Abstract
It has been reported that most children who showed anaphylaxis to measles, mumps and rubella vaccines containing bovine gelatin as a stabilizer have anti-bovine gelatin IgE. The present study was designed to investigate the reactivity of IgE in bovine gelatin-sensitive children to gelatins from various animals, and the antigenic cross-reactivity between the gelatins. Serum samples taken from 10 children who showed anaphylaxis to vaccines containing bovine gelatin were used in this study. The level of anti-bovine gelatin IgE in these serum samples ranged from 11·0 to 251 Ua/ml. The IgE in most of the children reacted to kangaroo and mouse gelatins, to which they had had little or no exposure as a food or a vaccine stabilizer. The IgE binding to kangaroo and mouse gelatins was completely inhibited by bovine gelatin, whereas reciprocal inhibition was not complete, indicating that antigenic cross-reactivity is present between the mammalian gelatins. Only one child had strong IgE reactivity to fish gelatins, and this reactivity was not inhibited by bovine gelatin, indicating that no antigenic cross-reactivity exists between bovine and fish gelatins. Most of the children who displayed sensitivity to bovine gelatin showed IgE reactivity to other mammalian gelatins. This reactivity may be due primarily to the antigenic cross-reactivity between mammalian gelatins.
INTRODUCTION
Anaphylactic reactions to measles, mumps and rubella vaccines and the combined measles–mumps–rubella (MMR) vaccine have been reported and have been suggested to be caused by allergy to egg proteins present in the vaccines.1 However, anaphylactic reactions have also been reported to occur following administration of the MMR vaccine in children who had demonstrated tolerance to egg proteins.2–6 Kelso et al.7 reported that a child who suffered from anaphylaxis following administration of MMR vaccine produced the IgE antibody to gelatin. Furthermore, in previous studies8–12 we have found that most children who show systemic immediate-type reactions, including anaphylaxis, to the live and inactivated virus vaccines have anti-gelatin IgE. We believe that most of the systemic immediate-type reactions occurring after vaccination are caused by the gelatin present in the vaccines as a stabilizer.
Gelatin has long been believed to be low-immunogenic and is thought to be weakly allergenic in humans. Therefore, gelatin has been widely used as a stabilizer in vaccines.13 Gelatins used in vaccines produced in Japan are derived from bovine or porcine sources; all live virus vaccines contain bovine gelatin, and the inactivated vaccines, such as acellular diphtheria-tetanus-pertussis and Japanese encephalitis virus vaccines, contain either bovine or porcine gelatin. Gelatin can be derived from collagen molecules present in all multicellular animals.14,15
The present study investigated the reactivity of IgE in bovine gelatin-sensitive children to gelatin from various animals by enzyme-linked immunosorbent assay (ELISA) and mast cell histamine release assay, as well as the antigenic cross-reactivity between the gelatins by ELISA inhibition.
MATERIALS AND METHODS
Children
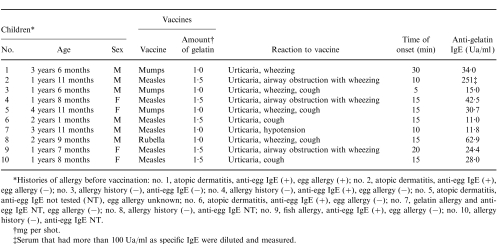
The subjects consisted of 10 children (six boys and four girls) (mean age±SD, 2 years 4 months±1 year) who showed anaphylaxis to live vaccines (Table 1). Serum samples from these children were submitted to the Japan National Institute of Infectious Diseases by the referring physicians and vaccine manufacturers. The anti-bovine gelatin IgE ranged from 11 to 250 Ua/ml (mean value±SD, 51±72 Ua/ml). Of the 10 children, six had received the measles vaccine, three the mumps vaccine, and one the rubella vaccine. Of these children, four children suffered from severe anaphylaxis – cutaneous signs (systemic urticaria) plus airway obstruction (with laryngeal oedema or wheezing) or anaphylactic shock (with hypotension and vascular collapse) – and six suffered mild anaphylaxis, e.g. systemic urticaria and/or wheezing and/or cough and/or other symptoms. The time of onset of anaphylaxis following vaccination ranged from 5 to 30 min.
