Abstract
A plasmid that contained the cytomegalovirus (CMV)-promoter-driven lacZ reporter gene (pCMV-lacZ) remained in the epaxial muscle of five of eight goldfish as covalently closed circles, the most functional form of plasmid, for at least 70 days at 22°. It was not present in the gills or elsewhere by polymerase chain reaction and was not integrated. Its expressed protein, Escherichia coliβ-galactosidase (β-gal), which was in the injected myofibres, was detected in all the fish at 4–21 days and in about half the fish from 28 days until the end of the experiment at 70 days. The numbers of cells that secreted antibody to β-gal in the kidney peaked at 14 days. Serum antibody and proliferating kidney cells to β-gal were in all fish from 14 days with a plateau of the responses from 21 days onwards. The plasmid did not induce autoimmune-like antibodies to itself or to single- or double-stranded salmon testis DNA. Plasmids can therefore induce long-term foreign protein expression whilst inducing humoral and cell-mediated immunity without autoimmunity or integration in goldfish.
INTRODUCTION
DNA antiviral vaccines are now under development for fish for several reasons: they induce B-cell and strong T-cell responses in mammals; they are more temperature-stable than protein; they can be produced cheaply and they avoid the risks of accidental release associated with traditional vaccines. Since 1993 most DNA vaccines have contained the immediate early antigen of human cytomegalovirus (CMV) as their promoter/enhancer.
DNA vaccines against the two main virus infections of salmonids, infectious haemopoietic necrosis virus and viral haemorrhagic septicaemia (VHS) are protective and induce antibody in some of the trout.1,2 The VHS vaccine induced T-cell-mediated immunity as measured indirectly by a reduction in expression of the coinjected luciferase gene (pCMV-luc), in transfected trout muscle fibres.3 Such protection experiments in fish require levels of containment which are often available only in government laboratories in the UK. We therefore used pCMV-lacZ, which expresses the Escherichia coliβ-galactosidase (β-gal) reporter gene and regularly induces antibody in fish,4 to study DNA vaccination in goldfish.
It is known that pCMV-lacZ induces cytotoxic T cells which destroy the β-gal-positive myofibres of mice.5–7 This release of β-gal, which is not normally secreted,5 may be the stimulus for the antibody response to β-gal.5 Fish make a more reproducible serum antibody response to β-gal than do mice after direct injection of the lacZ reporter gene4 and their antibody may also be triggered by the destruction of muscle fibres by cytotoxic T cells. We therefore counted β-gal-positive muscle fibres and anti-β-gal antibody-forming cells in goldfish over 70 days to look for an association between the two. We also used cell proliferation to β-gal to assess the T-cell immunity8 which should accompany any destruction of muscle fibres.
We found that β-gal-positive muscle fibres remained in a small majority of fish for 70 days and so we examined safety issues of DNA vaccination at 70 days with regard to three issues: first, the random integration of the injected plasmid although this does not occur in mice9 or trout;10 second, the transferance of the plasmid from muscle to other sites of the body, including the gills, as suggested for 1-g trout and zebrafish;3,10 and third, autoimmunity to single-strand (ss) and double-strand (ds) DNA, because mice made low levels of antibody to ssDNA and no antibody to dsDNA after three injections of a plasmid.11
MATERIALS AND METHODS
Preparation of the plasmids
The pCMV-lacZ was pcDNA3.1/His/LacZ plasmid (Invitrogen, Leek, The Netherlands). The control plasmid (pCMV-0) was pcDNA3 (Invitrogen). The plasmids were purified and resuspended at 1 mg plasmid per ml endotoxin-free saline.12
Animal experiment
One hundred and twenty-six common goldfish (Carassius auratus L.), which were in the weight range of 4 g to 10 g and bred in the UK, were obtained from Neil Hardy Aquatica Ltd, Carshalton, UK. They were maintained at 22±1° and were injected with plasmid as described earlier.4
Eighty-four fish received pCMV-lacZ whilst the other 42 fish received pCMV-0, the empty plasmid, in 50 μl saline. At 4, 7, 14, 21, 28, 35 and 70 days after injection 10 fish which had been injected with pCMV-lacZ and five fish which had been injected with pCMV-0 were killed with a lethal dose of anaesthetic for serum collection.4
The posterior kidney cells from six of 10 immune fish and three of five control fish were collected for culture of antibody-forming cells and proliferating lymphocytes at each time point. The fish were then eviscerated, embedded in optimal cutting tissue (OCT; Raymond Lamb, London, UK) and frozen in liquid nitrogen-cooled isopentane for sectioning to count β-gal-positive muscle fibres.
