambridge, UK) and phage particles were purified using polyethylene glycol (PEG).17 The selection of phage antibody and phage enzyme-linked immunosorbent assay (ELISA) has been described in detail.17 Briefly, immunotubes (Nunc Maxisorp, Life Technologies, Paisley, UK) were coated with 10 μg/ml of gp200-MR6 protein in 50 mm NaHCO3. Approximately 1013 transducing units (TU) of phage particles were PEG precipitated, resuspended in 5 ml PBS containing 2% Marvel (Premier Beverages, Stafford, UK), and added to the antigen-coated tube. After 2 hr at room temperature, the tube was washed extensively with PBS/0·1% Tween and finally with PBS. Bound phage were eluted and incubated with log-phase TG1 Escherichia coli as previously described.17 The library was subjected to a further five rounds of panning.
The specificity of isolated clones, after the fourth round of panning, was assessed by phage ELISA as described previously.17 Purified gp200-MR6 was coated overnight on polyvinylchloride plates (Nunc), blocked with 2% Marvel/PBS, and 50 μl of bacterial supernatant containing phage antibody was added to each well with an equal volume of 4% Marvel/PBS. Binding of phage antibody to purified gp200-MR6 was detected using a sheep anti-M13 polyclonal antibody (5 Prime-3 Prime, Herts, UK) followed by horseradish peroxidase (HRP)-conjugated rabbit anti-sheep immunoglobulin antibodies (Dako, High Wycombe, UK). Positive clones were further assessed by a cell-based ELISA using the gp200-MR6-positive adherent cell line HT29.18 Approximately 104 cells/well were plated into a 96-well plate (Nunc) and allowed to adhere overnight. Cells were fixed with paraformaldehyde18 and the ELISA was performed as described above.
To determine the exact number of independent clones selected, DNA fingerprinting analysis using the restriction endonuclease Bst NI (Sigma, Poole, UK) was performed as described.17 DNA encoding the variable region of positive clones was amplified by PCR, from the pHEN-1 vector,19 using the primers 5′-CAGTCTATGCGGCCCCATTCA-3′ (pHEN 3′-complementary to the sequence between the gene III and c-myc peptide tag) and 5′-ATGAAATACCTATTGCCTACG-3′ (pel 5′-sequence of the pel B leader). Reactions were performed in a volume of 20 μl at 94° for 30 seconds, 55° for 30 seconds and 72° for 1 min for 30 cycles using Taq DNA Polymerase (Promega, Southampton, UK). The amplified products were visualized on a 1·0% agarose gel and were used for DNA fingerprinting and sequence analysis. The nucleotide sequence encoding sFv was determined using the ABI automated sequencer (Perkin Elmer, Warrington, UK) and was analysed using the V-BASE sequence alignment program.20
Soluble sFv were produced by infecting E. coli non-suppressor strain SF110 (a kind gift of Dr J. de Kruif, University Hospital Utrecht, the Netherlands) with the appropriate phagemid; the bacteria were induced to express sFv in 1 l of bacterial supernatant with 1 mm isopropyl β-d-thiogalactoside.17 After centrifugation of the bacterial pellet, the supernatant was filtered using a 0·45-μm filter unit (Pall Gelman Sciences, Northampton, UK) and the sFv was then purified on protein A–Sepharose (Pharmacia, St. Albans, UK) as described.22 The sFv was eluted with 0·1 m glycine, 0·15 m NaCl pH 2·8 and neutralized with 1 m Tris–HCl, pH 9·0, dialysed against PBS for at least 24 hr and analysed by SDS–PAGE. Protein concentration was determined using the bicinchoninic acid (BCA) procedure.23 An anti-(4-hydroxy-5-iodo-3-nitrophenylacety) (anti-NIP) clone selected from this library,9 which uses the VH segment DP-47, that belongs to the VH3 family, was used as a negative control either expressed as phage antibody or soluble sFv in this study.
Western blotting
Purified gp200-MR6 or tissue lysates, prepared from frozen sections of mouse and pig thymus as previously described,15 were run on a 7·5% polyacrylamide gel and then electroblotted. Phage antibody was used to detect the homologous antigen in mouse and pig lysate and filters were incubated overnight at 4° with phage (1011 TU/ml) in 2% Marvel/PBS as described.9 Detection of purified gp200-MR6 was performed using soluble sFv.9 After washing, bound phage were detected with sheep anti-M13 followed by HRP-rabbit anti-sheep immunoglobulin antibody. For sFv, filters were incubated with the mAb 9E10, which detects the myc tag present on sFv,24 followed by HRP-rabbit anti-mouse immunoglobulin antibody (Dako). Peroxidase activity was detected using an ECL kit (Amersham International, Little Chalfont, UK).
Immunohistochemistry
Five-micrometre sections were cut from frozen thymic tissue, air dried, fixed in acetone and stained using the indirect immunoperoxidase method.25 Staining was performed using 100 μl of phage antibody particles mixed with 25 μl of 10% Marvel/PBS as described.26 Bound phage were detected using sheep anti-M13 polyclonal antibody, followed by HRP-rabbit anti-sheep immunoglobulin antibody. Monoclonal antibody MR6 was used neat from culture supernatant prepared in our laboratory and detected using HRP-rabbit anti-mouse immunoglobulin antibody.
Flow cytometry
Approximately 1×106 cells were preincubated with 100 μl of phage antibody particles mixed with 25 μl of 10% Marvel/PBS.26 Bound phage were detected using sheep anti-M13 polyclonal antibody, followed by fluorescein isothiocyanate (FITC)-conjugated rabbit anti-sheep immunoglobulin antibody (Dako). Labelled cells were analysed using the EPICS Profile flow cytometer (Coulter Electronics, Luton, UK) and the data were processed using EPICS XL software (Coulter Electronics).