Table 1.
Children with anaphylaxis to vaccine and anti-bovine gelatin IgE levels in the serum
*Histories of allergy before vaccination: no. 1, atopic dermatitis, anti-eff IgE (+), egg allergy (+): no. 2, atopic dermatitis, anti-egg IgE (+), egg allergy (−); no. 3, allergy history (−), anti-egg IgE (−); no. 4, allergy history (−), anti-egg IgE (+), egg allergy (−); no. 5, atopic dermatitis, anti-egg IgE not tested (NT), egg allergy unknown; no. 6, atopic dermatitis, anti-egg IgE (+), egg allergy (−); no. 7, gelatin allergy and anti-egg IgE NT, egg allergy (−); no. 8, allergy history (−), anti-egg IgE NT; no. 9, fish allergy, anti-egg IgE (+), egg allergy (−); no. 10, allergy history (−), anti-egg IgE NT.
†mg per shot.
‡Serum that had more than 100 Ua/ml as specific IgE were diluted and measured.
Gelatin
Gelatin for the ELISA was used after denaturation of native collagen at 100° for 10 min. Native collagen was prepared according to the following methods. Collagens from vertebrate animal species (bovine, guinea-pig, rat, mouse, chick, bullfrog tadpole and salmon) were prepared from skin dermis by 0·5 m acetic acid extraction, and purified by differential salt precipitation.16 Collagens from invertebrate animal species (shark, octopus and ascaris) were similarly prepared from dermis homogenate and purified.17 Identification and assessment of purity were routinely achieved by sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS-PAGE).18 Collagen and gelatin from other animals were purchased from the following sources: porcine collagen (Nitta Gelatin, Osaka, Japan); kangaroo and codfish gelatin (Sigma Chemicals, St Louis, MO).
Measurement of gelatin-specific IgE
The Pharmacia CAP system (Pharmacia, Uppsala, Sweden) was used to determine the concentration (Ua/ml) of IgE antibody to bovine gelatin (Wako Pure Chemical Industries, Osaka, Japan) and egg white.8
Specific IgE antibodies to gelatins from various animals were determined by fluorometric ELISA analysis as described previously.9 Microplates were coated with various animal gelatin (5 μg/ml). Following overnight incubation at 4°, the serum samples were added to the wells. After incubation for 3 hr at room temperature, anti-human IgE antibody conjugated with β-d-galactosidase (Pharmacia) was added. The enzyme reaction substrate was 0·2 mmol/l 4-methylumbelliferyl-β-d-galactoside (Sigma Chemicals). The fluorescence unit (FU) was measured using a fluorometric microplate reader (Fluoroskan; Flow Laboratory, McLean, VA).
Human mast cell
Cultured human mast cells were prepared as described previously.19 Briefly, mononuclear cells were obtained from umbilical cord blood and cultured in minimum essential medium (MEM) containing 20% fetal calf serum, in the presence of recombinant human stem cell factor (SCF) (100 ng/ml) and interleukin-6 (IL-6) (10 ng/ml; Kirin Brewery, Gunma, Japan). When the mast cells reached 100% purity, they were used for experiments.
