Abstract
In this study interleukin (IL)-15 was examined for its ability to modulate the expression of interferon-γ (IFN-γ) and IL-4 in activated human T lymphocytes. The effect of IL-15 was compared with IL-2 and IL-7, cytokines all known to use the IL-2 receptor γC chain. The results demonstrate that the extent of upregulation of IFN-γ and IL-4 mRNA was dependent on the applied cytokine (IL-2>IL-15>IL-7) and on the stimulatory signal. IFN-γ and IL-4 mRNAs were upregulated by IL-15 in concanavalin A- (twofold) and anti-CD3 plus anti-CD28- (fivefold) stimulated T lymphocytes. IFN-γ mRNA accumulation, but not IL-4 mRNA, was additively upregulated by IL-15 plus IL-7 (ninefold) in anti-CD3 stimulated T lymphocytes, and bypassed the requirement of CD28 signalling. Fluorescence-activated cell sorting (FACS) experiments demonstrated that IFN-γ mRNA was upregulated by IL-15 in both CD4+ and CD8+ T lymphocytes, whereas IL-4 mRNA accumulation predominantly occurred in CD4+ cells. Preincubation of highly purified CD4+ T lymphocytes during 7 days with IL-15 and/or IL-7, followed by activation, also showed enhanced IL-4 protein secretion, but predominantly upregulated IFN-γ protein. The net effect was a dramatically increased IFN-γ/IL-4 ratio. Taken together, IL-15 and IL-7 can act as costimulatory signals, which may favour a T helper 1 (Th1) immune response, particularly in the absence of sufficient CD28 costimulation.
INTRODUCTION
Interleukin (IL)-15, a recently identified cytokine, is produced by a wide variety of cells and tissues, such as epithelial and monocytic cells in response to endotoxin stimulation.1,2 The effects of IL-15 are target dependent. IL-15 enhances different monocytic functions, increases interferon-γ (IFN-γ) production by natural killer cells and is a chemoattractant for T lymphocytes.3,4 Recently, it has been demonstrated that IL-15 promotes the production of the T helper 2 (Th2)-like cytokine IL-5 by human helper T lymphocytes.5 The IL-15-mediated effects are transmitted by the IL-15 receptor which consists in part of the β-and γC chain of the IL-2 receptor.6
IL-7 is a cytokine which profoundly affects T lymphocyte functions.7–11 Although IL-7 enhances the production of cytokines such as IL-3 and IL-4,12,13 it preferentially stimulates the Th1-type mediated immune response by enhancing the production of IFN-γ14 and IL-2.15 The observed effects of IL-7 are mediated by the specific IL-7 receptor chain in conjunction with the IL-2 receptor γC chain.16,17 Thus, differential effects of IL-7 and IL-15 on Th1-and Th2-type cytokine expression are mediated partially by the shared IL-2R γC chain. Therefore, it might be of interest to study whether differences exist in effector functions of IL-15 and IL-7 on T lymphocytes, especially regarding the regulation of Th1- and Th2-type cytokines. In the present study we have analysed the effects of IL-15 and IL-7 on the gene expression of IFN-γ (Th1-type) and IL-4 (Th2-type) in activated human T lymphocytes. Although IL-15 and IL-7 enhanced IL-4 gene expression, the upregulatory effect of IL-15 and IL-7 on IFN-γ was most pronounced. The net result being a dramatically increased IFN-γ/IL-4 ratio. We conclude that IL-15 and IL-7 may act as costimulatory signals for T lymphocyte activation and may favour a Th1-type response.
MATERIALS AND METHODS
Preparation of cells
Peripheral blood cells were obtained from healthy volunteer platelet donors, and mononuclear cell suspensions were prepared by Ficoll–Hypaque (Lymphoprep, Nycomed, Oslo, Norway) density-gradient centrifugation as described before.12–14,18 T lymphocytes were isolated by 2-aminoethylisothiouronium bromide (AET) treated sheep red blood cell (SRBC) rosetting. The SRBC were lysed with 155 mmol/l NH4Cl, 10 mmol/l KHCO3, 0·1 mmol/l ethylenediamine tetra-acetic acid (EDTA) and the remaining cell preparations contained more than 98% T lymphocytes as assessed by flow cytometric analysis after staining with an anti-CD2 monoclonal antibody (Becton Dickinson, Mountain View, CA) and less than 1% CD14-positive cells (Becton Dickinson). After isolation, T lymphocytes were cultured overnight at 37° in RPMI-1640 media (Flow, Rockville, MD) containing 4% fetal bovine serum (FBS; Hyclone, Logan, UT) supplemented with 100 U/ml penicillin, 100 μg/ml streptomycin, 2 mmol/l l-glutamine and 6 ng/ml colistine. CD4+ and CD8+ T lymphocytes were obtained by a fluorescence cell sorting method on a FACS-Star flow cytometer (Becton Dickinson) using fluoroscein isothiocyanate (FITC)-labelled CD4-and phycoerythrin-labelled CD8-antibodies (Becton Dickinson). Purity of the obtained CD4+ and CD8+ subpopulations was >99% as assessed by re-analysis. These highly purified CD4+ T lymphocytes contained <1·0% CD56+ cells. Expression of CD25 and CD56 was performed by FACS-Star flow cytometry using FITC-labelled CD25 (Becton Dickinson) and CD56 (Becton Dickinson) antibodies.
