Abstract
Female C57BL/6 mice infected with the LP-BM5 leukaemia retrovirus developed murine acquired immune-deficiency syndrome (AIDS). Dehydroepiandrosterone (DHEA) and melatonin (MLT) modify immune dysfunction and prevent lipid peroxidation. We investigated whether DHEA and MLT could prevent immune dysfunction, excessive lipid peroxidation, and tissue vitamin E loss induced by retrovirus infection. Retrovirus infection inhibited the release of T helper 1 (Th1) cytokines, stimulated secretion of Th2 cytokines, increased hepatic lipid peroxidation, and induced vitamin E deficiency. Treatment with DHEA or MLT alone, as well as together, largely prevented the reduction of B- and T-cell proliferation as well as of Th1 cytokine secretion caused by retrovirus infection. Supplementation also suppressed the elevated production of Th2 cytokines stimulated by retrovirus infection. DHEA and MLT simultaneously reduced hepatic lipid peroxidation and prevented vitamin E loss. The use of DHEA plus MLT was more effective in preventing retrovirus-induced immune dysfunction than either DHEA or MLT alone. These results suggest that supplementation with DHEA and MLT may prevent cytokine dysregulation, lipid oxidation and tissue vitamin E loss induced by retrovirus infection. Similarly, hormone supplementation also modified immune function and increased tissue vitamin E levels in uninfected mice.
INTRODUCTION
Murine acquired immune-deficiency syndrome (AIDS) is induced in genetically susceptible strains of mice inoculated with the LP-BM5 murine leukaemia retrovirus mixture (MuLV). It is strikingly similar to human AIDS, even though human immunodeficiency virus (HIV) and murine retrovirus represent different retroviruses.1 Murine AIDS is characterized by splenomegaly, lymphadenopathy, reduced B- and T-cell function, loss of disease resistance, dysfunctional cytokine production, and tissue vitamin E deficiency.1 In retrovirus-infected people and mice, T helper 1 (Th1) cytokine [interleukin-2 (IL-2) and interferon-γ (IFN-γ)] production declines, while Th2 cytokine (IL-4, IL-5, IL-6, and IL-10) production increases.2–4 The excessive Th2 cytokines suppress Th1 cells, causing anergy of cell-mediated immunity, thus allowing the retrovirus as well as normal flora to reproduce and stimulate oxidative radical secretion by macrophages.5
Superoxide radicals including lipid peroxides are produced in greater quantities during retroviral infection as breached immune defences facilitate increased exposure to bacterial mitogens and endotoxins.6 Oxidation results in damage to lymphocyte DNA that inhibits cell function. Increased oxidants should promote disease progression from retrovirus infection to AIDS, by exacerbating nutritional deficiencies and suppressing immune cell function.7 Tissue levels of vitamin E and immune function were decreased during murine AIDS.5 In HIV-infected patients, serum vitamin E levels were reduced while supplementary vitamin E slowed the progression to AIDS.8
Dehydroepiandrosterone (DHEA) and DHEA sulfate (DHEAS) are major secretory products of the adrenal gland in humans and function as precursors for androgenic hormones. Serum DHEAS levels declined as HIV-1 infection progressed.9 DHEA supplementation could reduce the immune dysfunction of murine AIDS.10 DHEA moderated human immune dysfunction by overcoming cytokine dysregulation11 and preventing cell damage caused by free radicals.12
Melatonin (MLT), the principal pineal hormone secreted in response to photoperiod, influences many biological functions. MLT production declines with age when susceptibility to immune dysfunction, cancer, infectious disease and oxidative damage, increases. MLT enhances immunity and resistance to cancer in aged animals.13 Additionally, as a powerful free radical scavenger,14 it protects against lipid peroxidation. Although MLT has immunomodulatory properties, its actions on immune dysfunction induced by murine retrovirus infection have not been studied.
As both MLT and DHEA are antioxidant immunomodulators they would be expected to slow immune dysfunction and oxidation during AIDS. The current study investigated individual and synergistic effects of DHEA and MLT in preventing immune dysfunction, excessive lipid peroxidation, and vitamin E loss during murine retrovirus infection.
MATERIALS AND METHODS
Animals and murine retrovirus infection
Female C57BL/mice, 6-weeks old, were obtained from the Charles River Laboratories Inc. (Wilmington, DE). They were housed in transparent plastic cages with stainless steel wire lids (four mice per cage) at the University of Arizona animal facility. Animals were cared for as required by the University of Arizona Committee on Animal Research. The housing facility was maintained at 20–22° and 60–80% relative humidity, with a 12-hr light:dark cycle. Water and diet were freely available. After 2 weeks housing and consuming AIN 93 A (control) diet, these mice were randomly assigned to one of the following treatments with eight mice per group: uninfected and LP-BM5-infected mice given control diet and drinking water containing 0·05% ethanol; uninfected and infected mice given 0·02% DHEA supplemented diet for the first 3 weeks (0·9 mg DHEA/mouse/day) and then 0·06% DHEA supplemented diet for the following 9 weeks (2·7 mg DHEA/mouse/day) with 0·05% ethanol containing drinking water; uninfected and infected mice given control diet with 10·0 μg/ml MLT dissolved in 0·05% ethanol containing drinking water (49·8 μg MLT/mouse/day); uninfected and infected mice given 0·02% DHEA supplemented diet for the first 3 weeks and then 0·06% DHEA supplemented diet for the following 9 weeks with 10·0 μg/ml MLT in 0·05% ethanol-containing drinking water. The time and dose of DHEA and MLT treatment were derived from previous work by Araghi-Niknam10 and Mocchegiani,15 respectively.