Histamine release from cultured mast cell passive-sensitized with children’s sera
Histamine release from mast cells was conducted as described previously.20 Briefly, the mast cells were washed three times in MEM containing SCF (100 ng/ml) and IL-6 (10 ng/ml), then incubated overnight with the sera at 37°. During sensitization, SCF and IL-6 were added to the cultures at 100 and 10 ng/ml, respectively. After three washes, they were suspended in Tyrode–HEPES buffer (pH 7·4) containing 0·1% bovine serum albumin in the presence of SCF (10 ng/ml), then preincubated at 37° for 10 min. Next, the cells were challenged with various gelatins (5 μg/ml) or anti-human IgE monoclonal antibody (4 μg/ml) (Chemicon, Temecula, CA) at 37° for 30 min. The histamine contents in the supernatants and in the cell pellets were determined by an automated fluorometric procedure. The spontaneous release was assessed by the addition of a buffer instead of gelatin or anti-IgE monoclonal antibody. The percentage of histamine release was calculated as follows:
 |
Inhibition of gelatin-specific IgE
The antigenic cross-reactivity between bovine and other animal gelatins was analysed by fluorometric ELISA inhibition as described previously.21 Briefly, gelatin (5 μg/ml) was absorbed to microplate wells. After overnight incubation at 4°, the serum was mixed with anti-gelatin IgE and gelatin (100 μg/ml) as an inhibitor and added to the wells. After 3 hr of incubation at room temperature, anti-human IgE antibody conjugated with β-d-galactosidase was added. The following procedures were the same as those in the above-mentioned fluorometric ELISA. The percentage of inhibition was calculated as follows:
 |
RESULTS
IgE reactivity to gelatin from other animals
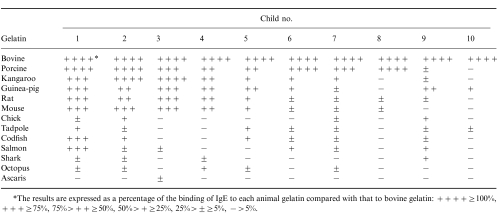
Sera from 10 children who showed anaphylaxis to vaccines and had relatively high levels of anti-bovine gelatin IgE were used to analyse the reactivity of IgE to gelatins isolated from other animals. Table 2 shows the reactivity of IgE in the sera to the gelatin isolated from 13 different animals. The values are expressed as a percentage of the binding of IgE to the various animal gelatins compared with that to bovine gelatin. Most of the children had specific IgE to porcine gelatin, which is contained in food and inactivated vaccines. Also, IgE antibody to gelatins from other mammals, such as kangaroo and mouse, gelatin to which they had never been exposed was detected in the sera of most of the children. Interestingly, only one child had strong IgE reactivity to gelatin from fishes (codfish and salmon).
Table 2.
Reactivity of anti-gelatin IgE to gelatin from various animals
*The results are expressed as a percentage of the binding of IgE to each animal gelatin compared with that to bovine gelatin: + + + + ≥ 100%, + + + ≥ 75%, 75% > + + ≥ 50%, 50% > + ≥ 25%, 25% ± ≥ 5%, − > 5%.
Gelatin-specific histamine release
A gelatin-specific histamine release assay was carried out with cultured mast cells passive-sensitized with three children’s sera (Fig. 1). The three children (nos 3–5) had relatively high levels of anti-bovine gelatin IgE. The mast cells sensitized with each serum or their pooled sera in the children showed bovine gelatin-specific histamine release. These children had little or no anti-chick or anti-shark gelatin IgE (Table 2). Accordingly, mast cells sensitized with their sera did not show chick or shark gelatin-specific histamine release (Fig. 1). As a positive control, the mast cells challenged with anti-human IgE showed histamine release.
Figure 1.
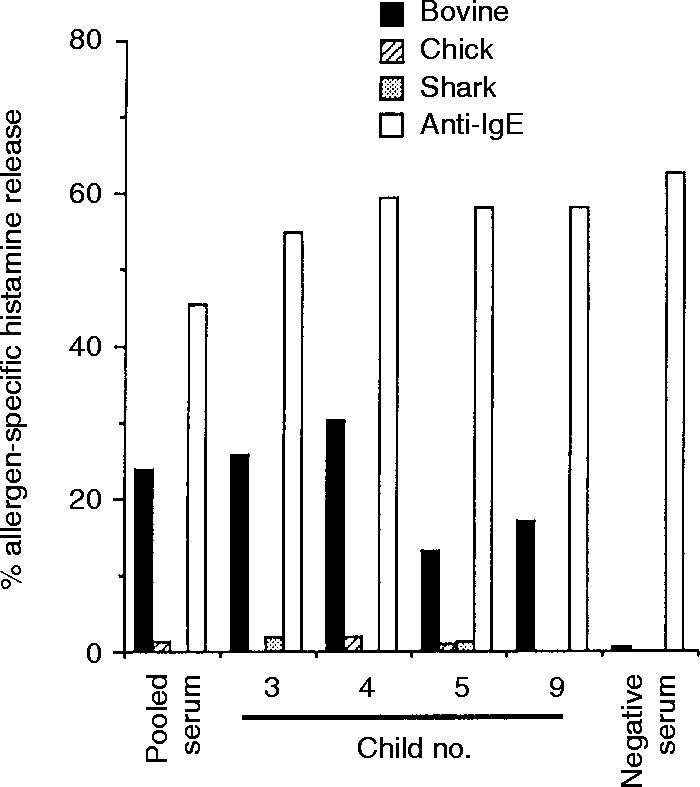
Histamine release from the mast cells passive-sensitized with the sera of bovine gelatin-sensitive children.