Proliferation assay
The mouse thymic cortical epithelial cell line TM25.F127 (a kind gift of Dr D. Kioussis National Institute for Medical Research, UK) was grown in Dulbecco’s modified Eagle’s medium (DMEM) with 4·5 g/l glucose supplemented with 10% fetal calf serum (FCS), penicillin 100 U/ml, streptomycin 0·1 mg/ml and l-glutamine 1 mm (all from Gibco, Paisley, UK) at 37°. The human colorectal carcinoma cell line HT29 was cultured in RPMI-1640 supplemented with 10% FCS, penicillin 100 U/ml, streptomycin 0·1 mg/ml and l-glutamine 1 mm (all from Gibco) at 37°.
The effect of antibodies and IL-4 on the proliferation of epithelial cell lines was performed as previously described.7 Cells were detached with dissociation solution (Sigma), washed and 5×104 (HT29) or 2×104 (TM25.F1) cells/well were incubated in a 96-well plate (Nunc) in a final volume of 100 μl. Cells were plated in triplicate and cultured either in medium alone, or in medium supplemented with either antibodies or IL-4 (100 U/ml) for 24 or 48 hr. The mAb MR6 and isotype-matched control (OKT8) were used at 10 μg/ml. Purified sFv was used at 2 μg/ml (the concentration used was determined by titration). Recombinant human IL-4 was purchased from Genzyme (West Malling, UK). Mouse IL-4 was a kind gift of Professor D. Gray (Department of Immunology, ICSM, Hammersmith Hospital). Cells were pulsed for 8 hr with [3H]thymidine (Amersham International) and the amount of radioactivity incorporated was measured. The results are shown as percentage inhibition of proliferation and the values were estimated by comparing the difference in proliferation of cells cultured in medium supplemented, with the experiment reagent, against cells cultured in medium only.
Fetal thymic organ culture (FTOC)
For FTOC, mouse thymic lobes from day 14 BALB/c embryos were cultured on nucleopore membrane filters28 in either medium (DMEM with 4·5 g/l glucose supplemented with 10% FCS, penicillin 100 U/ml, streptomycin 0·1 mg/ml and l-glutamine 1 mm) only or in medium supplemented with purified sFv (2 μg/ml) for 6 days. Cells were harvested and analysed by flow cytometry using the following fluorochrome-conjugated mAb (all obtained from PharMingen, San Diego, CA): phycoerythrin (PE)-anti-CD4 (clone GK1.5), FITC-anti-CD25 (clone 7D4), and anti-CD8β-biotin (clone 53-6.7) which was revealed with streptavidin–TRICOLOR (Caltag Laboratories, San Francisco, CA).28 Labelled cells were analysed using an EPICS Elite ESP (Coulter Electronics) and the data were processed with CELLQuest Software (Becton Dickinson, Mountain View, CA).
RESULTS
Phage selection
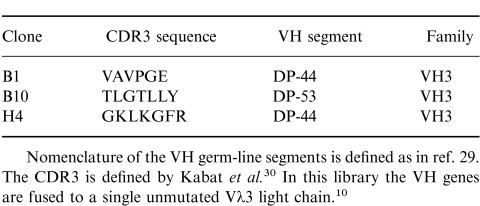
A phage display library expressing human sFv10 was used to isolate antibodies specific for the human thymic cortical epithelial antigen gp200-MR6. In this library a collection of 49 human germline VH gene segments have been cloned into the pHEN1 vector.10 The sequence corresponding to the CDR3 region was randomized in both sequence and length (between four and 12 amino acids) and paired with the single unmutated Vλ3 light-chain gene segment. Phage were selected by panning on antigen-coated immunotubes, and after six rounds of selection over 30 clones specific for gp200-MR6 were identified using ELISA against the purified antigen and a gp200-MR6-expressing cell line HT29 (data not shown). Nucleotide sequence analysis revealed that we had isolated three individual clones specific for gp200-MR6, each containing a distinct CDR3 (Table 1). Two clones (B1, H4) comprised the VH segment DP-44, while the other (B10) used DP-53; both these VH germline gene segments are members of the VH3 family.29,30 Western blotting analysis using purified gp200-MR6 probed with soluble sFv, produced from selected phage antibodies, further confirmed antibody specificity (Fig. 1). In some experiments a doublet was detected, which we have previously observed when using mAb MR6 in Western blotting analysis (our unpublished observations).
Table 1.
Deduced CDR3 amino acid sequence and germ-line VH gene segments used by phage antibodies selected against the human cortical antigen gp200-MR6
Nomenclature of the VH germ-line segments is defined as in ref. 29. The CDR3 is defined by Kabat et al. In this library the VH genes are fused to a single unmutated Vλ3 light chain.
Figure 1.
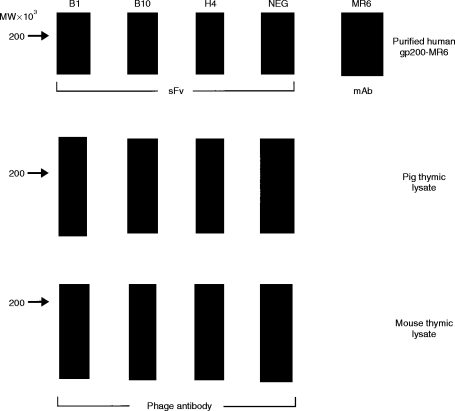
Detection of either purified human gp200-MR6 or the homologous antigen in thymic lysates, from pig and mouse, using specific soluble sFv and phage antibody, respectively. Two micrograms of purified antigen, or thymic lysates were electrophoresed on 7·5% acrylamide gels, electroblotted onto nitrocellulose and probed with either sFv (purified antigen) or phage antibody (pig and mouse thymic lysates). Soluble sFv from the individual clones (B1, B10, H4) isolated after six rounds of panning on antigen, bound specifically to purified human gp200-MR6 in a similar manner to mAb MR6. In contrast, the control anti-NIP sFv (NEG)10 (encoded by DP-47 which belongs to the VH3 family) does not bind to gp200-MR6. This antibody was used in all subsequent experiments as a negative control antibody either as phage or as soluble sFv. Phage antibody specific for gp200-MR6 detected a band at around 200 000 MW on pig and mouse lysate suggesting that these antibodies recognize the homologous antigen in mouse and pig thymus.