Stimulation
T cells (5×106/ml) from five different donors were stimulated with 25 μg/ml concanavalin A (Con A; Calbiochem, La Jolla, CA) or by an optimal concentration of anti-CD3 and anti-CD28 antibodies in a 5% hybridoma supernatant solution14,18 (gift from Dr B. J. Kroesen, Dept of Immunology, University of Groningen, The Netherlands). For the priming experiments purified CD4+ T lymphocytes (106/ml) were preincubated with medium (RPMI-1640), IL-15, IL-7 or combination of IL-15 plus IL-7 used at optimal concentrations.14 After 7 days of incubation the cells were washed twice and placed in fresh medium (RPMI-1640). The viability of the cells was >98% as determined by trypan blue exclusion. After counting the cells, 106 cells were activated with Con A plus phorbol myristate acetate (PMA; Sigma, St. Louis, MO) to maximally induce cytokine secretion. PMA was used at an optimal concentrations of 50 ng/ml. The exogenously added cytokines recombinant human (rh) IL-2 (Eurocetus, Amsterdam, The Netherlands), and rhIL-7 (Dr S. C. Clark, Genetics Institute, Cambridge MA) were used at optimal concentrations of 50 IU/ml and 10 ng/ml.12–14 RhIL-15 (Gift from Dr T. Troutt, Immunex, Seattle, WA) was used at an optimal concentration of 20 ng/ml. In order to determine the stability of mRNA new RNA synthesis was inhibited by Actinomycin D (Act D; Boehringer Mannheim, Mannheim, Germany) used at a concentration of 10 μg/ml.13
Extraction of mRNA and northern analysis
T cells from five different donors were pooled and total cellular RNA was extracted by the guanidinum isothiocyanate/cesium chloride method19 or by the single-step method as described by Chomczynski and Sacchi.20 Total RNA (15 μg) was electrophoresed in 2·2 mol/l formaldehyde, 1·1% agarose gels, and blotted onto nylon membranes (Hybond N+; Amersham, Buckinghamshire, UK).21 cDNA probes were labelled with [α-32*sref32*P]dCTP (3000 Ci/mol; Amersham) by a random hexamer priming method.22 The following cDNA probes were used: (1) the 0·3 kb Eco RI/Nhe I insert of human IL-4 cDNA purified from the pcD-IL-4 plasmid (gift from Dr S. Narula, Schering Plough); (2) the 0·6 kb Eco RI/Hin dIII insert of human IFN-γ cDNA purified from the pSP65 plasmid (gift from Dr C. B. Wilson, University of Washington, Seattle, WA) and (3) the Eco RI linearized pBR322 plasmid containing a 7·8-kb human 28S cDNA insert. Hybridization was performed at 65° for 18 hr in 0·5 mol/l Na2HPO4, pH 7·2, 1 mmol/l EDTA, 7% sodium dodecyl sulphate (SDS). Membranes were washed once in 2×saline sodium citrate (SSC), 0·1% SDS; once in 1×SSC, 0·1% SDS; and finally in 0·3×SSC, 0·1% SDS for 20 min at 65°. The membranes were exposed to Kodak X-omat XAR films (Eastman Kodak, Rochester, NY) at −80° using an intensifying screen. Quantification of mRNA levels was performed by densitometry using a Gel Scan laser densitometer (Pharmacia LKB, Uppsala, Sweden). The data shown are representative for at least three independently performed experiments.
Nuclear run-on transcription assay
To assess transcriptional mechanisms involved in the regulation of IFN-γ and IL-4 gene expression, nuclear run-on experiments were performed as previously described.12–14,18 T-lymphocytes (108) were pelleted at 500 g for 5 min, washed twice with ice-cold phosphate-buffered saline (PBS) and suspended in 4 ml of lysis buffer (10 mmol/l Tris–HCl, pH 7·5; 3 mmol/l MgCl2; 10 mmol/l NaCl; 0·5% NP-40). After gently vortexing, the suspension was incubated on ice for 5 min. Nuclei were pelleted at 500 g for 5 min and the lysis described above was repeated. The nuclei were resuspended in 100 μl glycerol-buffer (50 mmol/l Tris–HCl, pH 8·0, 40% glycerol, 5 mmol/l MgCl2, 0·1 mmol/l EDTA) and incubated at 26° for 20 min with 80 μl transcription buffer (12·5 mmol/l Tris–HCl, pH 8·0; 6·0 mmol/l MgCl2; 125 mmol/l KCl; 2 mmol/l dithiothreitol; 1 mmol/l each of adenosine triphosphate (ATP), cytidine triphosphate (CTP) and guanidine triphosphate (GTP) and 12·5 μl [α-32*sref32*P]UTP (3000 Ci/mol; Amersham). Transcription was terminated by the addition of 40 U DNAseI, 150 U RNAsin, 40 μg yeast RNA, and 200 μl stop buffer (10 mmol/l Tris–HCl, pH 7·4; 0·5 mol/l NaCl; 50 mmol/l MgCl2; 2 mmol/l CaCl2), and the solution was incubated at 37° for 20 min. After proteinase K (750 U/ml) digestion in 1% SDS, nuclear RNA was isolated by phenol–chloroform extraction and two times ethanol precipitated in 2·5 mol/l ammonium acetate. RNA was further purified by Sephadex G-50 column separation (Boehringer). Five micrograms of the following single stranded DNAs were immobilized on Hybond N+ membranes: (1) Eco RI linearized pGEM (negative control); (2) Eco RI linearized pcD-IL-4 plasmid containing a 0·3-kb human IL-4 cDNA fragment; and (3) Eco RI linearized plasmid containing a 0·6-kb human IFN-γ cDNA fragment; Hybridization with equal amounts of labelled RNA was performed as described above. Washing was performed as described above, with one extra wash-step with 2×SSC/1 μg/ml RNAse A (Boehringer).
Reverse transcription (RT) and polymerase chain reaction (PCR)
Reverse transcription
Total cellular RNA isolated from 106 CD4+ and CD8+ T lymphocytes was resuspended in 15 μl diethylpyrocarbonate (DEPC; Sigma) treated H2O and incubated at 68° for 10 min. After cooling on ice the samples were spun briefly in a microcentrifuge. Next, 10 μl of 10×RT mix (135 mm Tris–HCl pH 8·3, 204 mm KCl, 27 mm MgCl2, 0·24 mg/ml bovine serum albumin (BSA), 5·4 mm of each dinucleoside triphosphate (dNTP), 14·3% glycerol) and 3 units of reverse transcriptase (Promega) was added to the samples. The samples were spun briefly and incubated at 37° for 60 min.