At 70 days postinjection the remaining four of 10 immune fish and an additional four immune fish were frozen at −70°. Total DNA was extracted from the following sites: the injection site, the opposite uninjected block of muscle, gills, kidney, spleen, upper gut, middle gut, lower gut and thymus and stored in aliquots at 70°.
Counting of β-gal-positive muscle fibres and histology
Serial 10-μm transverse sections were cut in a cryostat at 1-mm intervals over the site of injection. Sections were stained with X-gal.7 The total number of β-gal-positive myofibres4,13 was determined microscopically from six sections per fish at regular intervals across the tissue block.
Enzyme-linked immunosorbent assay (ELISA) for antibody to β-gal protein
Twofold dilutions of fish sera at 1:50–1:12 800 were reacted overnight at 4° and binding was detected using mouse monoclonal antibody WCI 12 to carp immunoglobulin13,14 as described earlier.4,12
Antibody-forming cell assay
The assay was performed as described for dab15 with the following modifications. The kidney cells were gently pressed through nylon netting, the 96-well-filtration plates were coated with β-gal protein as in the above ELISA and the cells were incubated at 26° for 6 hr. The secreted antibody was detected as in the above ELISA and the reddish brown spots were counted under a Wild inverted microscope at a magnification of ×40. The number of anti-β-gal antibody-forming cells in 50 000 cells of each fish in each group was plotted as a column:scatter graph (GraphPad Prism™ Version 2·0, San Diego, CA).
Serum antibody to ss and dsDNA
All fish in the present study had received DNA. Pooled sera from six fish which were known to have serum antibody titres of 800–3200 to β-gal at 28 days after receiving 125 μg DNA and pooled sera from three control fish which had received no DNA at the start of a previous experiment12 were used instead.
DNA antigen for the ELISA was purified from salmon testis DNA and pCMV-lacZ by alcohol extraction16 and then treated either with S1 nuclease to give dsDNA or by boiling to give ssDNA.16 Fish antibodies to ds plasmid DNA, ds salmon testis DNA and ss salmon testis DNA were detected by ELISA16 using mouse monoclonal antibody WCI 12. Positive antibodies to DNA were from patients with systemic lupus erythematosus (SLE) (Inova Diagnostics, San Diego, CA) and their binding was detected by a rabbit anti-human peroxidase-conjugate (Dako, Copenhagen).
Lymphocyte proliferation assay
Kidney leucocytes at 106 per ml were cultured for 4 days at 27° in a 1:1 mixture of OPTIMEM and RPMI-1640 containing 0·5% pooled goldfish serum with 0·5 μg phytohaemagglutinin-P (Sigma, Poole, UK) per ml as a positive mitogen using triplicate 200-μl cultures in microwells.17 In the test wells β-gal was 20 μg per ml.18 Cultures were pulsed with 1 μCi [3H]thymidine per well during the last night and incorporation was assessed by liquid scintillation spectrometry. Results were expressed as the stimulation index of each fish relative to its unstimulated culture which incorporated 1000–4000 counts per minute (c.p.m.). The stimulation indices of all fish in each group were plotted as a column:scatter graph (GraphPad Prism™ Version 2·0). The kidney cells from the 21 control fish each had stimulation indices of less than 1·8 and are not shown except for comparison at day 70.
DNA isolation from tissues
DNA was extracted from 10 mg of the aforementioned tissues using the QIAamp tissue kit (Qiagen Ltd, Crawley, UK) according to the manufacturer’s instructions. The DNA was then ethanol-precipitated and resuspended at 1 μg DNA per μl sterile distilled water.
PCR analysis of tissues
A set of primers specific to plasmid backbone sequences (Gibco BRL, Paisley, UK) were made to amplify the region between the 5′ and 3′ end of the lacZ gene. S-pcDNA3 sense and A-pcDNA3 antisense primers produced an amplicon of ≈3·2 kilobases (kb).