Anti-gp200-MR6 phage antibodies recognize evolutionarily conserved epitopes
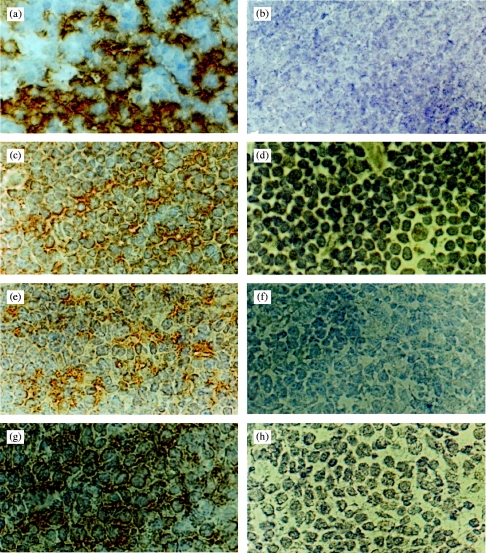
The mAb MR6 shows strong labelling of human cortical epithelium1–3 (Fig. 2). Immunohistochemical analysis revealed that the reactivity of the selected phage antibodies on human thymus showed a similar pattern of staining to that seen with mAb MR6 (Fig. 2), providing further evidence of their specificity. In addition to staining human thymus, we have previously shown that mAb MR6 also reacts with a 200 000 MW protein expressed on rabbit thymic cortical epithelium.31 This observation suggests that the antigen may be evolutionarily conserved. To determine whether the anti-gp200-MR6 phage antibodies also recognize this molecule in thymus from different species, immunohistochemistry was performed. Phage antibodies prepared from the three positive clones, stained mouse and pig thymic sections with a staining pattern similar to that observed with mAb MR6 on human thymus (Fig. 2). The mAb MR6 does not stain the thymus from these animals (data not shown). In addition, the phage antibodies also stained rabbit, chicken and rat thymus with a similar pattern to that observed on human and mouse thymus (data not shown). Western blotting analysis using the selected phage antibodies revealed that they recognized a 200 000 MW molecule in both mouse and pig thymic lysates (Fig. 1). These studies strongly suggest that the anti-gp200-MR6 phage antibodies recognize the homologous antigen from several species, indicating that this molecule is indeed evolutionarily conserved.
Figure 2.
Immunoperoxidase staining of frozen sections of normal human (a–d), mouse (e,f) and pig (g,h) thymus with either the mAb MR6 or the anti-gp200-MR6 specific phage antibody H4. This clone is used as a representative of the anti-gp200-MR6 antibodies isolated, which all gave similar staining results. The anti-gp200-MR6-specific phage antibody H4, labelled cortical epithelium from human (c), mouse (e) and pig (g) thymus with a similar pattern of staining as seen with mAb MR6 (a). The mAb MR6 did not stain mouse or pig thymus (data not shown). An irrelevant phage antibody anti-NIP (d,f,h) and the mAb OKT8 (b) were used as a negative control. Magnification ×800.
Anti-gp200-MR6 phage antibodies can recognize antigen in a native configuration
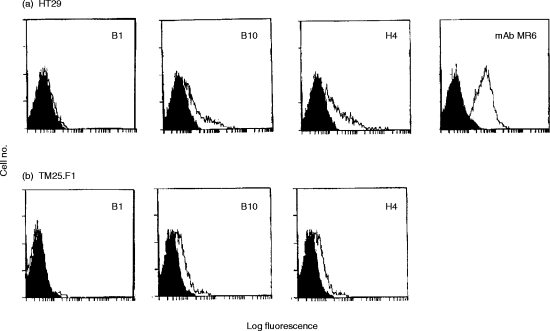
To determine whether the selected antibodies can bind gp200-MR6 present on the cell surface, flow cytometric analysis was performed using the HT29 cell line, which expresses high levels of surface gp200-MR615 (Fig. 3a). HT29 cells were incubated with the phage antibodies specific for gp200- MR6 (B1, B10, H4) and anti-NIP (4-hydroxy-5-iodo-3-nitrophenylacetyl; negative control9). The detection of bound phage on HT29 cells revealed that B10 and H4 sFv recognize gp200-MR6 on the cell surface (Fig. 3a), although at a reduced intensity in comparison to the mAb MR6. Similarly, only B10 and H4 sFv bound significantly to the surface of the mouse thymic cortical epithelial cell line TM25.F127 (Fig. 3b). In contrast, all the anti-gp200-MR6 antibodies prepared either as phage particles or as soluble sFv, bound to cytospin preparations of these cell lines (data not shown). This study revealed that the phage antibodies B10 and H4 can recognize native gp200-MR6 expressed on the cell surface, while B1 recognized an epitope that is either internal or requires denaturation. In addition, similarity in the pattern of reactivity of these phage antibodies on both mouse and human epithelial cell lines again suggests that they recognize the homologous antigen in these species.
Figure 3.
Flow cytometric analysis of the human colon carcinoma cell line HT29 cells (a), the mouse thymic cortical epithelial cell line TM25.F1 (b) using either the mAb MR6 or anti-gp200-MR6-specific phage antibodies (B1, B10, H4). The shaded area represents background using the negative control antibody (OKT8 for MR6 or anti-NIP for phage antibodies). The mAb MR6 did not stain the TM25.F1 cell lines (data not shown).
Purification of anti-gp200-MR6 sFv
For functional studies, we purified the antigen-specific soluble sFv (B1, B10 and H4) and the negative control sFv (anti-NIP) using protein A. Protein A binds to human VH domains that are encoded by genes belonging to the VH3 family,32 and therefore sFv can be purified directly from bacterial supernatant. Eluted fractions were analysed by SDS–PAGE, transferred to a membrane and probed with the mAb 9E10, which detects the myc tag present on sFv (Fig. 4).24 The yield of sFv was determined to be ≈0·1 mg/l after purification.