Polymerase chain reaction
The following specific primer pairs for β-2-microglobulin (β2μG), IFN-γ and IL-4 were synthesized on a Gene Assembler Plus DNA synthesizer (Pharmacia) and purified using NAP™ 10 colums. β2μG: 5′CCAGCAGAGAATGGAAAGTC3′ (sense) and 5′GATGCTGCTTACATGTCTCG3′ (antisense); IFN-γ: 5′GCAGAGCCAAATTGTCTCCT3′ (sense) and 5′ATGCTCTTCGACCTCGAAAC3′ (antisense); IL-4: 5′TGCCTCCAAGAACACAACTG3′ (sense) and 5′AACGTACTCTGGTTGGCTTC3′ (antisense). One μl of 100 μm sense and antisense of β2μG were added to each RT-reaction sample and DEPC-treated H2O was added to a final volume of 100 μl. Next, the samples were heated for 3 min at 94°, spun briefly in a microcentrifuge and placed on ice. Taq DNA polymerase 0·5 μl of 5 U/μl (Promega) was added to each sample. The tubes were placed in a thermal cycler (Perkin-Elmer, Foster City, CA). PCR was performed for 20, 25, or 32 cycles: 1 min denaturation at 94°, 0·5 min annealing at 55°, and 0·5 min extension at 72°. With these primers fragments spanning 268 basepair (bp), 290 bp and 224 bp were amplified for β2μG, IFN-γ and IL-4, respectively. After 20 PCR cycles 10 μl of the reaction mixture was run on a 2% agarose gel, containing 0·2 μg/ml ethidium bromide in 1×TAE (0·04 m tris-acetate, 0·001 m EDTA) buffer. To be sure that equivalent amounts of cDNA were used in each PCR reaction, bands were quantified with respect β2μG. Then, equal amounts of cDNA were amplified with primers for IFN-γ or IL-4. A 100-bp ladder (Pharmacia) was used as DNA marker
Measurement of IFN-γ and IL-4 protein
Human T lymphocytes (1–3×106/ml) were stimulated as indicated in the text in the presence or absence of maximal effective concentrations of IL-15 and/or IL-7 for 12 and 24 hr. Viability of cells was controlled by trypan blue exclusion. Secreted IFN-γ and IL-4 protein was quantified in cellfree supernatants using a human IFN-γ or human IL-4 enzyme-linked immunosorbent assay (ELISA) kit (Central Laboratory of the Netherlands Red Cross Blood Transfusion Service, Amsterdam, The Netherlands) as recommended by the manufacturers. The data for CD4+ T lymphocytes are expressed as picogram (pg) protein/106 cells.
Statistical analysis
For the protein measurements, statistical analysis was performed using Student’s t-test for paired observations. Statistical significance of the secretion data was set at P < 0·05.
RESULTS
IL-15 enhances IFN-γ mRNA accumulation in activated T lymphocyte
T lymphocytes stimulated with IL-15 alone did not demonstrate IFN-γ and IL-4 transcripts (data not shown). However, T lymphocytes costimulated with Con A demonstrated a dose dependent increase in the IFN-γ mRNA expression in response to IL-15 stimulation. Optimal expression was observed at a dose of 20 ng/ml and this concentration was further used in all experiments.
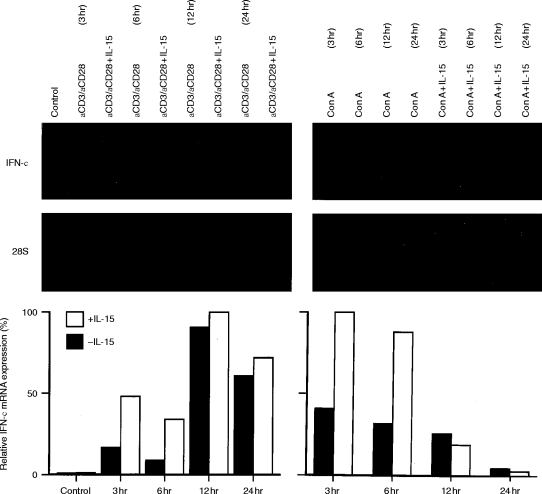
Time curve experiments with both Con A and anti-CD3/anti-CD28 in the absence and presence of IL-15 demonstrated that IL-15 did not change the kinetics of IFN-γ mRNA expression but rather enhanced the degree of stimulation (Fig. 1). Surprisingly, the pattern of accumulation for Con A and anti-CD3/anti-CD28-induced IFN-γ mRNA were distinctly different. Con A-induced IFN-γ mRNA was transiently expressed, whereas anti-CD3/anti-CD28-induced message for IFN-γ was ongoing in time and still present after 24 hr of stimulation. However, in both Con A- and anti-CD3/anti-CD28-stimulated cells, the upregulatory effect of IL-15 was an early event: IL-15 had its maximal effect after 3–6 hr of stimulation.
Figure 1.
Kinetics of IFN-γ mRNA accumulation in anti-CD3/anti-CD28 (left panel) and Con A (right panel) stimulated T lymphocytes in the absence and presence of IL-15 (indicated at top). Northern analysis of total RNA extracted after 3, 6, 1 2 and 24 hr of activation. The bar diagrams represent relative IFN-γ mRNA levels after normalization to the respective 28S signals. The data shown are representative for three independent experiments.
IL-15 and IL-7 additively enhance IFN-γ mRNA, but not IL-4 mRNA
As shown in Fig. 2, IL-15 was able to enhance anti-CD3 induced IFN-γ mRNA accumulation compared to CD3 stimulation alone, and the effect was comparable to IL-7 (about fivefold). A combination of IL-15 plus IL-7 acted additively on the anti-CD3-induced IFN-γ mRNA accumulation, demonstrating a ninefold increase compared to stimulation with anti-CD3 alone, and was able to bypass the signal normally delivered by CD28. Although antibodies against CD3 induced very low levels of IL-4 mRNA, both IL-15 and IL-7 had no obvious effect on the accumulation of IL-4 mRNA in anti-CD3-activated T lymphocytes.
Figure 2.