S-pcDNA3 5′-TAATACGACTCACTATAGGG-3′
A-pcDNA3 5′-TAGAAGGCACAGTCGAGG-3′
Hot-start polymerase chain reaction (PCR) amplification by adding the DNA polymerase at 95° was performed in a thermocycler. The cycling conditions included a 3-min initial denaturation step at 95° followed by 35 cycles at 94° for 45 seconds, 55° for 50 seconds and 72° for 3 min with a final extension step at 72° for 8 min.
The final concentrations for all PCR components in a 50-μl volume were as follows: 10 nmol of each dNTP, 40 pmol of each primer, 1 μg of extracted genomic DNA, 5 units Taq polymerase (Promega, Southampton, UK) and 1× reaction buffer [50 mm KCl, 10 mm Tris–HCl, pH 9·0, 0·1% (v/v) Triton-X-100] supplemented with 2·5 mm MgCl2 and 10% (v/v) dimethyl sulphoxide (DMSO). The PCR products were run on 0·8% agarose gels and photographed with Polaroid film.
Southern blot analysis of tissues
Ten micrograms of tissue DNA was digested with either Hin dIII, to linearize the 8·6-kb pcDNA3.1/His/LacZ, or with Hin dIII and Xba I to release the 3·2-kb lacZ gene. Enzymes, buffers and full instructions were supplied from Promega. Digested/undigested DNA were separated in a 1% electrophoresis gel, transferred to a Hybond-N+ nylon membrane (Amersham, Little Chalfont, UK) and visualised by enzyme-linked chemiluminescence using probes to lacZ or pcDNA3.1 prepared by a hexamer random prime fluorescent labelling kit (Amersham).
RESULTS
Counting of β-gal-positive muscle fibres
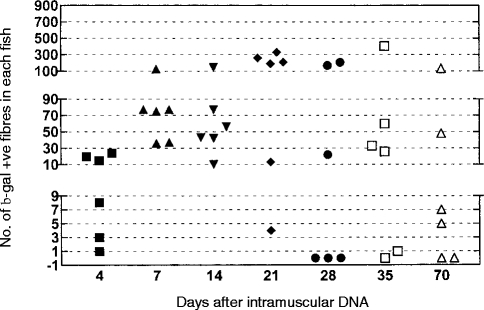
The expression of β-gal started in all six fish at 4 days and peaked at 21 days when a majority of the fish contained more than 100 positive fibres (Fig. 1). Some negative fish started to appear from 28 days onwards. One fish contained more than 100 positive fibres at the end of the experiment at 70 days (Fig. 1). Control fish were negative at each time-point and lacZ-injected fish were negative at their uninjected side.
Figure 1.
Counting of β-gal-positive muscle fibres at different times after intramuscular administration of pCMV-lacZ. Counts from the control fish were negative and are not shown.
Antibody-forming cell assay to β-gal
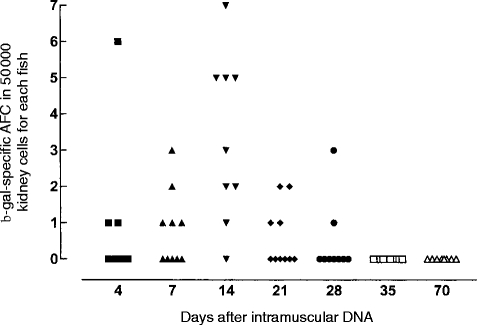
The response peaked at 14 days, which was the only time-point when over half the fish had a response (Fig. 2). The response started at 4 days and had disappeared by 35 days.
Figure 2.
Antibody-forming cell assay to β-gal after lacZ plasmid injection. Symbols for times of sampling are the same as in Fig. 1, control fish were negative and are not shown.
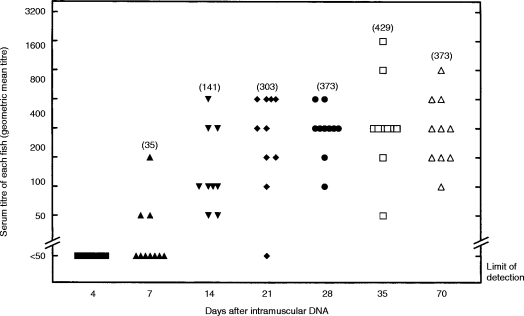
Serum antibody to β-gal
The response was absent at 4 days, increased between days 7 and 14 and then was on a plateau from day 21 to the end of the experiment at day 70 (Fig. 3). Three of 10 fish responded at day 7; 48 of 49 fish responded between days 14 and 70.