Figure 4.
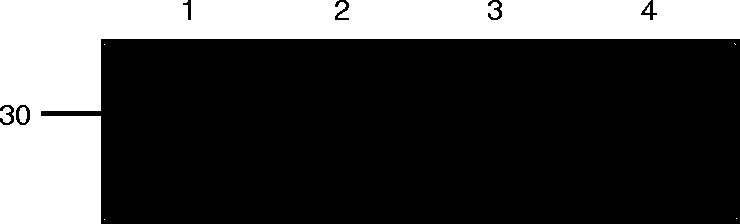
Immunoblot of a 15% SDS–PAGE analysis showing purified sFv proteins using protein A chromatography (lane 1, anti-NIP; lane 2, B1; lane 3, B10; and lane 4, H4). Single-chain Fv was purified from bacterial supernatant and eluted protein was visualized with the mouse mAb 9E10, anti-myc tag,24 followed by rabbit anti-mouse peroxidase. The position of 30 000 MW is indicated on the left.
Anti-gp200-MR6 sFv inhibit both human and mouse epithelial cell proliferation
We have previously shown that the mAb MR6 inhibits the proliferation of the colon carcinoma cell line HT29 (Fig. 5a).7 Therefore, to determine whether the gp200-MR6-specific antibodies exhibit a similar functional activity to the mAb MR6, we incubated these antibodies, prepared as soluble sFv, with HT29 cells. In the presence of H4 sFv, the proliferation of HT29 cells was reduced to a level similar to that seen with the mAb MR6 and human IL-4 (Fig. 5a). In contrast, neither B1 nor B10 sFv inhibited HT29 proliferation (Fig. 5a).
Figure 5.
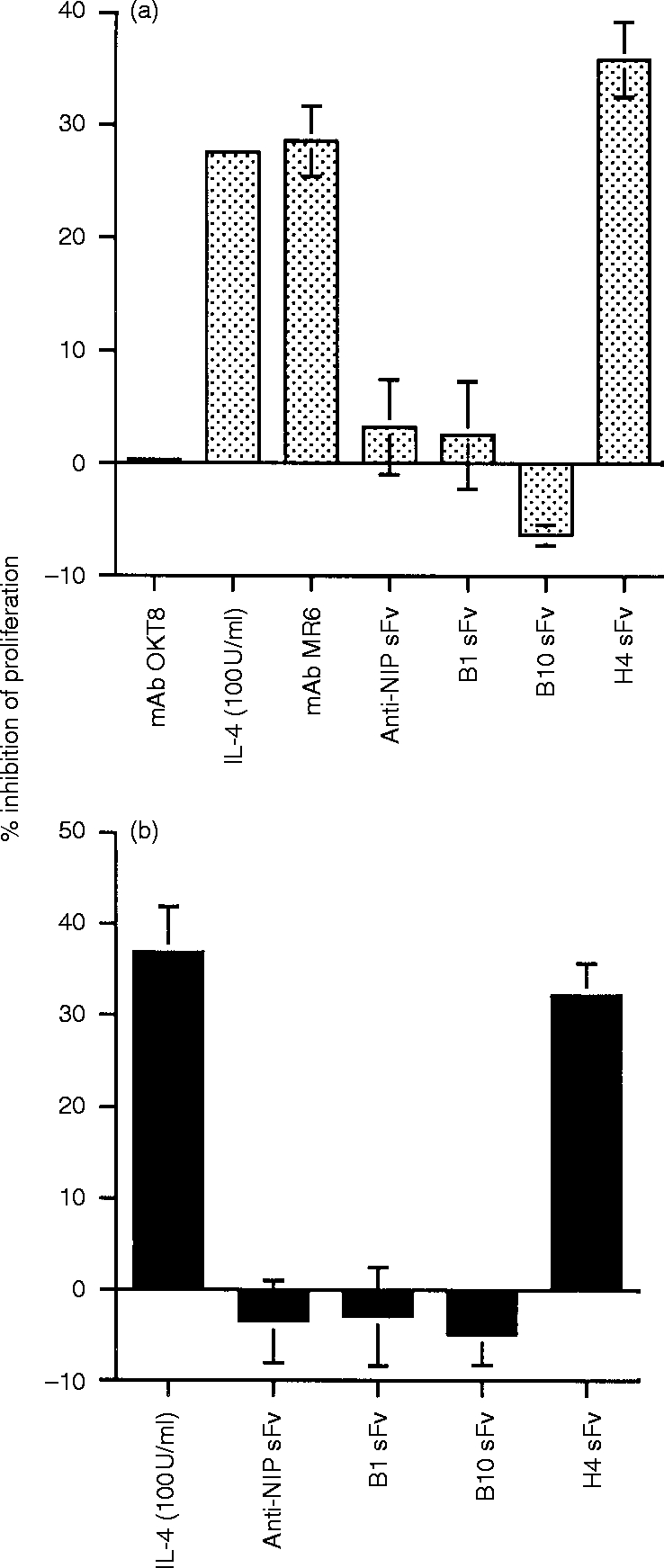
The effect of mAb MR6, purified sFv and IL-4 on the proliferation of the human colon carcinoma cell line HT29 (a) and the mouse thymic cortical epithelial cell line TM25.F1 (b). HT29 cells were cultured either in medium alone or in medium supplemented with the experimental reagent for 48 hr and pulsed with [3H]thymidine for a further 8 hr and the amount of radioactivity (c.p.m.) incorporated was measured. The mAb MR6 and isotype-matched control OKT8 were used at 10 μg/ml. Recombinant human IL-4 was used at 100 U/ml. Purified sFv was used at 2 μg/ml. TM25.F1 cells were cultured either in medium alone or in medium supplemented with the experimental reagent for 24 hr and pulsed with [3H]thymidine for a further 8 hr and the amount of radioactivity (c.p.m.) incorporated was measured. Mouse IL-4 was used at 100 U/ml. Purified sFv was used at 2 μg/ml. The results are shown as percentage inhibition of proliferation and the values were estimated by comparing the difference in proliferation of cells cultured in medium supplemented, with the experiment reagent, against cells cultured in medium only. Columns and bars represent the mean±SD of triplicate determinants. In some columns the error bars are not visible due to the low value. For HT29 (a), the mAb MR6, IL-4 and H4 sFv and for TM25.F1 (b), IL-4 and H4 sFv significantly reduced cell proliferation; P < 0·01. The proliferation for HT29 and TM25.F1 cultured in medium only was 8·1×104 c.p.m. and 2·1×105 c.p.m., respectively.