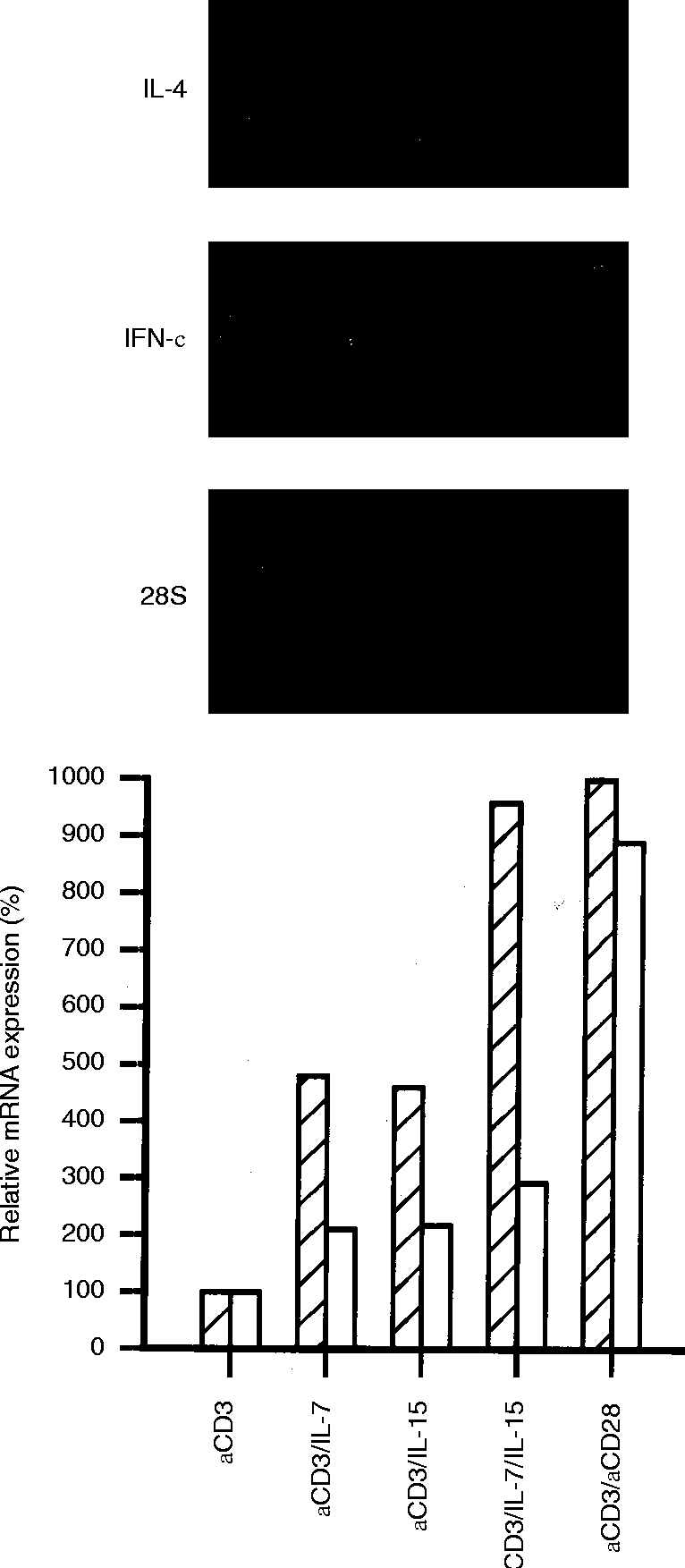
Effect of IL-15 on the accumulation of IFN-γ and IL-4 mRNA in T lymphocytes stimulated with different activators. Northern analysis of total RNA extracted after 5 hr of stimulation with anti-CD3 (lane 1), in the presence of IL-7 (lane 2), IL-15 (lane 3), IL-7 plus IL-15 (lane 4), or anti-CD28 (lane 5). The bar diagrams represent relative IFN-γ (hatched bars) and IL-4 mRNA (open bars) after normalization to the 28S signal. The data shown are representative for three independent experiments.
To further compare the effects of cytokines which make use of the IL-2 receptor γC, anti-CD3/anti-CD28-stimulated T lymphocytes were preincubated with IL-2, IL-7 and IL-15 at optimal concentrations. IFN-γ and IL-4 mRNAs were most potently enhanced by IL-2, followed by IL-15 and IL-7 (Fig. 3).
Figure 3.
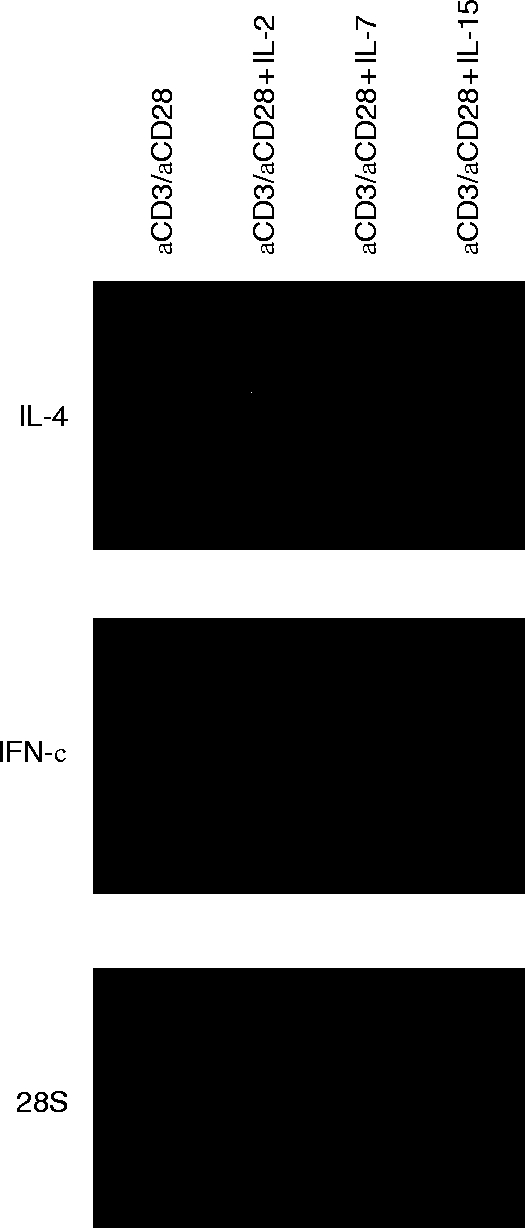
Effects of IL-2, IL-7 and IL-15, which share the IL-2 receptor γC chain on the accumulation of IFN-γ and IL-4 mRNA in anti-CD3/anti-CD28-stimulated T lymphocytes. Northern analysis of total RNA extracted after 5 hr of stimulation with anti-CD3/anti-CD28 (lane 1), anti-CD3/anti-CD28 plus IL-2 (lane 2), anti-CD3/anti-CD28 plus IL-7 (lane 3), and anti-CD3 /anti-CD28 plus IL-15 (lane 4). The data shown are representative for three independent experiments.