Antigenic cross-reactivity among gelatins by ELISA inhibition
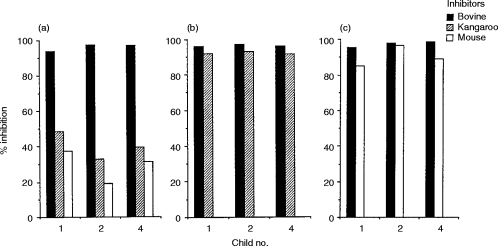
Antigenic cross-reactivity between mammalian gelatins was investigated by ELISA inhibition. The sera of the children (nos 1, 2 and 4) in Table 2 were used for the analysis. Incubation of the homologous bovine gelatin with the serum inhibited binding by more than 95% (Fig. 2a). IgE binding to bovine gelatin was not completely inhibited by either kangaroo or mouse gelatin (Fig. 2a), whereas reciprocal inhibition was complete (Fig. 2b, c). A similar pattern was found in the inhibition between bovine and rat gelatins (data not shown). These results indicate that there is antigenic cross-reactivity between bovine and other mammalian gelatins.
Figure 2.
Cross-reactivity between mammalian gelatins by ELISA inhibition analysis using sera of bovine gelatin-sensitive children. (a) Immobilized antigen:bovine gelatin; (b) immobilized antigen:kangaroo; (c) immobilized antigen:mouse gelatin.
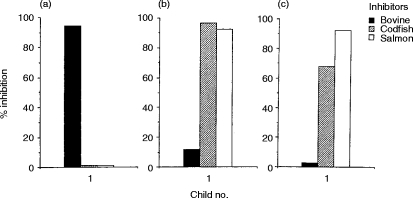
Next, cross-reactivity between bovine and fish gelatins was studied by ELISA inhibition. Serum of the child (no. 1) who had both anti-bovine and anti-fish gelatin IgE was used for the analysis. IgE binding to bovine gelatin was not inhibited by codfish or salmon gelatin (Fig. 3a), and no reciprocal inhibition was observed (Fig. 3b,c). These results indicate that there is little cross-reactivity between bovine and fish gelatins. In contrast, IgE binding to codfish gelatin was completely inhibited by salmon gelatin (Fig. 3b) and reciprocal inhibition was detected (Fig. 3c). Therefore, a strong cross-reactivity was observed between fish gelatins.
Figure 3.
Cross-reactivity between bovine and fish gelatin by ELISA inhibition analysis using the serum of a bovine and fish gelatin-sensitive child. (a) Immobilized antigen:bovine gelatin; (b) immobilized antigen:codfish gelatin; (c) immobilized antigen:salmon gelatin. ¤
DISCUSSION
In previous studies, we have found that most children who have had anaphylaxis to live vaccines containing bovine gelatin had anti-bovine gelatin IgE.8,9 It was suggested that there is a strong relationship between anaphylaxis to the vaccines and the presence of anti-bovine gelatin IgE. In the present study, mast cells sensitized with each serum or the pooled sera from children who had showed anaphylaxis to vaccines induced bovine gelatin-specific histamine release. This result reconfirms a relationship between anaphylaxis to the vaccines and bovine gelatin-sensitivity.