Figure 3.
Serum antibody to β-gal at different times after lacZ plasmid injection showing individual ELISA titres. Symbols for times of sampling are the same as in Fig. 1. The titre was the last serum dilution to give a binding of twice the conjugate control, i.e.>0·44. By this criterion 19/20 fish injected with empty plasmid and sampled at 7, 14, 3 5, or 70 days had no antibody to β-gal at their starting serum dilution of 1/25.
Lymphocyte proliferation to β-gal
The response was absent at 4 days, was increasing over days 7 and 14 and then peaked at days 21 and 28 when six of six and eight of eight fish had stimulation indices of >4, respectively (Fig. 4). The response was less robust at 70 days when two of six fish had stimulation indices of <4. Lymphocyte proliferation, serum antibody and the number of β-gal-positive muscle fibres were all high at 21 days postinjection.
Figure 4.
Proliferation of kidney cells to β-gal at different times after lacZ plasmid injection. Symbols for times of sampling are the same as in Fig. 1. The unstimulated cultures incorporated 1000–4 000 c.p.m. The stimulation indices of the fish which had been injected with lacZ are shown whereas those of the control fish were less than 2·0 and are not shown except at 70 days.
Safety – serum antibody to ss and dsDNA
The binding of the serum from the human patient with SLE was at least seven times background which indicated that the salmon testis ssDNA, salmon testis dsDNA and plasmid DNA were antigenic. The pooled serum from the uninjected control fish gave a low level of binding, about twice background, at the starting dilution of 1:25, to all three preparations of DNA. The binding of the immune serum was less than that of the control serum (Fig. 5).
Figure 5.
Serum antibody to plasmid and salmon testis ds and ss DNA by ELISA using dilutions of these sera: standard human SLE serum to dsDNA (*); standard human SLE serum to ssDNA (♦); pooled serum from three fish before DNA (□); pooled serum from eight fish 70 days after LacZ (▪).
Safety – integration of pCMV-lacZ or its dispersal from the site of injection at 70 days
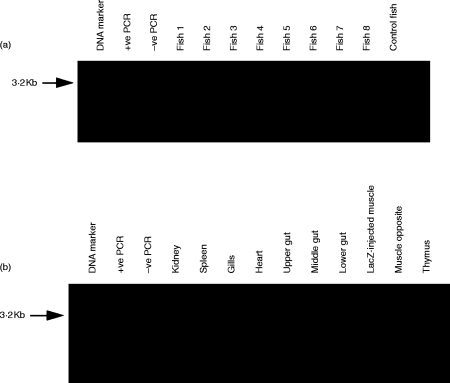
PCR was used to detect plasmid lacZ in DNA from the different tissues. The gene was amplified to the expected size of ≈3·2 kb from the injected muscle of five of eight fish (Fig. 6a). It was present at the injection site but nowhere else (Fig. 6b). Southern blotting of DNA which had been digested using the restriction endonuclease sites adjacent to the lacZ insert showed a band of the same molecular weight as the plasmid insert to be in the injection site of three of five fish (Fig. 7a). The reason that two of five fish gave no signal is probably owing to the lower sensitivity of the Southern blot technique compared to PCR.
Figure 6.
PCR analysis was used to detect the lacZ gene of pCMV-lacZ at 70 days after injection. (a) The 3·2-kb fragment was present in five of the eight fish (numbers 1, 3, 4, 5 and 7), and not in the fish injected with the empty plasmid (control fish). (b) The fragment was in the muscle but in no other tisssue from a pooled material from the three strongly positive fish.
Figure 7.