We also observed that H4 sFv, but not the other anti-gp200-MR6 sFv, was able to reduce the proliferation of the mouse thymic epithelial cell line TM25.F1 (Fig. 5b) to a level similar to that seen with murine IL-4 (Fig. 5b). Thus the H4 sFv exhibits similar functional activity to the mAb MR6 on both murine and human epithelial cells, indicating that the murine gp200-MR6 molecule may have functional properties identical to human gp200-MR6.
The effect of anti-gp200-MR6 sFv on T-cell development using FTOC
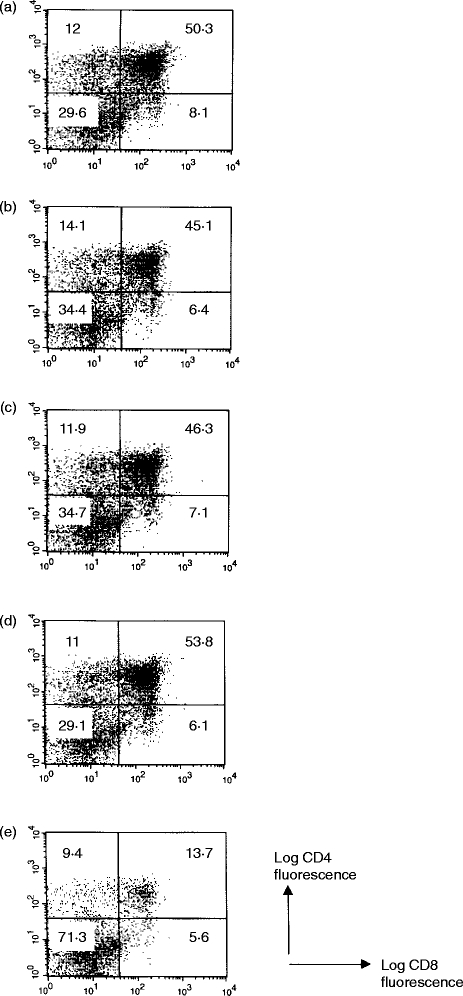
To determine what effect the anti-gp200-MR6 sFv may have on T-cell development within the thymus, we cultured day 14 fetal thymic lobes supplemented in medium containing either purified anti-gp200 sFv (B1, B10 and H4) or control sFv (anti-NIP). After 6 days in culture, cells were harvested and the cell surface phenotype of recovered cells was analysed using flow cytometry with anti-CD4, anti-CD8 and anti-CD25 antibodies. Thymocytes recovered from fetal lobes cultured in the presence of either B1 or B10 sFv showed no difference in cell number and phenotype compared to control sFv or medium only (Fig. 6). In contrast, the number of thymocytes recovered from lobes cultured in the presence of H4 sFv were reduced fivefold (Fig. 6e). Furthermore, over 70% of the cells recovered were double negative (DN) compared to 35% in control cultures, and as a result only 13% of thymocytes had reached the double positive (DP) stage of development, compared to ≈50% in control cultures. The number of CD25+ thymocytes were reduced, by twofold in the presence of H4 in comparison to control cultures (Table 2).
Figure 6.
The effect of purified gp200-MR6-specific sFv on thymocyte development in FTOC. Mouse fetal thymic lobes were cultured in medium only (a) or medium supplemented with either control sFv (b: anti-NIP), B1 (c), B10 (d), or H4 (e). Single-chain Fv were purified by protein A and used at a concentration of 2 μg/ml. After 6 days in culture, thymic lobes were recovered and analysed for cell surface expression using flow cytometry with anti-CD4, anti-CD8 and anti-CD25. Thymocytes recovered in thymic lobes cultured in H4 sFv were reduced in number by ≈ fivefold, with over 70% at the DN stage of development compared to 35% DN in control culture (b). In addition, the total numbers of CD25+ thymocytes were reduced twofold in the presence of H4 sFv in comparison to control (see Table 2). This study suggests that gp200-MR6 plays an important role in the maturation of DN thymocytes to the DP stage of development. Each culture contained four thymic lobes, and the total number of thymocytes recovered from each culture were: a, 1·5×105; b, 1·2×105; c, 1·6×105; d, 1·6 ×105; e, 3·2×104. Three further experiments gave similar results.
Table 2.
Total number of CD25+ thymocytes recovered from thymic lobes cultured in either medium or purified sFv*
*Data from the experiment shown in Fig. 6 see figure legend for details.
DISCUSSION
Analysis of key molecules within the human thymus has been hampered by a lack of good functional assay systems comparable to those available for the mouse. We have therefore taken advantage of the phage display technique, where, bypassing in vivo deletion of autoreactive specificites, antibodies to evolutionarily conserved epitopes can be isolated.9–13 Using this technology we have isolated three clones that recognize not only the human gp200-MR6 cortical epithelial cell surface molecule, the selecting antigen, but also the gp200-MR6 homologue in several other species including mouse and pig. Together with our previous biochemical analysis using mAb MR6 on rabbit thymus,31 these studies provide further evidence that the gene product of gp200-MR6 is highly conserved amongst different species and phyla.