In Japan, live virus vaccines contain only bovine gelatin, and inactivated vaccines contain either bovine or porcine gelatin. In a previous study, we evaluated the IgE response to bovine and porcine gelatins in the sera of children who showed anaphylaxis to the vaccines.9 In this study, we measured the specific IgE to gelatins from other mammals, and found that most of the children had the specific IgE to gelatin from other mammals, such as kangaroo, guinea-pig, mouse and rat, to which they had had little or no exposure as a food or vaccine stabilizer. To investigate the possibility of antigenic cross-reactivity among these gelatins, ELISA inhibition with the children’s sera was performed. IgE binding to bovine gelatin was not completely inhibited by other mammalian gelatin, whereas reciprocal inhibition was complete. These results show that cross-reactivity exists among mammalian gelatins, implying that mammalian gelatins may share a common antigenic structure.
In the present study, we measured the specific IgE to gelatin from non-mammalian animals such as chick and fish. Most of the children had weak or no reactivity to these gelatin. Furthermore, the mast cells sensitized with the sera that had no anti-chick or no anti-shark gelatin IgE showed no corresponding histamine release.
Only one child (no. 1) had a high level of specific IgE to fish gelatin. The serum of this child was analysed by ELISA inhibition and it was shown that there was little cross-reactivity between bovine and fish gelatins. At present, the vaccines available in Japan do not contain fish gelatin. However, fish meat and skin contain collagen from which gelatin is derived, and it is likely that the child may become sensitized by fish gelatin in food. For this child, no information on fish allergy was available, but he had anti-codfish and anti-salmon meat IgE by CAP methods (data not shown).
In conclusion, the children with bovine gelatin sensitivity also had the specific IgE to other mammalian gelatins. There is antigenic cross-reactivity among mammalian gelatins, whereas mammalian and fish gelatins show no cross-reactivity. One child showed a strong IgE reactivity to fish gelatin, which was independent of the anti-bovine gelatin IgE.
Acknowledgments
We thank the physicians and the staff of the vaccine manufacturers for their help in the serum sampling.
Glossary
Appreviations
- ELISA
enzyme-linked immunosorbent assay
- FU
fluorescence unit
- MEM
minimum essential medium
- MMR
combined measles–mumps–rubella vaccine
- NT
not tested
- SCF
stem cell factor
References
- 1.Zimmerman B, Zimmerman RS. Adverse reactions to vaccines. In: Middeleton E, Reed CE, Ellis EF, Adkinson NF, Yunginer JW, Busse WW, editors. Allergy: Principles and Practice. 4. Vol. 1. St Louis, MO: C. V. Mosby; 1993. p. 469. [Google Scholar]
- 2.Aukrust L, Almeland TL, Refsum D, Aas K. Severe hypersensitivity or intolerance reactions to measles vaccines in six children. Allergy. 1980;35:581. doi: 10.1111/j.1398-9995.1980.tb01808.x. [DOI] [PubMed] [Google Scholar]
- 3.Pollok TM, Morris J. A 7-year survey of disorders attributed to vaccination in North West Thames region. Lancet i. 1983:753. doi: 10.1016/s0140-6736(83)92037-8. [DOI] [PubMed] [Google Scholar]
- 4.Thurston A. Anaphylactic shock reaction to measles vaccine. JR Coll Gen Pract. 1987;37:41. [PMC free article] [PubMed] [Google Scholar]
- 5.Jantunen-Backman K, Peltola H, Backman A, Saol OP. Safe immunization of allergic children against measles, mumps, and rubella. Am J Dis Child. 1987;141:1103. doi: 10.1001/archpedi.1987.04460100081032. [DOI] [PubMed] [Google Scholar]
- 6.Businco L. Measles, mumps, rubella immunization in egg-allergic children: a long-lasting debate. AnnAllergy. 1994;72:1. [PubMed] [Google Scholar]