Southern blotting. (a) 10 μg DNA from the injection site of five PCR-positive fish (fish a–e), and one control fish (CMV-0 injected muscle), were digested with Kpn I and Xba I restiction endonucleases to release a 3·2-kb lacZ fragment which was detected by a fluorescein-labelled lacZ probe in fish c, d and e. (b) Undigested DNA from differing tissues was detected by a fluorescein-labelled probe for lacZ. The injected muscle, but no other tissue, shows the three forms of plasmid DNA: linear, closed and open circles. The three samples of gut each contained a different lacZ band with a molecular weight which was distinct from that of any other sample. (c) 10 μg DNA from differing tissues was cleaved with Hin dIII and detected by the probe to the plasmid backbone. The fragment was only in the muscle and was of the same length as pCMV-lacZ.
To detect the conformation of the plasmid, undigested DNA from the fish tissues was probed for lacZ. DNA with the three conformations of pCMV-lacZ (covalently closed circles, open circles and linear), was present at the injected site but was absent at the opposite side or elsewhere (Fig. 7b). Single bands of DNA which hybridized to lacZ and were of different molecular weight to pCMV-lacZ were in each section of the gut. β-gal was detected in epithelial cells of the gut of untreated fish by X-gal and immunostaining with a monoclonal antibody to β-gal (GAL 13, Sigma) (results not shown).
Tissue DNA and pCMV-lacZ were linearized by a single cut with Hin dIII and probed with pcDNA3.1 to indicate whether the plasmid was integrated. Plasmid DNA, which was indistinguishable from pCMV-lacZ, was in the injected muscle but in no other site (Fig. 7c). Integration of the plasmid could therefore not be demonstrated.
DISCUSSION
We used the reporter gene lacZ, which induces antibody in fish and mice,4 to study the longevity of expression of β-gal and the kinetics of T- and B-cell immunity to DNA vaccines in fish.
β-gal-positive myofibres were present at comparable numbers in mice and fish at 7 days postinjection. The numbers of β-gal-positive fibres averaged 71 per fish (Fig. 1) compared to 236 per murine rectus femoris.19 The disappearance of these muscle fibres occurred from 28 days onwards but some individual fish (Fig. 1) and mice19 remained as obvious expressors at 70 days.
If the anterior tibialis muscle of mice is pretreated with a myotoxic agent the site becomes infiltrated by T cells20 and the destruction of β-gal-positive fibres precedes the antibody response.5 Normal goldfish differed from myotoxin-treated mice in that the disappearance of β-gal-positive fibres did not start until after the antibody-forming cell response had peaked and the serum antibody response was already high (Figs 1,2 and 3). Myotoxic agents were not tested in goldfish because in an earlier experiment they reduced antibody responses to lacZ 10-fold.12
The goldfish with intact muscle fibres were unlikely to be deficient in T cells because their kidney cells proliferated to β-gal in vitro(Fig. 4) and such assays detect primarily T cells.8 This is the first report of T-cell-like proliferation after DNA immunization in fish. The tank temperature of 22° was below the optimum of 32° for scale rejection between goldfish.21 The cytotoxic T-cell response of mice after pCMV-lacZ is empowered by CpG motifs or other T-cell immunostimulants in the DNA inoculum.17 Increasing the water temperature or coinjecting T-cell immunostimulants might therefore have altered the kinetics of fibre destruction and antibody production in goldfish.
When plasmid is injected into mouse muscle it is present at only trace amounts by Southern blotting after 3 hr but can be detected by PCR for 28 days.22 Trout19 and goldfish (Fig. 7) are different to mice because Southern blotting revealed much open circular plasmid DNA for at least 1 month. Circular DNA, especially the closed form, is more functional than linear DNA. The survival of circular DNA in fish muscle might explain why fish are much better than mice at expressing luciferase from CMV-luc.3
In goldfish (Fig. 7), but not trout,10 covalently closed circular DNA was present at 60–70 days. This difference may be technical because conversion from closed to open circular DNA occurs during sample extraction for Southern blotting23 and our sample extraction method using the QIAamp tissue kit would have been gentler than Maniatis’ method as used for trout DNA.10
How circular DNA is protected from nucleases is unknown in fish but the short myotomes of goldfish might reduce the time for DNA to find a possible shelter in micronucleoli as discussed for myofibres of young and old mice.22 The DNA may also be less susceptible to nucleases at the lower temperatures of fish compared to mice. Once fibres are lysed their plasmid DNA is likely to be lost and this might explain why the fractions of fish with β-gal-positive fibres (four of six) and plasmid DNA (five of eight) were similar at 70 days postinjection. In this regard it is interesting that one of the few reports of trace amounts of open circular DNA persisting in mouse muscle for 1 month was made for the luciferase gene23,24 and myofibres expressing this gene survive relatively long-term compared to myofibres expressing β-gal or the surface protein of hepatitis B.5
The induction of persistent cell-mediated immunity and antibody by DNA injection was not accompanied by any obvious shortfall in safety. The lacZ plasmid was not integrated into the muscle and did not spread to other tissues in common with earlier results with CMV-luc in 50 g trout10 and it did not induce the marginal levels of antibodies to DNA which have been reported in mice.11,25
In conclusion we have shown how DNA induces antibody and memory T-like cells in all goldfish in a safe manner. This is accompanied by the long-term expression of DNA in the muscle of about half the fish. The commercial potential of DNA for fish vaccines is therefore strengthened.