IL-15 induces upregulation by post-transcriptional mechanisms
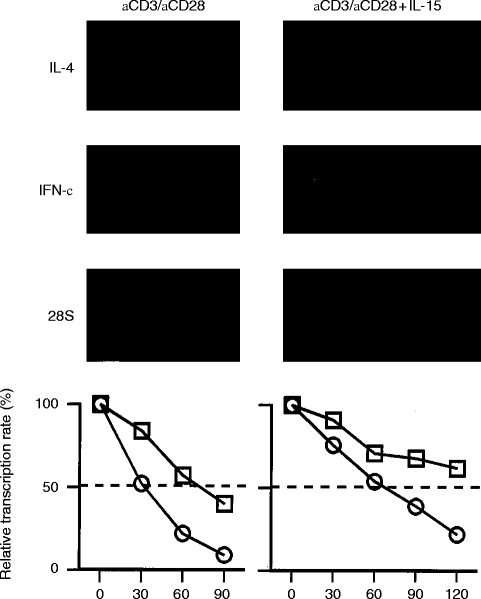
To determine whether the induced upregulation by IL-15 is the result of changes at the post-transcriptional level, half-life studies were performed using the actinomycin D chase technique. Anti-CD3/anti-CD28-induced IFN-γ mRNA decayed with a t½ of ≈30 min (Fig. 4). In the presence of anti-CD3/anti-CD28 plus IL-15, the t½ of IFN-γ mRNA increased to ≈60 min. Similar results were obtained for Con A-activated T lymphocytes. Here, in the presence of IL-15, the t½ increased from ≈40 min to >90 min. IL-4 mRNA decayed with a t½ of ≈60 min in anti-CD3/anti-CD28-stimulated T lymphocytes. In the presence of IL-15 the stability of IL-4 mRNA transcripts increased to >120 min.
Figure 4.
Effect of IL-15 on the stability of anti-CD3/anti-CD28 induced IL-4 and IFN-γ mRNA. T lymphocytes were stimulated with anti-CD3/anti-CD28 in the absence (left panel) and presence (right panel) of IL-15 for 6 hr. After addition of actinomycin D, mRNA was extracted at different time points (indicated in minutes). mRNA decline was meausered and plotted in a graph (bottom panels). From the slopes, the t½ values were calculated (IL-4, square; IFN-γ, circle). The data shown are representative for three independent experiments.
In addition, the transcription rates of the IFN-γ and IL-4 gene were studied by nuclear run-on studies. Previously, we have shown that the anti-CD3/antiCD28-induced IFN-γ transcription rate was twofold enhanced by IL-7,14 while leaving IL-4 gene transcription unaffected. In contrast, IL-15 did not significantly affect the anti-CD3/anti-CD28-induced transcription rate of both IFN-γ and IL-4 genes, indicating that the promotive effect of IL-15 is mediated at post-transcriptional level (data not shown).
Comparison of IL-15 and IL-7 on IFN-γ and IL-4 protein secretion
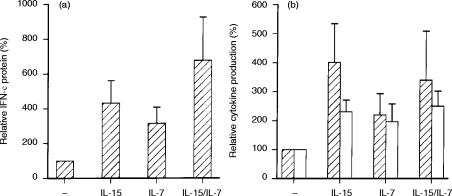
Next, we assessed whether the observed mRNA data corresponded with protein secretion in T lymphocytes. Because the upregulatory effects of IL-15 and IL-714 are early events, supernatants were collected after 12 and 24 hr of stimulation and analyzed for IFN-γ and IL-4 protein, respectively. The data given in Fig. 5 are expressed as mean percentages with respect to stimulated cells in the absence of the cytokine(s) (100%) and are calculated per individual donor.
Figure 5.
Modulation of IFN-γ (hatched bars) and IL-4 (open bars) protein secretion in activated T lymphocytes in the absence and presence of IL-15 and/or IL-7. IFN-γ and IL-4 protein levels are expressed as percentage (x±SEM, n = 5) of the production after stimulation with the mitogen in the absence of IL-15 and IL-7.(a) Relative IFN-γ protein levels in supernatants collected after 12 hr of stimulation with anti-CD3 alone (mean: 151 pg/ml, range: 92–235 pg/ml), or in the presence of IL-15 (mean: 540 pg/ml, range: 182–1299 pg/ml), IL-7 (mean: 316 pg/ml, range 122–696 pg/ml) or a combination of IL-15 plus IL-7 (mean: 915 pg/ml, range: 298–2423 pg/ml). (b) Relative IFN-γ and IL-4 protein levels in supernatants collected after 12 hr (IFN-γ) and 24 hr (IL-4) of stimulation with anti-CD3/anti-CD28 (IFN-γ mean=100%=959 pg/ml, range: 431–1941 pg/ml; IL-4 mean=100%=171 pg/ml, range 39–422 pg/ml) in the absence and presence of IL-15, IL-7 or a combination of IL-15 plus IL-7.
T lymphocytes stimulated for 24 hr with anti-CD3 did not secrete detectable levels of IL-4 protein (<40 pg/ml), whether or not costimulated with IL-15, IL-7 or a combination of IL-15 plus IL-7. As shown in Fig. 5(a), 12 hr of stimulation with anti-CD3 resulted in the secretion of IFN-γ protein (range 92–235 pg/ml; 100%). After 12 hr of stimulation with anti-CD3 plus IL-15 or IL-7 the secretion of IFN-γ protein increased. In accordance with the mRNA data, costimulation with anti-CD3/anti-CD28 and IL-15 plus IL-7 further enhanced IFN-γ protein secretion.
Figure 5(b) shows that T cells stimulated with anti-CD3/anti-CD28 for 24 hr secreted low levels of IL-4 protein (range 39–422 pg/ml; 100%). Costimulation with anti-CD3/anti-CD28 and IL-15 or IL-7 increased the production of IL-4 protein. Costimulation with anti-CD3/anti-CD28 and IL-15 plus IL-7 did not further increase IL-4 protein secretion. Similar results were obtained for IFN-γ protein secretion after 12 hr of stimulation. Anti-CD3/anti-CD28 T lymphocytes secreted high levels of IFN-γ protein (range 431–1941 pg/ml; 100%). Costimulation with anti-CD3/anti-CD28 plus IL-15 or IL-7 increased IFN-γ productions. Costimulation with IL-15 plus IL-7 could not further increase the production of IFN-γ protein.
Although the amount of secreted IFN-γ and IL-4 protein was highly variable between donors, the observed increment of IFN-γ and IL-4 protein induced by IL-15, IL-7 and IL-15 plus IL-7 was significant in all donors (P < 0·02).
T lymphocyte subpopulations are responsible for IFN-γ and IL-4 production
To exclude the possibility that a subset of IFN-γ-producing CD8+ natural killer (NK) cells is involved in our observations on IFN-γ production, we extended our experiments with highly purified CD4+ T lymphocytes containing <1·0% CD56+ cells as assessed by FACS analysis. By semiquantitative RT–PCR it was demonstrated that the accumulation of IFN-γ and IL-4 mRNAs were enhanced by IL-15 in activated CD4+ T lymphocytes. Although IFN-γ mRNA was upregulated by IL-15 in activated CD4+ T lymphocytes, the upregulatory effect of IL-15 was even greater in activated CD8+ lymphocytes. In contrast, IL-4 mRNA accumulation predominantly occurred in activated CD4+ T lymphocytes (Fig. 6).