- 7.Kelso JM, Jones RT, Yunginger JW. Anaphylaxis to measles, mumps and rubella vaccine mediated by IgE to gelatin. J Allergy Clin Immunol. 1993;91:867. doi: 10.1016/0091-6749(93)90344-f. [DOI] [PubMed] [Google Scholar]
- 8.Sakaguchi M, Ogura H, Inouye S. IgE antibody to gelatin in children with immediate-type reactions to measles and mumps vaccines. J Allergy Clin Immunol. 1995;96:563. doi: 10.1016/s0091-6749(95)70304-7. [DOI] [PubMed] [Google Scholar]
- 9.Sakaguchi M, Nakayama T, Inouye S. Food allergy to gelatin in children with systemic immediate-type reactions including anaphylaxis to vaccines. J Allergy Clin Immunol. 1996;98:1058. doi: 10.1016/s0091-6749(96)80191-6. [DOI] [PubMed] [Google Scholar]
- 10.Sakaguchi M, Yoshida T, Asahi T, Aoki T, Miyatani Y, Inouye S. Development of IgE antibody to gelatin in children with anaphylactic reactions to vaccines. J Allergy Clin Immunol. 1997;99:720. doi: 10.1016/s0091-6749(97)70038-1. [DOI] [PubMed] [Google Scholar]
- 11.Sakaguchi M, Yoshida M, Kuroda W, Harayama O, Inouye S. Systemic immediate-type reactions to gelatin including in Japanese encephalitis vaccines. Vaccine. 1997;15:121. doi: 10.1016/s0264-410x(96)00170-3. [DOI] [PubMed] [Google Scholar]
- 12.Sakaguchi M, Yamanaka T, Ikeda K, et al. IgE-mediated systemic reactions to gelatin included in the varicella vaccine. J Allergy Clin Immunol. 1997;99:263. doi: 10.1016/s0091-6749(97)70108-8. [DOI] [PubMed] [Google Scholar]
- 13.Hashizume S. Vaccine components and side effects. Clin Virol. 1994;22:140. (in Japanese) [Google Scholar]
- 14.Alberrts B, Bray D, Lewis J. Cell junctions, cell adhesion, and the extracellular matrix. In: Albert B, Bray D, Lewis J, Raff M, Roberts K, Watson JD, et al., editors. Molecular Biology of the Cell. 3. New York, NY: Garland Publishing; 1994. p. 978. [Google Scholar]
- 15.Budavari S. Gelatin. In: Budavari S, editor. The Merck Index. 12. Whitehouse Station, NJ: Merch & Co; 1996. p. 742. [Google Scholar]
- 16.Miller EJ, Rhodes RK. Preparation and characterization of the different types of collagen. In: Cunningham LW, Frederiksen DW, editors. Methods in Enzymology, Structural and Contractile Proteins. Part A. Extracellular Matrix. Vol. 82. New York, NK: academic press; 1982. p. 33. [DOI] [PubMed] [Google Scholar]
- 17.Murray LW, Waite JH, Tanzer ML, Hauschka PV. Preparation and characterization of invertebrate collagens. In: Cunningham L W, Frederiksen D W, editors. Methods in Enzymology, Structural and Contractile Proteins. Part A. Extracellular Matrix. Vol. 82. New York, NY: Academic Press; 1982. [Google Scholar]
- 18.Hori H, Keene DR, Sakai LY, et al. Repeated helical epitopes of defined amino acid sequence in human type III collagen identified by monoclonal antibodies. Mol Immunol. 1992;29:759. doi: 10.1016/0161-5890(92)90186-2. [DOI] [PubMed] [Google Scholar]
- 19.Yanagida M, Fukamachi H, Ohgami K, et al. Effects of T-helper 2-type cytokines, interleukin-3 (IL-3), IL-4, IL-5, and IL-6 on the survival of cultured human mast cells. Blood. 1995;86:3705. [PubMed] [Google Scholar]
- 20.Yanagida M, Fukamachi H, Takei M, et al. Interferon-γ promotes the survival and FcεRI-mediated histamine release in cultured human mast cells. Immunology. 1996;89:547. doi: 10.1046/j.1365-2567.1996.d01-768.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sakaguchi M, Hashimoto M, Nigi H, et al. Comparison of specific IgE antibody to B-cell epitopes of the major allergen (Cry j 1) of Japanese cedar (Cryptomeria japonica) pollen in humans and monkeys with pollinosis. Immunology. 1997;91:161. doi: 10.1046/j.1365-2567.1997.00255.x. [DOI] [PMC free article] [PubMed] [Google Scholar]