LP-BM5 retrovirus was administered i.p. to mice in 0·1 ml with an esotropic titer (XC) of 4·5 log10 plaque-forming units (PFU)/ml, which induces disease with a time course comparable to that previously published.16 Uninfected mice were injected with complete culture medium [CM, RPMI-1640 containing 10% fetal calf serum (FCS), 2 mm glutamine, 100 U/ml penicillin and streptomycin] as controls. Infection of adult female C57BL/6 mice with LP-BM5 murine leukaemia leads to the rapid induction of clinical symptoms with virtually no latent phase. Supplementation with DHEA and MLT was begun 2 weeks after LP-BM5 infection. DHEA was a kind gift from Edenland, Inc. (Baybush, Kildare, Ireland). The 0·02% DHEA-supplemented diet and 0·06% DHEA- supplemented diet were prepared by Diets Inc. (Bethlehem, PA) using the same pelleted AIN 93 A diet. MLT (Sigma, St Louis, MO) was dissolved in 95% ethanol and then diluted with distilled water. The final concentration of MLT in the drinking water containing 0·05% ethanol was 10·0 μg/ml. The treatment period was 12 weeks for all groups.
Enzyme-linked immunosorbent assays (ELISA) for cytokines
IL-2, IFN-γ, IL-4, IL-6, IL-10 and tumour necrosis factor-α (TNF-α) were produced by splenocytes as described previously.17 Rat anti-murine IL-2, IFN-γ, IL-4, IL-6, IL-10 and TNF-α monoclonal antibody (mAb), biotin–rat anti-murine IL-2, IFN-γ, IL-4, IL-6, IL-10 and TNF-α mAb, and standard murine rIL-2, rIFN-γ, rIL-4, rIL-6, rIL-10 and rTNF-α were obtained from Pharmingen (San Diego, CA).
Briefly, spleens were collected after killing the mice under ether anaesthesia. Mononuclear cells were obtained by gently teasing with forceps in complete medium (CM), producing a single cell suspension of spleen cells. Red blood cells (RBC) were lysed by addition of a lysis buffer (0·16 m ammonia chloride Tris buffer, pH 7·2) at 37° for 3 min. Then the cells were washed twice with CM. The cell concentration was counted and adjusted to 1×107 cells/ml. Splenocyte viability was more than 95%, as determined by trypan blue exclusion. Splenocytes (0·1 ml/well; 1×107 cell/ml) were cultured in triplicate on 96-well flat-bottomed culture plates (Falcon 3072, Lincoln Park, NJ) with CM. Splenocytes were then stimulated with concanavalin A (Con A; 0·1 ml/well; 10 μg/ml; Sigma) for induction of IL-2, IL-4 and IL-10 with 24 hr incubation, and for induction of IFN-γ with 72 hr incubation at 37° in a 5% CO2 incubator. Splenocytes were also stimulated by lipopolysaccharide (LPS; 0·1 ml/well; 10 μg/ml; Gibco, Grand Island, NY) for 24 hr to induce IL-6 and TNF-α production. After incubation, the plates were centrifuged for 10 min at 800 g. Supernatant fluids were collected and stored at −70° until analysis. They were determined by sandwich ELISA as described previously.17
Mitogenesis of splenocytes
Splenic B- and T-cell proliferation was determined by [3H]thymidine incorporation as described previously.18 Briefly, splenocytes in 0·1 ml of CM (1×107 cell/ml) were cultured in 96-well flat-bottomed culture plates (Falcon, Becton Dickinson Labware, Franklin Lakes, NJ) with LPS (0·1 ml/will; 10 μg/ml) and Con A (0·1 ml/well; 10 μg/ml). They were incubated at 37° in a 5% CO2 incubator for 44 hr for LPS-induced B-cell- and Con A-induced T-cell proliferation, and then pulsed with [3H]thymidine (0·5 μCi/well; New England Nuclear, Boston, MA). After 4 hr, they were harvested by a cell sample harvester (Cambridge Technology, Cambridge, MA). Radioactivity was determined by a liquid scintillation counter (Tri-Carb, 2200 CA, Packard, Lagunahills, CA). Data were presented as counts per min (c.p.m.).
Determination of vitamin E
Vitamin E levels in liver were measured by high-performance liquid chromatography (HPLC) as described previously.19 Briefly, about 0·2 g of tissue was homogenized in 1 ml of water. Butylated hydroxytoluene was added to prevent oxidation of α-tocopherol. Pentane, ethanol and sodium dodecyl sulfate were used to extract α-tocopherol from the homogenate. Extracts were evaporated under a steady flow of nitrogen gas at 20° and then redissolved in 0·5 ml of methanol injection onto a C18 column (3·9×150 mm NovaPak, Millipore, Bedford, MA). A mobile phase composed of methanol:sodium acetate (1 mol/l) in the ratio of 98:2 (v/v) at a flow rate of 1·5 ml/min was used. A-Tocopherol, eluting at 6·5 min, was monitored by a fluorescence detector (Millipore) at 290 nm excitation and 320 nm emission wavelength.