Figure 6.
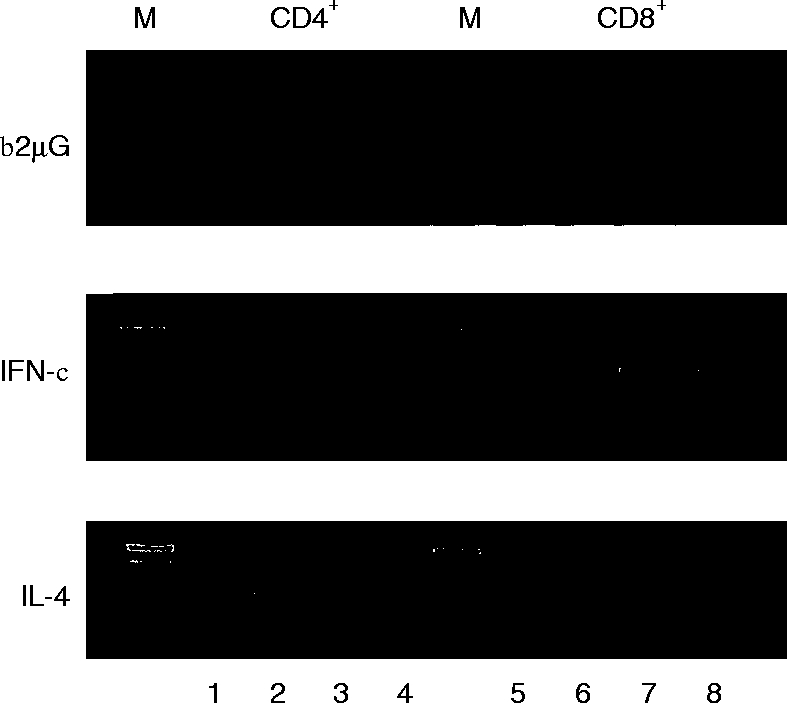
Effect of IL-15 on FACS-sorted CD4+ and CD8+ T lymphocytes stimulated during 6 hr with anti-CD3 (lane 1 and 5), anti-CD3 plus IL-15 (lane 2 and 6), anti-CD3/anti-CD28 (lane 3 and 7), and anti-CD3/anti-CD28 plus IL-15 (lane 4 and 8). Ethidium bromide stained agarose gel demonstrating RT–PCR-amplified products corresponding to IFN-γ (25 cycles) and IL-4 (32 cycles). The signals for β2μG (20 cycles) show comparable amounts of amplified product in each lane. The data shown are representative for three independent experiments. M=100 bp marker.
Priming with IL-15 and/or IL-7 increases IFN-γ/IL-4 ratio
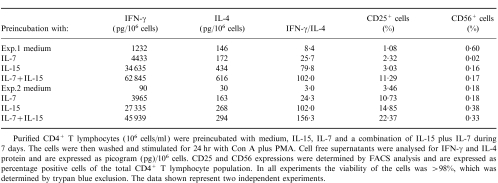
In the described experiments IL-15 was added in conjunction with the T-cell activators. However, recent studies have demonstrated that IL-15 alone can activate different intracellular signalling pathways like phosphorylation of JAK-3 and activation of STAT-5.16 In view of these results we questioned whether pre-incubation with IL-15 might influence the IFN-γ and IL-4 protein secretion. Highly purified CD4+ T lymphocytes (106/ml) were obtained after cell sorting and pre-incubated with medium (control), IL-15, IL-7 or a combination of IL-15 plus IL-7. After 7 days of incubation the cells were washed twice, counted and 106 cells were placed in fresh medium. CD4+ T lymphocytes preincubated with IL-15 and/or IL-7 alone, did not secrete detectable levels of IFN-γ, IL-4 or IL-2 protein (data not shown). Priming of CD4+ T lymphocytes with IL-15 and/or IL-7, followed by activation with Con A plus PMA for 24 hr, had differential effects on IFN-γ and IL-4 protein secretion with respect to the extent of upregulation. As demonstrated in Table 1, pre-incubation with IL-15 or IL-7 dramatically enhanced the IFN-γ/IL-4 ratio. The presence of IL-15 plus IL-7 during 7 days even further increased the IFN-γ/IL-4 ratio. In addition, it is shown that priming with IL-15 or IL-7 upregulated the expression of CD25, whereby the most prominent effect was observed with the combination of IL-15 plus IL-7. In contrast, the 7 days priming with IL-15 and/or IL-7 did not expand the CD56+ cell population.
Table 1.
Effect of preincubation with medium, IL-15, IL-7 or a combination of IL-15 plus IL-7 on the secretion of IFN-γ and IL-4 protein and on the expression of CD25 and CD56 by CD4+T lymphocytes
Purified CD4+ T lymphocytes (106 cells/ml) were preincubated with medium, IL-15, IL-7 and a combination of IL-15 plus IL-7 during 7 days. The cells were then washed and stimulated for 24 hr with Con A plus PMA. Cell free supernatants were analysed for IFN-γ and IL-4 protein and are expressed as picogram (pg)/106 cells. CD25 expressions were determined by FACS analysis and are expressed as Percentage positive cells of the total CD4+ T lymphocyte population. In all experiments the viability of the cells was > 98%, which was determined by trypan blue exclusion. The data shown represent two independent experiments.