Acknowledgments
This work was supported by a ROPA grant from the BBSRC and a visiting fellowship to Professor A. Ambali from the Wellcome Trust. We thank Dr J. Rombout, Wageningen Agricultural University (Wageningen, The Netherlands), for providing monoclonal antibody WCI 12, and DR D. Wells, Imperial College Medical School, London for advice.
References
- 1.Anderson ED, Mourich DV, Fahrenkrug SC, Lapatra S, Shepherd J, Leong J-A C. Genetic immunization of rainbow trout (Oncorhynchus mykiss) against infectious hematopoietic necrosis virus. Mol Mar Biol Biotechnol. 1996;5:114. [PubMed] [Google Scholar]
- 2.Lorenzen N, Lorenzen E, Einer-Jensen K, Heppell J, Wu T, Davis H. Protective immunity to VHS in rainbow trout trout (Oncorhynchus mykiss, Walbaum) following DNA vaccination. Fish and Shellfish Immunology. 1998;8:261. [Google Scholar]
- 3.Heppell J, Lorenzen N, Armstrong NK, et al. Development of DNA vaccines for fish: vector design, intramuscular injection and antigen expression using viral haemorrhagic septicaemia virus genes as model. Fish and Shellfish Immunology. 1998;8:271. [Google Scholar]
- 4.Russell PH, Kanellos T, Sylvester ID, Chang KC, Howard CR. Nucleic acid immunisation with a reporter gene results in antibody production in goldfish (Carassius auratus L.) Fish and Shellfish Immunology. 1998;8:121. [Google Scholar]
- 5.Davis HL, Brazolot Millan CL, Watkins SC. Immune-mediated destruction of transfected muscle fibers after direct gene transfer with antigen-expressing plasmid DNA. Gene Therapy. 1997;4:181. doi: 10.1038/sj.gt.3300380. [DOI] [PubMed] [Google Scholar]
- 6.Tighe H, Corr M, Roman M, Raz E. Gene vaccination: plasmid DNA is more than just a blueprint. Immunol Today. 1998;19:89. doi: 10.1016/s0167-5699(97)01201-2. [DOI] [PubMed] [Google Scholar]
- 7.Wells KE, Maule J, Kingston R, et al. Immune responses, not promoter inactivation, are responsible for decreased long term expression following plasmid gene expression following plasmid gene transfer into skeletal muscle. FEBS Lett. 1997;407:164. doi: 10.1016/s0014-5793(97)00329-3. [DOI] [PubMed] [Google Scholar]
- 8.Bradley LM. Cell proliferation. In: Mishell B M, Shiigi SS, editors. Selected Methods in Cellular Immunology. Vol. 154. San Francisco: W.H.Freeman; 1980. [Google Scholar]
- 9.Nichols WW, Ledwith BJ, Manam SV, Troilo PJ. Potential DNA vaccine integration into host cell genome. Ann N Y Acad Sci. 1998;772:30. doi: 10.1111/j.1749-6632.1995.tb44729.x. [DOI] [PubMed] [Google Scholar]
- 10.Anderson ED, Mourich DV, Leong J-AC. Gene expression in rainbow trout (Oncorhynchus mykiss) following intramuscular injection of DNA. Mol Mar Biol Biotechnol. 1996;5:105. [PubMed] [Google Scholar]
- 11.Katsumi AK, Nobuhiko E, Abe A, Hasegawa Y, Ito M, Sato H. Humoral and cellular immunity to an encoded protein induced by direct DNA injection. Human Gene Therapy. 1994;5:1335. doi: 10.1089/hum.1994.5.11-1335. [DOI] [PubMed] [Google Scholar]
- 12.Kanellos T, Sylvester ID, Howard CR, Russell PH. DNA is as effective as protein at inducing antibody in fish. Vaccine. 1999;17:965. doi: 10.1016/s0264-410x(98)00312-0. [DOI] [PubMed] [Google Scholar]