Determination of conjugated dienes
Approximately 0·5 g of liver tissue was homogenized in 10 ml of Folch solution (2:1 v/v chloroform:methanol). Afterprotein separation, a 0·1-ml fraction was dried in a steady flow of nitrogen gas at 55° and used to determine conjugated dienes as previously described.20 The hepatic levels of conjugated dienes were determined by obtaining absorbency of the solution at 237 nm in a Shimdzo UV 160 UV recording spectrophotometer (Tokyo, Japan) using an appropriate blank.
Determination of phospholipid
The phospholipid content of liver was determined by the method of Raheja et al.21 This method does not require predigestion of the phospholipid. Briefly, 0·5 ml chloroform was added then followed by 0·2 ml of a colouring reagent and 3·0 ml of carbon tetrachloride. Phospholipid was determined by obtaining absorbency of the chloroform solution at 710 nm in a Shimadzo UV 160 UV recording spectrophotometer (Tokyo, Japan). Dipalmitoyl phosphatidylcholine was used as a standard.
Determination of total cholesterol
The total cholesterol content of liver was determined by the method of Zak.22 Briefly 0·3 ml of Folch extract was dried under air at 70°. Then 3 ml Zak’s reagent was added followed by 2 ml of sulfuric acid. Total cholesterol was determined by obtaining the absorbency of the solution at 570 nm in a Zhimadzo UV 170 UV recording spectrophotometer (Yokyo, Japan) using cholesterol standards (Sigma).
Statistics
The statistical tests for comparison among groups were finished in the Number Cruncher Statistical System (NCSS) programme (Kaysville, UT) using Friedman’s Block/Treatment test, followed by Duncan’s multiple range test between any two groups. P < 0·05 was considered significant difference between two groups.
RESULTS
Body weight
There was no change in diet and water consumption due to murine retrovirus infection or treatment with DHEA or MLT (data not shown). Body weights were not affected by DHEA or MLT treatment post-retrovirus infection (data not shown). Spleen and lymph node weights (data not shown) were significantly (P < 0·05) elevated in infected mice, indicating that infection had progressed to murine AIDS as shown previously.1,6 However, neither DHEA nor MLT significantly reduced the elevated spleen weights.
Proliferation of splenocytes
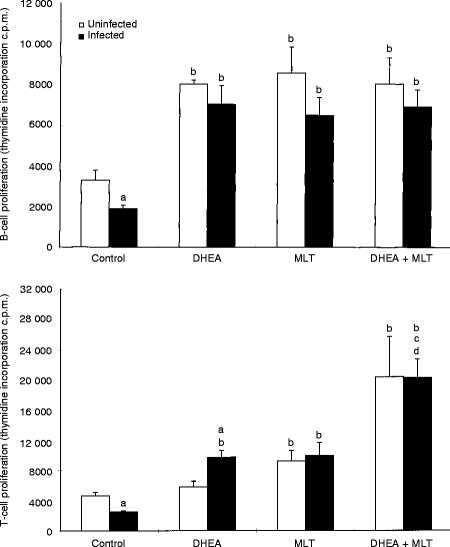
Proliferation of LPS- and Con A-induced splenocytes was significantly (P < 0·05) reduced by murine retrovirus infection (Fig. 1). This was significantly (P < 0·05) prevented by DHEA, MLT, and DHEA+MLT supplementation as compared with untreated, infected mice (Fig. 1). DHEA, MLT, and DHEA+MLT similarly significantly (P < 0·05) increased B-cell proliferation in uninfected mice. MLT, DHEA+MLT, but not DHEA, increased proliferation of T cells from uninfected mice (Fig. 1). Infected mice treated with DHEA+MLT had significantly (P < 0·05) higher T-cell proliferation than infected mice given either DHEA or MLT alone did.
Figure 1.
Effects of DHEA, MLT and DHEA+MLT on LPS-stimulated B-cell- and Con A-stimulated T-cell proliferation of splenocytes. Every sample from each mouse was measured in triplicate. The bars are the mean±SE for eight mice. Letters indicate significant differences at P < 0·05. (a) Compared with uninfected mice receiving the same treatment; (b) compared with their respective untreated controls; (c) compared with their respective DHEA-treated mice; (d) compared with their respective MLT-treated mice.
Cytokine production by splenocytes
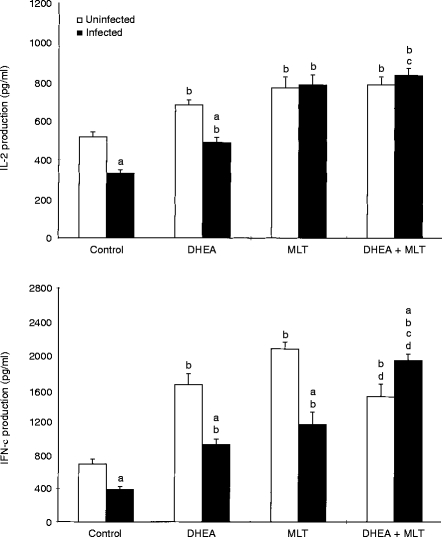
In vitro production of Th1 cytokines (IL-2 and IFN-γ) by Con A-stimulated splenocytes was significantly (P < 0·05) suppressed in retrovirus-infected mice (Fig. 2). Treatment with DHEA, MLT and DHEA+MLT significantly (P < 0·05) stimulated IL-2 and IFN-γ release by mitogen-stimulated splenocytes from both uninfected and infected mice as compared with their untreated controls (Fig. 2). Infected mice treated with DHEA+MLT had significantly (P < 0·05) higher IL-2 production than mice treated with DHEA alone did. In addition, these mice produced significantly (P < 0·05) more IFN-γ than infected mice given either DHEA or MLT did.