DISCUSSION
In the present report, we have examined IL-15 for its ability to stimulate T lymphocytes in their capacity to produce IFN-γ and IL-4. The results were compared with IL-2 and IL-7, which also share the IL-2R γC chain for lymphocyte activation. Although IL-15 alone has been shown to induce proliferation in T lymphocytes,23 it was not capable of inducing IFN-γ and IL-4 mRNA expression when used as the only stimulus. In the presence of a second mitogenic signal, like the combination of anti-CD3 plus anti-CD28 or Con A, IL-15 exhibited a promotive effect on both IFN-γ and IL-4 mRNA accumulation. Interestingly, anti-CD3 activated T lymphocytes costimulated with a combination of IL-15 plus IL-7 showed a additive effect on the IFN-γ mRNA accumulation and together they were able to mimic the signal(s) normally delivered by CD28. This additive effect may indicate that IL-15 delivers its signal mainly through the β-chain of the IL-2 receptor,3 whereas IL-7 uses the γC-chain in conjunction with the specific IL-7 receptor α-chain.24 This suggestion is supported by observations that antibodies to the IL-2 receptor β-chain abolished the stimulatory effect of IL-15 on IFN-γ and IL-4 protein secretion, leaving the IL-7-induced upregulation unaffected (unpublished observations). The differences in the upregulation of IFN-γ and IL-4 in response to IL-7 and IL-15 may in part be ascribed to different cellular origins. Although IFN-γ was produced by CD4+ cells, the main source of IFN-γ were CD8+ cells. In contrast, IL-4 mRNA accumulation was predominantly observed in CD4+ cells.
As has been demonstrated for IL-7,14 the IFN-γ mRNA and the protein secretion data designate IL-15 as an early acting cytokine that is able to partially bypass the requirement for CD28 stimulation. In contrast, although very low levels of IL-4 mRNA were detectable, IL-4 protein was not secreted in the absence of the costimulatory signal delivered by CD28. Thus, in the absence of sufficient CD28 costimulation IL-15 may direct towards a Th1 immune response. The observation with IL-15 on IFN-γ is supported by investigations demonstrating that antigen-specific unresponsiveness can be overcome by activation of the IL-2 receptor γ-chain, a component of both IL-15 and IL-7 receptor complexes.6,17,24 These findings may have important implications for antigen-presenting properties of cells to T lymphocytes, for instance in respiratory tissue. It has been shown that human alveolar macrophages derived from the respiratory tract are impaired in their capacity to present antigen as a result of a diminished expression of B7-1/2 (CD80/86), the physiological ligands for CD28.25 IL-15 produced by lung tissue1 may thus induce T-cell activation without the necessity of triggering the CD28 signalling pathway. It has also been demonstrated that (lung) epithelial cells are a major source of IL-15.26 Because IL-15 has chemoattractive and activating properties for T lymphocytes,4,23 the latter observation may have implications for asthmatic subjects. Recently, it has been shown that T lymphocytes obtained from asthmatics show enhanced expression of CD25.27 This may be the result of IL-15 locally produced by epithelial cells, as we demonstrated that CD25 can be substantially upregulated by IL-15. In addition, locally produced IL-7 (for instance by B-cells28) may further enhance CD25 expression. Although it can be reasoned that the observed upregulation of CD25 expression may be the result of IL-2 produced during the incubation period, we were not able to demonstrate IL-2 protein in supernatants incubated during 7 days with IL-15 and/or IL-7 alone. Therefore, the observed enhanced activation status of T lymphocytes obtained from asthmatics may result from augmented local production of IL-15 and/or IL-7 in context with the allergen. This view is supported by the observation that allergen-specific Th2 clones produce IL-5 in response to IL-15, without requiring additive activation signal(s).5
The priming experiments with IL-15-and IL-7-preincubated CD4+ T lymphocytes demonstrate that T lymphocytes become more prone to activating signals, reflected by enhanced secretion of both IL-4 and IFN-γ protein. This phenomenon might be a result of the intracellular accumulation of STAT-5 and subsequent binding to its specific DNA sequence,18 thereby facilitating the transcription of genes that STAT-5 binds to. This is supported by recent observations in our laboratory demonstrating that tyrosine phosphorylation of STAT-5 depended on IL-7, whereas serine phosphorylation of STAT-5 and transactivation depended on T-cell receptor activation.29 The ultimate effect of priming with either IL-15, IL-7 or the combination of IL-15 plus IL-7 resulted in a Th1-like cytokine profile: high levels of IFN-γ and low levels of IL-4. These data are different from the observations of Mori et al.,5 who showed that IL-5 (a Th2-like cytokine) could be induced by IL-15 in T helper clones, implicating Th2-directing properties for IL-15. This discrepancy may be due to the studied cell type: T helper clones5 versus fresh human T lymphocytes used in our study. However, we provide evidence that IL-15 is rather an overall T-cell activator which may promote Th1-like immune responses. This was most convincingly demonstrated by the increased IFN-γ/IL-4 ratio after priming with IL-15 for 7 days.
In this paper we demonstrate that IL-15 and IL-7 share many proporties with respect to the (up)regulation of IFN-γ and IL-4 gene expression. We show that IL-15 only affects the stability of IFN-γ mRNA, demonstrating a functional difference compared to IL-7, which also enhanced IFN-γ gene transcription.14 With respect to IL-4 gene expression no obvious differences between the cytokines were observed, because both IL-15 and IL-714 only affected IL-4 mRNA stabilization. Taken together, IL-7 and IL-15 are important mediators in the ultimate outcome of a specific cytokine pattern. Specifically, in the absence of the costimulatory signal(s) triggered by CD28, IL-15 and the combination of IL-7 plus IL-15 dramatically enhanced the IFN-γ/IL-4 ratio, and may thus enhance a Th1-like response.
Acknowledgments
We thank Dr S. Narula and Dr C. B. Wilson for providing the IL-4 probe and the IFN-γ probe, respectively; Dr T. Troutt for providing rhIL-15; Dr S. C. Clark for providing rhIL-7. We thank Dr B. J. Kroesen for the anti-CD3 and anti-CD28 Abs. This study was financially supported by a grant from the ‘Dutch Asthma Foundation’ (Grant no. 92·30).