We initially studied the human system and purified sFv from one of these selected clones, H4, reduced the proliferation of the human HT29 cell line in a manner comparable to that seen with mAb MR6 (Fig. 5a), indicating that H4 sFv has a similar biological activity to the mAb MR6. However, B10 had no such effect. This may result from the recognition of different epitopes on gp200-MR6, since the VH gene segment and CDR3 region from both these clones are distinct (Table 1). Alternatively, the two antibodies may differ in their degree of ‘multimerization’, since sFv purified using protein A may associate to form multimers;9 the resulting enhanced avidity would therefore contribute to the functional efficacy of the sFv, as previously shown for phage-derived Fab fragments specific for hepatocyte growth factor/scatter factor.14 However, this latter explanation seems unlikely since our preliminary studies have shown that the Fab fragments of the mAb MR6 can also reduce proliferation of HT29 cells (Al -Tubuly and Ritter, unpublished observation).
The evolutionary conservation of the gp200-MR6 molecule enabled us then to ‘walk’ from the human to the murine thymus for functional studies. H4 sFv reduced the proliferation of the mouse thymic cell line TM25.F1 in a similar manner to that seen with mouse IL-4 (Fig. 5b). This observation suggests that the functional activity of H4 sFv on mouse epithelium is comparable to that observed with either H4 sFv or mAb MR6 on HT29 cells, indicating that ligation of gp200-MR6 on either human or mouse epithelium produces similar biological effects. It is therefore reasonable to conclude that the effects seen with H4 sFv in murine FTOC can be extrapolated to human thymic development. Furthermore, the ability of H4 sFv to exhibit functional activity on cell lines from two different species is an important finding, since few studies14 exist which demonstrate sFv with these properties.
The addition of H4 sFv to murine FTOC had a marked effect on thymocyte development, blocking the majority of thymocytes at the DN stage of differentiation (Fig. 6e), resulting in a dramatic loss of DP thymocytes (13-fold compared to control sFv) and therefore reducing the total number of thymocytes fivefold. As with the epithelial cell line proliferation studies, no such effect was seen with either B1 or B10 sFv. The total numbers of CD25+ thymocytes recovered from thymic lobes cultured with H4 sFv were reduced twofold compared to the control, indicating that the action of the gp200-MR6 molecule is at the development of these cells. However, the reduced numbers of CD25+ thymocytes are unlikely to explain fully the dramatic reduction of DP thymocytes, since only a small proportion of CD25+ cells give rise to progeny that down-regulate CD25 expression and subsequently become DP thymocytes.33 Moreover, our preliminary studies, using day 16 fetal thymic lobes cultured in vitro for 6 days showed that whereas both control and H4 sFv-treated cultures contained more than 80% DP thymocytes, in the presence of H4 the total number of cells was reduced ≈10-fold (Palmer and Crompton, unpublished observations). These data suggest that gp200-MR6 is also important at this later DP stage of thymocyte development. Interestingly, while the development of DN, CD44+ CD25+ thymocyte precursors requires both thymic epithelial cells and mesenchymal cells.34 The necessary signals required to continue the maturation of their immediate descendants and subsequently the differentiation of DP thymocytes can be provided sufficiently by epithelial cells alone.35 A functional role for gp200-MR6 at both these stage of thymocyte development fits well with its distribution on the surface of cortical epithelial cells.
The action of H4 sFv may be to directly disrupt thymocyte–stromal cell interactions, which are known to be important not only for thymocyte maturation36 but also in the maintenance of the thymic microenvironment.25,37,38 Thus the effect seen with H4 sFv could result from the direct loss of a gp200-MR6-mediated signal via a specific ligand on the thymocyte surface. In addition, or alternatively, the initial effect may occur at the epithelial cell surface and subsequently affect the development and function of the thymic stroma. The latter possibility is supported by the effects observed with H4 on the mouse thymic cortical epithelial cell line TM25.F1 (Fig. 5b) and our previous data showing mAb MR6-induced differentiation of colonic epithelial cells.7 However, although gp200-MR6 may be involved in maintenance of the thymic microenvironment, it is unlikely that gp200-MR6 is involved in thymus organogenesis, since antibodies that affect organogenesis do not mediate their effect in intact thymic lobes.39
We have previously demonstrated a functional link between gp200-MR6 and the IL-4 system on human cells;5–7 our current observation that ligation of gp200-MR6 mimics the antiproliferative effect of IL-4 on a mouse thymic epithelial cell line (Fig. 5b), suggests that this association may also occur on murine cells. Moreover, addition of IL-4 to mouse fetal thymic organ culture leads to a severe reduction in the numbers of DP thymocytes.40,41 Similar effects are also seen in the thymus of IL-4 transgenic mice,42–44 while thymocyte development appears normal in IL-4-deficient mice.45 These data are consistent with the effects seen with H4 sFv on thymic development and further support our hypothesis that gp200-MR6 has similar functional properties to the IL-4 receptor within the thymus.
In summary, we have used the phage display technique to select for antibodies to epitopes on the human gp200-MR6 cortical thymic molecule that are evolutionarily conserved, thus permitting functional analysis in murine FTOC. These studies therefore highlight the advantages of using phage libraries for selection and in the production of soluble sFv for functional studies. Our data suggest that the cell surface cortical epithelial gp200-MR6 plays an important role in T-cell development and although the precise function is unknown it seems likely to involve delivering proliferation/survival signals to thymocytes.
Acknowledgments
We thank Dr J. Dyson and Professor H. Reiser for discussion and reading of the manuscript, Dr I. Tomlinson for advice, Drs D. Kioussis and E. Spanopoulou for providing the TM25.F1 cell line, Drs J. de Kruif and T. Logtenberg for providing the E. coli bacterial strain SF110, and Professor D. Gray for providing mouse IL-4. We are indebted to Dr G. Winter for providing the phage display library. This work was funded by the Wellcome Trust.