Figure 2.
Effects of DHEA, MLT and DHEA+MLT on Th1 cytokine production by splenocytes. Every sample from each mouse was measured in triplicate. The bars are the mean±SE for eight mice. Letters indicate significant differences at P < 0·05. (a) Compared with uninfected mice receiving the same treatment; (b) compared with their respective untreated controls; (c) compared with their respective DHEA-treated mice; (d) compared with their respective MLT-treated mice.
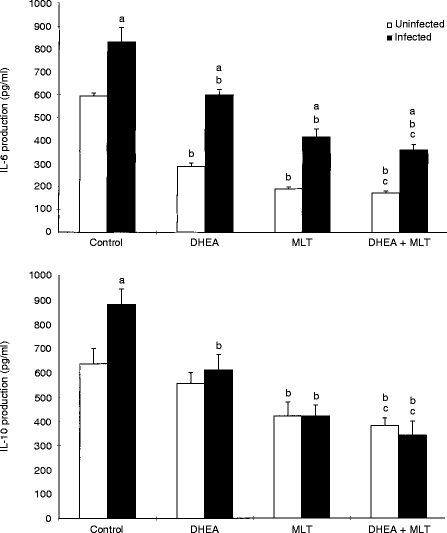
Release of Th2 cytokines (IL-4, IL-6 and IL-10) (Fig. 3, data not shown for IL-4) as well as TNF-α (data not shown), from mitogen-stimulated splenocytes was significantly (P < 0·05) elevated in retrovirus-infected mice. Treatment with DHEA, MLT, and DHEA+MLT significantly (P < 0·05) inhibited production of Th2 cytokines and TNF-α in both uninfected and infected mice (Fig. 3). However, DHEA had no significant effect on IL-10 production in uninfected mice. Cells from infected mice treated with DHEA+MLT produced significantly (P < 0·05) less IL-4, IL-6 and IL-10 than those from DHEA-treated infected mice.
Figure 3.
Effects of DHEA, MLT and DHEA+MLT on Th2 cytokine production by splenocytes. Every sample from each mouse was measured in triplicate. The bars are the mean±SE for eight mice. Letters indicate significant differences at P < 0·05. (a) Compared with uninfected mice receiving the same treatment; (b) compared with their respective untreated controls; (c) compared with their respective DHEA-treated mice.
Hepatic vitamin E and lipid peroxidation
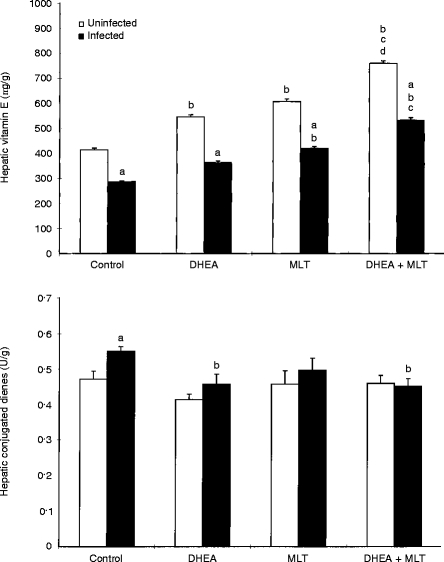
Hepatic vitamin E concentrations were significantly (P < 0·05) decreased by retrovirus infection (Fig. 4). Treatment with MLT and DHEA+MLT significantly (P < 0·05) increased vitamin E levels in both uninfected and infected mice as compared with untreated controls (Fig. 4). DHEA+MLT was more effective than DHEA at increasing vitamin E given to uninfected and infected mice, and it was also more effective than MLT fed to uninfected mice.
Figure 4.
Effects of DHEA, MLT and DHEA+MLT on hepatic vitamin E concentrations and conjugated diene production. Every sample from each mouse was measured in triplicate. The bars are the mean±SE for eight mice. Letters indicate significant differences at P < 0·05. (a) Compared with uninfected mice receiving the same treatment; (b) compared with their respective untreated controls; (c) compared with their respective DHEA-treated mice; (d) compared with their respective MLT-treated mice.
Retrovirus infection significantly (P < 0·05) increasedhepatic diene conjugates, a major product of lipid peroxidation (Fig. 4). Treatment of infected mice with DHEA and DHEA+MLT significantly (P < 0·05) normalized hepatic diene conjugates to levels near those of uninfected mice (Fig. 4). The supplements had no significant effect on diene conjugate levels in uninfected mice.
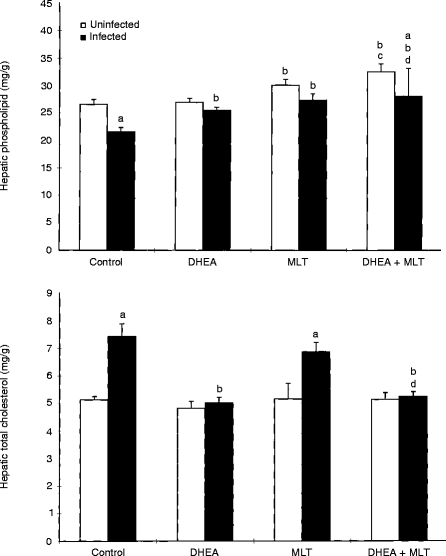
Hepatic phospholipids and total cholesterol
Retrovirus infection significantly (P < 0·05) reduced hepatic phospholipids(Fig. 5). Treatment with MLT and DHEA+ MLT significantly (P < 0·05) increased phospholipid levels in uninfected and infected mice compared with their respective untreated controls. DHEA treatment significantly (P < 0·05) elevated phospholipid levels only in infected mice (Fig. 5).