- 13.Hansen E, Fernandez K, Goldspink G, Butterworth P, Umeda PK, Chang K-C. Strong expression of foreign genes following direct injection into fish muscle. FEBS letts. 1991;290:73. doi: 10.1016/0014-5793(91)81229-2. [DOI] [PubMed] [Google Scholar]
- 14.Secombes CJ, Van Groningen JJM, Egberts E. Separation of lymphocytes in carp Cyprinus carpio L. by monoclonal antibodies: immunohistochemical studies. Immunology. 1983;48:165. [PMC free article] [PubMed] [Google Scholar]
- 15.Secombes CJ, White A, Fletcher TC, Houlihan DF. The development of an ELISPOT assay to quantify total and specific antibody-secreting cells in dab Limanda limanda(L.) Fish and Shellfish Immunology. 1991;1:87. [Google Scholar]
- 16.Wu Z-Q, Drayton D, Pisetsky DS. Specificity and immunological properties of antibodies to bacterial DNA in sera of normal human subjects and patients with systemic lupus erythematosus (SLE) Clin Exp Immunol. 1997;109:27. doi: 10.1046/j.1365-2249.1997.4301328.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Troutaud DLE, Morvan C, Deschaux P. A serum-reduced culture medium for carp lymphocyte in-vitro mitogen-induced proliferation. Fish and Shellfish Immunology. 1995;5:81. [Google Scholar]
- 18.Raz E, Tighe H, Corr M, et al. Preferential induction of a Th1 immune response and inhibition of a specific IgE antibody formation by plasmid DNA immunization. Proc Natl Acad Sci USA. 1996;93:5141. doi: 10.1073/pnas.93.10.5141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Doh SG, Vahlsing HL, Hartikka J, Liang X, Manthorpe M. Spatial-temporal patterns of gene expression in mouse skeletal muscle after injection of lacZ plasmid DNA. Gene Therapy. 1997;4:648. doi: 10.1038/sj.gt.3300460. [DOI] [PubMed] [Google Scholar]
- 20.Mcmahon J, Wells KE, Bamfo JE, Cartwright MA, Wells DJ. Inflammatory reponses following direct injection of plasmid DNA into skeletal muscle. Gene Therapy. 1998;5:1283. doi: 10.1038/sj.gt.3300718. [DOI] [PubMed] [Google Scholar]
- 21.Hildemann WH. Scale homotransplantation in goldfish (Carassius auratus) Ann NY Acad Sci. 1957;64:775. doi: 10.1111/j.1749-6632.1957.tb52472.x. [DOI] [PubMed] [Google Scholar]
- 22.Levy MY, Barron LG, Meyer KB, Szoka FC., Jr Characterisation of plasmid DNA transfer into mouse skeletal muscle: evaluation of uptake mechanism, expression and secretion of gene products into blood. Gene Therapy. 1996;3:201. [PubMed] [Google Scholar]
- 23.Wolff JA, Malone RW, Williams P, et al. Direct gene transfer into mouse muscle in vivo. Science. 1990;247:1465. doi: 10.1126/science.1690918. [DOI] [PubMed] [Google Scholar]
- 24.Wolff JA, Ludtke JJ, Acsadi G, Williams P, Jani A. Long-term persistence of plasmid DNA and foreign gene expression in mouse muscle. Human Molecular Genetics. 1992;1:363. doi: 10.1093/hmg/1.6.363. [DOI] [PubMed] [Google Scholar]
- 25.Donnelly JJ, Ulmer JB, Shiver JW, Liu MA. DNA vaccines. Annu Rev Immunol. 1997;15:617. doi: 10.1146/annurev.immunol.15.1.617. [DOI] [PubMed] [Google Scholar]