Glossary
Abbreviations
- DN
double-negative
- DP
double-positive
- FTOC
fetal thymic organ culture
- sFv
single-chain antibody fragment
References
- 1.De Maagd RA, MacKenzie WA, Schuurman H-J, et al. The human thymus microenvironment: heterogeneity detected by monoclonal anti-epithelial cell antibodies. Immnuology. 1985;54:745. [PMC free article] [PubMed] [Google Scholar]
- 2.Larche M, Ladyman H, Ritter MA. Human thymic epithelial cells and lymphocytes may share common epitopes. In: Mcmicheal AJ, editor. Leukocyte Typing III. Oxford: Oxford University Press; 1987. p. 257. [Google Scholar]
- 3.Von Gaudecker B, Kendall M, Ritter MA. Immuno-electron microscopy of the thymic microenvironment. Microscopy Research and Technique. 1997;38:1. doi: 10.1002/(SICI)1097-0029(19970801)38:3<237::AID-JEMT5>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 4.Mat I, Larche M, Melcher D, Ritter MA. Tumour-associated upregulation of IL-4 receptor complex. Br J Cancer. 1990;62(Suppl. X):96. [PMC free article] [PubMed] [Google Scholar]
- 5.Larche M, Lamb J, O’Hehir R, et al. Functional evidence for a monoclonal antibody that binds to the human IL-4 receptor. Immunology. 1988;65:617. [PMC free article] [PubMed] [Google Scholar]
- 6.Imami N, Larche M, Ritter MA. Inhibition of alloreactivity by mAb MR6: differential effects on IL-2- and IL-4-producing human T cells. Int Immunol. 1994;6:1575. doi: 10.1093/intimm/6.10.1575. [DOI] [PubMed] [Google Scholar]
- 7.Al Tubuly AA, Spijker R, Pignatelli M, Kirkland SC, Ritter MA. Inhibition of growth and enhancement of differentiation of colorectal carcinoma cell lines by mAb MR6 and IL-4. Int J Cancer. 1997;71:605. doi: 10.1002/(sici)1097-0215(19970516)71:4<605::aid-ijc16>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 8.Jenkinson EJ, Anderson G. Fetal thymic organ cultures. Curr Opin Immunol. 1994;6:293. doi: 10.1016/0952-7915(94)90104-x. [DOI] [PubMed] [Google Scholar]
- 9.Lerner RA, Kang AS, Bain JD, Burton DR, Barbas CF. Antibodies without immunization. Science. 1992;258:1313. doi: 10.1126/science.1455226. [DOI] [PubMed] [Google Scholar]
- 10.Nissim A, Hoogenboom HR, Tomlinson IM, et al. Antibody fragments from a ‘single pot’ phage display library as immunochemical reagents. EMBO J. 1994;13:692. doi: 10.1002/j.1460-2075.1994.tb06308.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Winter G, Griffiths AD, Hawkins RE, Hoogenboom HR. Making antibodies by phage display technology. Annu Rev Immunol. 1994;12:433. doi: 10.1146/annurev.iy.12.040194.002245. [DOI] [PubMed] [Google Scholar]
- 12.George AJT. Antibody engineering: Potential application for immunoassays. In: Price CP, Newman DJ, editors. Principles and Practice of Immunoassay. 2. Macmillian Press: London; 1994. p. 66. [Google Scholar]
- 13.De Kruif J, Terstappen L, Boel E, Logtenberg T. Selection and application of human single chain Fv antibody fragments from a semi-synthetic phage antibody display library with designed CDR3 regions. J Mol Biol. 1995;248:97. doi: 10.1006/jmbi.1995.0204. [DOI] [PubMed] [Google Scholar]
- 14.Zaccolo M, Griffiths AD, Prospero TD, Winter G, Gherardi E. Dimerization of Fab fragments enables ready screening of phage antibodies that affect hepatocyte growth factor/scatter factor activity on target cells. Eur J Immunol. 1997;27:618. doi: 10.1002/eji.1830270307. [DOI] [PubMed] [Google Scholar]
- 15.Al Tubuly AA, Luqmani YA, Shousa S, Melcher D, Ritter MA. Down regulation of the IL-4 receptor-associated molecule gp200-MR6 in invasive carcinoma and differential expression in benign hyperplasia of the breast. Br J Cancer. 1996;74:1005. doi: 10.1038/bjc.1996.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;277:680. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 17.Marks JD, Hoogenboom HR, Boonet TP, McCafferty J, Griffiths AD, Winter G. By-passing immunization. Human antibodies from V-gene libraries displayed on phage. J Mol Biol. 1991;222:581. doi: 10.1016/0022-2836(91)90498-u. [DOI] [PubMed] [Google Scholar]
- 18.Palmer DB, Kevany M, Mackworth-Young C, Batchelor R, Lombardi G, Lechler RI. Generation and characterisation of an anti-DRα chain monoclonal antibody using L cell transfectants expressing human and murine class II major histocompatibility dimers. Immunogentics. 1992;33:201. doi: 10.1007/BF00211690. [DOI] [PubMed] [Google Scholar]
- 19.Hoogenboom HR, Griffiths AD, Johnson KS, Chiswell DJ, Hudson P, Winter G. Multi-subunit proteins on surface of filamentous phage: methodologies for displaying antibody (Fab) heavy and light chains. Nucl Acids Res. 1991;19:4133. doi: 10.1093/nar/19.15.4133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tomlinson IM, Williams SC, Corbett SJ, Cox JPL, Winter G. Hills Road, Cambridge, CB2, 2QH, UK: MRC Centre for Protein Engineering; 1996. The V BASE Directory of Human Variable Gene Sequences. ( http://www.mrc-cpe.cam.ac.uk/imt-doc/vbase-home-page.htm1). [Google Scholar]
- 21.Meerman HJ, Georgiou G. Construction and characterization of a set of E.coli strains deficient in all know loci affecting the proteolytic stability of secreted recombinant proteins. Bio/Technology. 1994;12:1107. doi: 10.1038/nbt1194-1107. [DOI] [PubMed] [Google Scholar]