Figure 5.
Effects of DHEA, MLT and DHEA+MLT on hepatic phospholipid content and total cholesterol levels. Every sample from each mouse was measured in triplicate. The bars are the mean±SE for eight mice. Letters indicate significant differences at P < 0·05. (a) Compared with uninfected mice receiving the same treatment; (b) compared with their respective untreated controls; (c) compared with their respective DHEA-treated mice; (d) compared with their respective MLT-treated mice.
Significantly (P < 0·05) higher hepatic total cholesterol was induced by retrovirus infection (Fig. 5). Treatment of infected mice with DHEA and DHEA+MLT maintained near-normal cholesterol levels that were significantly (P < 0·05) lower than those in infected, untreated mice (Fig. 5). The treatments had no effect on cholesterol levels in uninfected mice, nor did MLT alone suppress the retrovirus-induced increase.
DISCUSSION
The current study showed that retrovirus infection induced immune dysfunction, lipid peroxidation, and vitamin E loss. Murine retrovirus infection inhibited proliferation of B and T cells, repressed release of Th1 cytokines, and stimulated secretion of Th2 cytokines as well as TNF-α. Retrovirus infection also increased hepatic lipid peroxidation and induced tissue vitamin E deficiency. DHEA and MLT treatment significantly prevented immune dysfunction, excessive lipid peroxidation, and loss of tissue vitamin E induced by retrovirus infection. DHEA and MLT also significantly increased B-cell proliferation, Th1 cytokine production, and decreased Th2 cytokine production in uninfected mice. While DHEA and MLT increased hepatic vitamin E and phospholipids in uninfected mice as well, they had little effect on conjugated dienes or cholesterol.
DHEA modestly downregulated HIV-1 expression in infected human cells.23 DHEA blocks cytokine-induced NF-κB activation by inhibiting NADPH-dependent oxidativeintermediates, thus preventing HIV gene expression.24 As HIV infection progresses, the mean DHEAS levels fall and the cortisol/DHEAS ratio rises,25 predicting disease progression from HIV-1 infection to AIDS.26 Since DHEA may have a direct antiglucocorticoid activity, reduced DHEA could allow cortisol to act more effectively, enhancing immunosuppression.27 Retrovirus infection may shift precursor adrenal hormones from androgenic pathways into cortisol pathways, excessively stimulating cortisol by reducing DHEA production. There was a clear positive relationship between CD4+ lymphocytes and DHEAS values in AIDS patients.25 DHEA and DHEAS may be natural mediators of T-cell responses.28 High-affinity DHEA binding sites in human and murine T cells are regulated by DHEA during the process of signal-induced activation.29 DHEA binding to its receptor is directly related to stimulation of IL-2 production.30 Lymphocytes from DHEA-treated tolerant mice produced more IFN-γ and less IL-4 and IL-6 than those from untreated mice did.31 Thus, DHEA supplementation was expected to stimulate Th1 cells in both uninfected and infected mice.
DHEA prevented suppression of hepatic phospholipids and increase of total cholesterol during murine AIDS. As membrane fluidity is a necessary component for the function of signal transduction, DHEA may work by improving membrane fluidity which should facilitate lymphocyte function.32
We found that MLT treatment prevented retrovirus-induced reduction in B- and T-cell proliferation and in Th1 cytokine secretion, as well as overproduction of Th2 cytokines and TNF-α. MLT also significantly increased Th1 cytokine production and decreased Th2 cytokine production inuninfected mice. These results support the hypotheses of MLT’s role in cytokine regulation. MLT increased immune components deficient in HIV-infected patients including lymphoid cells and Th1 cytokines.33 Human circulating CD4+ lymphocytes contain specific34 and high-affinity35 binding sites for MLT, and MLT has been shown to enhance IL-2 and IFN-γ production.36 MLT also affects adrenal and sexual glands with immunomodulatory roles.37
MLT was more effective than vitamin E in scavenging peroxyl radicals.14 MLT supplementation significantly increased hepatic vitamin E and phospholipid levels in uninfected mice as well as their suppressed quantities in infected mice. Thus, MLT may protect against lipid peroxidation and chronic immune dysfunction in murine AIDS as well as in uninfected mice by reducing the production of free radicals.
We also observed that infected mice treated with DHEA+MLT had significantly higher splenic T-cell proliferation and IFN-γ production than infected mice given either DHEA or MLT alone. DHEA+MLT treatment was more effective at increasing IL-2 production and decreasing Th2 cytokine production in infected mice than DHEA alone. In addition, DHEA+MLT was more effective at increasing tissue vitamin E levels than either DHEA or MLT alone. Although both DHEA and MLT decline with age,13,27 this is the first study where their combined treatment and actions were examined in retrovirus-infected mice and uninfected young mice. Lymphocytes from uninfected and infected mice respond similarly to these hormones. Thus, these cells are not unresponsive owing to infection. In addition, not only do DHEA and MLT overcome the damage caused by infection, but also they can further stimulate cells from uninfected young mice who are at the peak of their immune function. Nevertheless these hormones do not totally overcome this damage, as the activities of cells from infected mice do not fully reach those of cells from uninfected mice.