Glossary
Abbreviations
- Act D
actinomycin D
- IL
interleukin
- Th
T helper
References
- 1.Grabstein KH, Eisenman J, Shanebeck K, et al. Cloning of a T cell growth factor that interacts with the β chain of the interleukin-2 receptor. Science. 1984;264:965. doi: 10.1126/science.8178155. [DOI] [PubMed] [Google Scholar]
- 2.Doherty TM, Seder RA, Sher A. Induction and regulation of IL-15 expression in murine macrophages. J Immuno. 1996;156:735. [PubMed] [Google Scholar]
- 3.Carson WE, Giri JG, Lindemann MJ, et al. Interleukin (IL) 15 is a novel cytokine that activates human natural killer cells via components of the IL-2 receptor. J Exp Med. 1994;180:1395. doi: 10.1084/jem.180.4.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wilkinson PC, Liew FY. Chemoattraction of blood T lymphocytes by interleukin-15. J Immunol. 1995;181:1255. doi: 10.1084/jem.181.3.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mori A, Suko M, Kaminuma O, et al. IL-15 promotes cytokine production of human T helper cells. J Immunol. 1996;156:2400. [PubMed] [Google Scholar]
- 6.Giri JG, Adhie M, Eisenmann J, et al. Utilization of the β and γ chains of the IL-2 receptor by the novel cytokine IL-15. EMBO J. 1994;13:2822. doi: 10.1002/j.1460-2075.1994.tb06576.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Touw I, Pouwels K, van Agthoven T, et al. Interleukin-7 is a growth-factor of precursor B and T acute lymphoblastic leukemia. Blood. 1990;75:2097. [PubMed] [Google Scholar]
- 8.Hickman CJ, Crim JA, Mostowski HS, Siegel JP. Regulation of human cytotoxic T lymphocyte development by IL-7. J Immunol. 1990;145:2415. [PubMed] [Google Scholar]
- 9.Alderson MR, Sassenfeld HM, Widmer MB. Interleukin 7 enhances cytolytic T lymphocyte generation and induces lymphokine-activated killer cells from human peripheral blood. J Exp Med. 1990;172:577. doi: 10.1084/jem.172.2.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grabstein KH, Namen AE, Shanebeck K, Voice RF, Reed SG, Widmer MB. Regulation of T cell proliferation by IL-7. J Immunol. 1990;144:3015. [PubMed] [Google Scholar]
- 11.Armitage RJ, Namen AE, Sassenfeld HM, Grabstein KH. Regulation of human T cell proliferation by IL-7. J Immunol. 1990;144:938. [PubMed] [Google Scholar]
- 12.Dokter WHA, Sierdsema SJ, Esselink MT, Halie MR, Vellenga E. IL-7 enhances the expression of IL-3 and granulocyte–macrophage CSF mRNA in activated human T cells by post-transcriptional mechanisms. J Immunol. 1993;150:2584. [PubMed] [Google Scholar]
- 13.Dokter WHA, Sierdsema SJ, Esselink MT, Halie MR, Vellenga E. Interleukin-4 mRNA and protein in activated human T cells are enhanced by IL-7. Exp Hematol. 1994;22:74. [PubMed] [Google Scholar]
- 14.Borger P, Kauffman HF, Postma DS, Vellenga E. IL-7 differentially modulates the expression of IFN-γ and IL-4 in activated human T lymphocytes by transcriptional and post-transcriptional mechanisms. J Immunol. 1996;156:1333. [PubMed] [Google Scholar]
- 15.de Gringhuis SILF, Leij EW, Verschuren P, Borger, Vellenga E. Interleukin (IL) -7 upregulates the IL-2 gene expression in activated human T lymphocytes at the transcriptional level by enhancing the DNA binding activities of both nuclear factor of activated T cells and activator protein 1. Blood. 1997;90:2690. [PubMed] [Google Scholar]
- 16.Lin JX, Migone TS, Tsang M, et al. The role of shared receptor motifs and common Stat proteins in the generations of cytokine pleiotropy and redundancy by IL-2, IL-4, IL-7, IL-13 and IL-15. Immunity. 1995;2:331. doi: 10.1016/1074-7613(95)90141-8. [DOI] [PubMed] [Google Scholar]
- 17.Boussiotis VA, Barber DL, Nakarai T, et al. Prevention of T cell anergy by signaling through the γc chain of the IL-2 receptor. Science. 1994;266:1039. doi: 10.1126/science.7973657. [DOI] [PubMed] [Google Scholar]
- 18.Borger P, Kauffman HF, Postma DS, Vellenga E. Interleukin-4 gene expression in activated human T lymphocytes is regulated by the cyclic adenosine monophosphate-dependent signaling pathway. Blood. 1996;87:691. [PubMed] [Google Scholar]
- 19.Vellenga E, Rambaldi A, Ernst TJ, Ostapovicz D, Griffin JD. Independent regulation of M-CSF and G-CSF gene expression in human monocytes. Blood. 1988;71:1529. [PubMed] [Google Scholar]
- 20.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate–phenol chloroform extraction. Anal Biochem. 1987;162:156. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 21.Maniatis T, Fritsch EF, Sambrook A. Molecular Cloning: a Laboratory Manual. Harbor, NY: Cold Spring Harbor Laboratory, Cold Spring; 1982. [Google Scholar]
- 22.Feinberg AP, Vogelstein B. A technique for labeling DNA restriction endonuclease fragments with high specific activity. Anal Biochem. 1983;132:6. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- 23.Nishimura H, Heromatsu K, Kabayashi N, et al. IL-15 is a novel growth factor for murine γδ T cells induced by Salmonella infection. J Immunol. 1996;156:663. [PubMed] [Google Scholar]
- 24.Ziegler SF, Morella KK, Anderson D, et al. Reconstitution of a functional interleukin (IL) -7 receptor demonstrates that the IL-2 receptor γ chain is required for IL-7 signal transduction. Eur J Immunol. 1995;25:399. doi: 10.1002/eji.1830250214. [DOI] [PubMed] [Google Scholar]
- 25.Chelen CJ, Fang Y, Freeman GJ, et al. Human alveolar macrophages present antigen ineffectively due to defective expression of B7 costimulatory cell surface molecules. J Clin Invest. 1994;95:1415. doi: 10.1172/JCI117796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Holton RH, Bost KL. Interleukin 15 (IL-15) is upregulated in mucosal tissues, following oral inoculation with Salmonella: a possible role for IL-15 in the immune response against this pathogen. FASEB J. 1996;10(A):1167. [Google Scholar]
- 27.Corrigan CJ, Kay AB. T cells and eosinophils in the pathogenesis of asthma. Immunol Today. 1992;13:501. doi: 10.1016/0167-5699(92)90026-4. [DOI] [PubMed] [Google Scholar]
- 28.Benjamin D, Sharma V, Knobloch TJ, Armitage RJ, Dayton MA, Goodwin RG. B-cell IL-7. human B-cell lines constitutively secrete IL-7 and express IL-7 receptors. J Immunol. 1994;152:4749. [PubMed] [Google Scholar]
- 29.de Gringhuis SI, de Leij LFMH, Vellenga E. Serine phosphorylation of STAT 5 in response to interleukin-7 and T cell receptor activation is mediated by the mitogen-activated protein kinase p38/Mpk2 in human T lymphocytes. 1998 submitted. [Google Scholar]