- 22.Hoogenboom HR, Winter G. By-passing immunisation. Human antibodies from synthetic repertories of germ line VH segments rearranged in vitro. J Mol Biol. 1992;227:381. doi: 10.1016/0022-2836(92)90894-p. [DOI] [PubMed] [Google Scholar]
- 23.Smith PK, Krohn RI, Hermanson GT, et al. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985;150:76. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- 24.Evan GI, Lewis GK, Ramsey G, Bishop MJ. Isolation of monoclonal antibodies specific for human c-myc-proto-oncogene product. J Mol Cell Biol. 1985;5:3610. doi: 10.1128/mcb.5.12.3610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Palmer DB, Viney JL, Ritter MA, Hayday AC, Owen MJ. Expression of the αβ T-cell receptor is necessary for the generation of the thymic medulla. Dev Immunol. 1993;3:175. doi: 10.1155/1993/56290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Palmer DB, George AJ.T, Ritter MA. Selection of antibodies to cell surface determinants on mouse thymic epithelial cells using a phage display library. Immunology. 1997;91:473. doi: 10.1046/j.1365-2567.1997.00262.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Spanopoulou E, Early A, Elliott J, et al. Complex lymphoid and epithelial thymic tumors in Thy 1-myc transgenic mice. Nature. 1989;342:185. doi: 10.1038/342185a0. [DOI] [PubMed] [Google Scholar]
- 28.Crompton T, Gilmour KC, Owen MJ. The map kinase pathway controls differentiation from double-negative to double-positive thymocytes. Cell. 1996;86:243. doi: 10.1016/s0092-8674(00)80096-3. [DOI] [PubMed] [Google Scholar]
- 29.Tomlinson IM, Walter G, Marks JD, Llewelyn MB, Winter G. The repertoire of human germline VH sequences reveals about fifty groups of VH segments with different hypervariable loops. J Mol Biol. 1992;227:77. doi: 10.1016/0022-2836(92)90223-7. [DOI] [PubMed] [Google Scholar]
- 30.Kabat EA, Wu TT, Perry HM, Gottesman KS, Foeller C. Sequence of Immunological Interest. 5. Bethesda: Department of Human Health and Human Services, Public Health Service, National Institute of Health; 1991. [Google Scholar]
- 31.Sivolapenko GB, Imami N, Larche M, Epenetos AA, Ritter MA. Enhanced in vivo immunogenicity induced by an antibody to the IL-4 receptor-associated gp200–MR6 molecule. Scand J Imunol. 1996;44:1. doi: 10.1046/j.1365-3083.1996.d01-292.x. [DOI] [PubMed] [Google Scholar]
- 32.Sasso EH, Silverman GJ, Mannik M. Human IgA and IgG F (ab′) 2 that bind staphylococcal protein A belong to the VHIII subgroup. J Immunol. 1991;147:1877. [PubMed] [Google Scholar]
- 33.Penit C, Lucas B, Vasseur F. Cell expansion and growth arrest phases during the transition from precursor (CD4−) to immature (CD4+8+) thymocytes in normal and genetically modified mice. J Immunol. 1995;153:103. [PubMed] [Google Scholar]
- 34.Anderson G, Jenkinson EJ, Moore NC, Owen JJT. MHC class II+ thymic epithelial cells and mesenchyme cells are both required for T cell development in the thymus. Nature. 1993;362:70. doi: 10.1038/362070a0. [DOI] [PubMed] [Google Scholar]
- 35.Anderson G, Anderson KL, Tchilian EZ, Owen JJT, Jenkinson EJ. Fibroblast dependency during early thymocyte development maps to the CD25+ CD44+ stage and involves interaction with fibroblast matrix molecules. Eur J Immunol. 1997;27:1200. doi: 10.1002/eji.1830270522. [DOI] [PubMed] [Google Scholar]
- 36.Anderson G, Moore NC, Owen JJT, Jenkinson EJ. Cellular interactions in thymocyte development. Annu Rev Imunol. 1996;14:73. doi: 10.1146/annurev.immunol.14.1.73. [DOI] [PubMed] [Google Scholar]
- 37.Ritter MA, Boyd RL. Development in the thymus: it takes two to tango. Immunol Today. 1993;14:462. doi: 10.1016/0167-5699(93)90250-O. [DOI] [PubMed] [Google Scholar]
- 38.Van Ewijk W, Shores EW, Singer A. Crosstalk in the mouse thymus. Immunol Today. 1994;15:214. doi: 10.1016/0167-5699(94)90246-1. [DOI] [PubMed] [Google Scholar]
- 39.Muller KM, Luedecker CJ, Udey MC, Farr AG. Involvement of E-cadherin in thymus organogenesis and thymocyte maturation. Immunity. 1997;6:257. doi: 10.1016/s1074-7613(00)80328-3. [DOI] [PubMed] [Google Scholar]
- 40.Plum J, De Smedt M, Leclercq G, Tison B. Inhibitory effect of murine recombinant IL-4 on thymocyte development in fetal thymus organ cultures. J Immunol. 1990;145:1066. [PubMed] [Google Scholar]
- 41.Suda T, Zlotnik A. IL-7 maintains the T cell precursor potential of CD3−CD4−CD8− thymocytes. J Immunol. 1991;146:3068. [PubMed] [Google Scholar]
- 42.Tepper RI, Levinson DA, Stanger BZ, Campos-Torres J, Abbas AK, Leder P. IL-4 induces allergic-like inflammatory disease and alters T cell development in transgenic mice. Cell. 1990;62:457. doi: 10.1016/0092-8674(90)90011-3. [DOI] [PubMed] [Google Scholar]
- 43.Lewis DB, Yu CC, Forbush KA, et al. Il-4 expressed in situ selectively alter thymocyte development. J Exp Med. 1991;173:89. doi: 10.1084/jem.173.1.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Erb KJ, Holtschke T, Muth K, Horak I, Schimpl A. T cell subset distribution and B cell hyperreactivity in mice expressing interleukin-4 under the control of major histocompatibility complex class I regulatory element. Eur J Immunol. 1994;24:1143. doi: 10.1002/eji.1830240520. [DOI] [PubMed] [Google Scholar]
- 45.Kuhn R, Rajewsky K, Muller W. Generation and analysis of interleukin-4 deficient mice. Science. 1991;254:707. doi: 10.1126/science.1948049. [DOI] [PubMed] [Google Scholar]