Our results also suggest an association between immune dysfunction, lipid peroxidation, and tissue vitamin E levels during retrovirus infection. Immune dysfunction caused by retrovirus infection decreases host resistance to opportunistic pathogens.38 Increased infections stimulate phagocytes to release more free radicals, explaining the increased lipid peroxidation.1 Free radicals react with antioxidant vitamins and also increase TNF-α production which further excites oxidative stress by activating macrophage and neutrophils.39 The antioxidant, vitamin E, is an important immune modulator,particularly in murine AIDS.5 Vitamin E deficiency could accentuate retroviral immunosuppression since vitamin E supplementation partially restored immune function in retrovirus-infected mice.5 Oxidative stress stimulates of HIV replication in vitro through activation of NF-κB which stimulates HIV gene expression by acting on the promoter region of the viral long-terminal repeats.40 In addition, oxidative stress in AIDS may lead to the damage and death of T helper cells, thus weakening the immune system. In murine AIDS, increased lipid peroxidation, together with decreased phospholipids and elevated cholesterol levels may be responsible for reduced membrane fluidity and elevated membrane viscosity,32 interfering with the signal transduction processes.41 Such changes in HIV-infected people could help explain their increased risk for heart disease.
Increased hepatic lipid peroxidation and decreased vitamin E may be due to the retrovirus-altered cytokine production.5 Th1 cells secrete IL-2 and IFN-γ, which are involved in stimulating cell-medicated responses. Th2 cells, in contrast, secrete IL-4, IL-6 and IL-10, which activate antibody production and suppress Th1 cells. In vivo, activated B cells and macrophages from HIV-infected patients, produced high levels of IL-6 and TNF-α,42 as do LPS-stimulated splenocytes and peritoneal macrophages in MuLV retrovirus-infected mice.43 When IL-4-deficient (IL-4 gene knockout) mice were infected with LP-BM5 retrovirus, there was no lethality and development of T-cell abnormalities was delayed.44 Excessive Th2 cytokines could be responsible for B-cell dysfunction during murine AIDS. Elevated levels of IL-6 have also been associated with stimulating HIV replication in macrophages and T cells.45 IFN-γ administration significantly retarded murine AIDS by preventing activation of Th2 cytokines.43 Thus, the reduction of Th2 cytokines by hormone supplementation could be a potential treatment for AIDS.27 Our data demonstrated that T-cell proliferation and Th1 cytokines declined while Th2 cytokines increased during retrovirus infection. Additionally we showed that these effects could be attenuated by hormone supplementation. These results are in accordance with the hypothesis of Clerici et al.2 which implicates a cytokine imbalance in the pathogenesis of human AIDS.
Acknowledgments
This work is supported by grants from Wallace Genetic Foundation and NIH L59794.
References
- 1.Liang B, Wang JY, Watson RR. Murine AIDS, a key to understanding retrovirus-induced immunodeficiecy. Viral Immunol. 1996a;98:225. doi: 10.1089/vim.1996.9.225. [DOI] [PubMed] [Google Scholar]
- 2.Clerici M, Hakim FT, Venzon DJ, et al. Changes in interleukin-2 and interleukin-4 production in asymptomatic, human immunodeficiency virus-seropositive individuals. J Clin. 1993;9:759. doi: 10.1172/JCI116294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bradley WG, Ogata N, Good RA, Day NK. Alteration of in vivo cytokine gene expression in mice infected with a molecular clone the defective MAIDS virus. J AIDS. 1993;7:1. [PubMed] [Google Scholar]
- 4.Gazzinelli RT, Makino M, Chattopadhyay SK, et al. CD4+ subset regulation in viral infection. Preferential activation of Th2 cells during progression of retrovirus-induced immunodeficiency in mice. J Immunol. 1992;148:182. [PubMed] [Google Scholar]
- 5.Wang Y, Huang DS, Liang B, Watson RR. Nutritional status and immune response in mice with murine AIDS are normalized by vitamin E supplementation. J Nutr. 1994;124:2024. doi: 10.1093/jn/124.10.2024. [DOI] [PubMed] [Google Scholar]
- 6.Bruan DP, Kessler H, Falk I, et al. Monocytefunctional studies in asymptomatic human immunodeficiency disease virus (HIV) infected individuals. J Clin Immunol. 1988;8:486. doi: 10.1007/BF00916955. [DOI] [PubMed] [Google Scholar]
- 7.Baruchel S, Wainberg MA. The role of oxidative stress in disease progression in individuals infected by the human immunodeficiency virus. J Leukoc Biol. 1992;52:111. doi: 10.1002/jlb.52.1.111. [DOI] [PubMed] [Google Scholar]
- 8.Abrams B, Duncan D, Hertz-Picciotto I. A prospective study of dietary intake and acquired immune deficiency syndrome in HIV-seropositive homosexual men. J AIDS. 1993;6:949. [PubMed] [Google Scholar]
- 9.Jacobson MA, Flusaro RE, Galmarrini M, Lang W. Decreased serum dehydroepiandrosterone is not associated with an increased CD4 cell count of 200. J Infect Dis. 1991;164:864. doi: 10.1093/infdis/164.5.864. [DOI] [PubMed] [Google Scholar]
- 10.Araghi-Nikam M, Zhang Z, Inserra P, Watson RR. Cytokine dysregulation and increased oxidation is prevented by dehydroepiandrosterone in mice infected with murine leukemia retrovirus. Proc Soc Exp Bio Med. 1997;216:386. doi: 10.3181/00379727-216-44186. [DOI] [PubMed] [Google Scholar]
- 11.Suzuki T, Suzuki N, Daynes RA, Engleman EG. Dehydroepiandrosterone enhances of IL-2 production and cytotoxic effector function of human T-cells. Clin Immunol Immunopathol. 1991;61:202. doi: 10.1016/s0090-1229(05)80024-8. [DOI] [PubMed] [Google Scholar]
- 12.Aragno M, Tamagno E, Boccuzzi G, et al. Dehydroepiandrosterone pretreatment protects rats against the pro-oxidant and necrogenic effects of carbon tetrachloride. Biochem Pharm. 1993;46:1689. doi: 10.1016/0006-2952(93)90572-e. [DOI] [PubMed] [Google Scholar]
- 13.Zhang Z, Inserra P, Liang B, Watson RR. Melatonin, immune modulation, and aging. Autoimmunity. 1997;26:43. doi: 10.3109/08916939709009549. [DOI] [PubMed] [Google Scholar]
- 14.Pieri C, Marra M, Moroni F, Recchioni R, Marcheselli F. Melatonin: a peroxyl radical scavenger more effective than vitamin E. Life Sci. 1994;55:271. doi: 10.1016/0024-3205(94)00666-0. [DOI] [PubMed] [Google Scholar]
- 15.Moccegiani E, Bulian D, Sanatarelli L, et al. The immuno-reconstituting effect of melatonin or pineal grafting and its relation to zinc pool in aging mice. J Neuroimmunol. 1994;53:189. doi: 10.1016/0165-5728(94)90029-9. [DOI] [PubMed] [Google Scholar]
- 16.Liang B, Ardestani S, Marchalonis JJ, Watson RR. T-cell-receptor dose and the time of treatment during murine retrovirus infection for maintenance of immune function. Immunology. 1996b;87:198. doi: 10.1046/j.1365-2567.1996.449551.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang Y, Huang DS, Wood S, Watson RR. Modulation of immune function and cytokine production by various levels of vitamin E supplementation during murine AIDS. Immunopharmacology. 1995;29:225. doi: 10.1016/0162-3109(95)00061-w. [DOI] [PubMed] [Google Scholar]
- 18.Ernst DN, Hobbs MV, Torbett BE, et al. Differences in the expression profiles of CD45RB, Pgp-1, and 3G11 membrane antigens and in the patterns of lymphokine secretion by splenic CD4+ T cells from young and aged mice. J Immunol. 1990;145:1295. [PubMed] [Google Scholar]
- 19.Burton GW, Webb A, Ingold KU. A mild, rapid, and efficient method of lipid extraction for use in determining vitamin E/lipid ratios. Lipids. 1985;20:29. doi: 10.1007/BF02534359. [DOI] [PubMed] [Google Scholar]
- 20.Iverson SA, Cawood P, Madigan MJ, Lawson AM, Dormandy TL. Identification of a diene conjugated component of human lipid as octadeca-9, 11 dienoic acid. FASEBS Lett. 1984;141:320. doi: 10.1016/0014-5793(84)80512-8. [DOI] [PubMed] [Google Scholar]
- 21.Raheja RK, Kaur C, Singh A, Bhatia IS. New colorimetric method for the quantitative estimation of phospholipids without acid digestion. J Lipid Res. 1973;14:695. [PubMed] [Google Scholar]
- 22.Zak B. Standard Methods of Clinical Chemistry. New York, NY: Academic press; 1965. Total and free cholesterol; p. 79. [Google Scholar]
- 23.Henderson EE, Yang JY, Schwartz A. Dehydroepiandrosterone and synthetic DHEA analogs are modest inhibitors of HIV-1 IIIB replications. AIDS Res Hum Retroviruses. 1992;6:459. doi: 10.1089/aid.1992.8.625. [DOI] [PubMed] [Google Scholar]
- 24.Ursini MV, Lettieri T, Braddock M, Martini G. Enhanced activity of human G6PD promoter transfected in Hela cell producing high levels of HIV-1 Tat. Virology. 1993;196:338. doi: 10.1006/viro.1993.1485. [DOI] [PubMed] [Google Scholar]
- 25.Wisniewski TL, Hilton CW, Morse EV, Frank S. The relationship of serum DHEA-S and cortisol levels to measures of immune function in human immunodeficiency virus-related illness. Am J Med Sci. 1993;305:79. doi: 10.1097/00000441-199302000-00003. [DOI] [PubMed] [Google Scholar]
- 26.Mulder JW, Jos Frissen PH, Krijnen P, et al. Dehydroepiandrosterone as predictor for progression to AIDS in asymptomatic human immunodeficiency virus-infected men. J Infect Dis. 1992;165:413. doi: 10.1093/infdis/165.3.413. [DOI] [PubMed] [Google Scholar]
- 27.Watson RR, Chung S, Huls A, Araghiniknam M. Dehydroepiandrosterone (DHEA) and diseases of aging. Drugs Aging. 1996;9:274. doi: 10.2165/00002512-199609040-00005. [DOI] [PubMed] [Google Scholar]
- 28.Regelson W, Loria R, Kalimi M. Dehydroepiandrosterone (DHEA) – the ‘mother steroid’: I. immunologic action. Am N Y Acad Sci. 1994;719:553. doi: 10.1111/j.1749-6632.1994.tb56859.x. [DOI] [PubMed] [Google Scholar]
- 29.Okabe T, Haji M, Takayanagi R, et al. Up-regulation of high-affinity dehydroepiandrosterone binding activity by dehydroepiandrosterone in activated human T lymphocytes. Clin Endocrinol Metab. 1995;80:2993. doi: 10.1210/jcem.80.10.7559886. [DOI] [PubMed] [Google Scholar]
- 30.Meikle AW, Dorchuck RW, Araneo BA, et al. The presence of a dehydroepiandrosterone-specific receptor binding complex in murine T-cells. J Steriod Biochem Mol Biol. 1992;42:293. doi: 10.1016/0960-0760(92)90132-3. [DOI] [PubMed] [Google Scholar]
- 31.Kim HR, Ryu SY, Kim HS, et al. Administration of dehydroepiandrosterone reverses the immune suppression induced by high dose antigen in mice. Immunol Invest. 1995;24:583. doi: 10.3109/08820139509066859. [DOI] [PubMed] [Google Scholar]
- 32.Hengner D, Platt D. Effect of essential phospholipid on the properties of ATPase of isolated rat liver plasma membrane of young and old animals. Mech Ageing Dev. 1975;4:191. doi: 10.1016/0047-6374(75)90021-4. [DOI] [PubMed] [Google Scholar]
- 33.Maestroni GI. T-helper-2 lymphocytes as peripheral target of melatonin signaling. J Pineal Res. 1995;18:84. doi: 10.1111/j.1600-079x.1995.tb00144.x. [DOI] [PubMed] [Google Scholar]
- 34.Calvo JR, Rafii-El-Idrissi M, Pozo D, Guerrero JM. Immunomodulatory role of melatonin: specific binding sites in human and rodent lymphoid cells. J Pineal Res. 1995;18:119. doi: 10.1111/j.1600-079x.1995.tb00149.x. [DOI] [PubMed] [Google Scholar]
- 35.Gonzalez-Haba MG, Garcia-Maurino S, Calvo JR, Goberna R, Guerrero JM. High-affinity binding of melatonin by human circulating T lymphocytes (CD4+) FASEB J. 1995;9:1331. doi: 10.1096/fasebj.9.13.7557023. [DOI] [PubMed] [Google Scholar]
- 36.Garcia-Maurino S, Gonzalez-Haba MG, Calvo JR, et al. Melatonin enhances IL-2, IL-6, and IFN-γ production by human circulating CD4+ cells. J Immunol. 1997;159:574. [PubMed] [Google Scholar]
- 37.Guerrero JM, Reiter RJ. A brief survey of pineal gland-immune system interrelationships. Endocrinol Res. 1992;18:91. doi: 10.1080/07435809209035401. [DOI] [PubMed] [Google Scholar]
- 38.Liang B, Marchalonis JJ, Watson RR. Prevention of immune dysfunction and loss of Cryptosporidium resistance during murine retrovirus infection by T cell receptor peptide immunization. Nutr Res. 1997;17:677. [Google Scholar]
- 39.Greenspan HC, Arouma O. Oxidative stress and apoptosis in HIV infection: a role for plant-derived metabolites with synergistic antioxidant activity. Immunol Today. 1994;15:209. doi: 10.1016/0167-5699(94)90245-3. [DOI] [PubMed] [Google Scholar]
- 40.Allard JP, Aghdassi E, Chau, J, Salit I, Walmsley S. Oxidative stress and plasma antioxidant micronutrients in humans with HIV infection. Am J Clin Nutr. 1998;67:143. doi: 10.1093/ajcn/67.1.143. [DOI] [PubMed] [Google Scholar]
- 41.Spencer NF, Poynter ME, Hennebold JD, Ehler LA, Fauci AS. Does DHEAS restore immune competence in aged animals through its capacity to function as a natural modulator of peroxisomeactivities? Ann N Y Acad Sci. 1995;774:200. doi: 10.1111/j.1749-6632.1995.tb17382.x-i1. [DOI] [PubMed] [Google Scholar]
- 42.Chun TW, Engel D, Mizell SB, Ehler LA, Fauci AS. Induction of HIV-1 replication in latently infected CD4+ T cells using a combination of cytokines. J Exp Med. 1998;188:83. doi: 10.1084/jem.188.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gallichio VS, Tse KF, Morrow J, Hughes NK. Suppression of haemotopoeitic support function is associated with overexpression of IL-4 and transforming growth factor-β1 in LP-BMS murine leukaemia virus-infected stromal cell lines. Acta Haematol. 1996;95:204. doi: 10.1159/000203879. [DOI] [PubMed] [Google Scholar]
- 44.Cohen OJ, Kinter A, Fauci AS. Host factors in the pathogenesis of HIV disease. Immunol Rev. 1997;159:31. doi: 10.1111/j.1600-065x.1997.tb01005.x. [DOI] [PubMed] [Google Scholar]
- 45.Poli G, Bressler P, Kinter A, et al. Interleukin 6 induces human immunodeficiency virus expression in infected monocytic cells alone and in synergy with tumor necrosis factor alpha by transcriptional and post-transcriptional mechanisms. J Exp Med. 1990;172:151. doi: 10.1084/jem.172.1.151. [DOI] [PMC free article] [PubMed] [Google Scholar]