Abstract
The balance between T helper type 1 (Th1) and Th2 cytokines is thought to be important in the initiation and outcome of autoimmune diseases. The goal of the present study was to compare the production of interferon-γ (IFN-γ) and interleukin-4 (IL-4) by synovial fluid (SF) and peripheral blood (PB) CD4+ and CD8+ cells from patients with rheumatoid arthritis (RA) using three-colour immunofluorescence staining and flow cytometry, and to investigate the capacity of IL-4, IL-10 and IL-12 to modify the cytokine production profile of SF T cells. The frequency of IFN-γ-producing CD4+ and CD8+ cells was significantly increased in SF when compared with PB. In contrast to IFN-γ, the expression of IL-4 in SF and PB T cells was comparable. The majority of IL-4-producing cells in SF belonged to Th0/T cytotoxic (Tc) type 0 phenotype, whereas there were significantly more Th2/Tc2 cells in PB than in SF. Interestingly, IL-4 was unable to induce differentiation of non-adherent SF mononuclear cells (SFMC) into Th2 cells, whereas PB mononuclear cells (PBMC) under similar culture conditions differentiated into cells producing high levels of IL-4, IL-10 and IL-13. In contrast, there were no major differences in the effects of IL-10 and IL-12 on the cytokine production profile of SFMC when compared with PBMC. Taken together, the present results suggest that SF T cells from patients with RA are terminally differentiated into Th1/Tc1-like phenotype, and Th2/Tc2 differentiation-inducing agents, such as IL-4, may not be able to reverse the inflammatory process occurring in the joints.
INTRODUCTION
In rheumatoid arthritis (RA) activated T cells, macrophages and plasma cells accumulate into the joints. T cells specific for the yet unknown antigens have been suggested to be important in the induction and maintenance of synovial inflammation, which eventually leads to cartilage degradation.1,2 Synovial T cells are activated when judged by their phenotype3,4 and most of them express memory cell marker CD45RO.5 The genetic association of RA with the major histocompatibility complex (MHC) class II alleles, human leucocyte antigen (HLA)-DR1 and HLA-DR4, also argues for a role of antigen-specific T cells in RA. In addition, synovial T cells derived from patients with RA have been shown to cause inflammatory arthritis when transferred into severe combined immunodeficient (SCID) mice.6 Synovial T cells are likely to regulate the function of other cells via secretion of cytokines. Cytokines produced by T cells, such as interferon-γ (IFN-γ), interleukin-2 (IL-2), IL-4 and IL-13 have been demonstrated in rheumatoid joints, although the expression levels of these cytokines in vivo appear to be relatively low.7–11
The outcome of several infectious and inflammatory/autoimmune diseases has been suggested to depend on the cytokine production profile of specific T helper cells, based on which human and mouse CD4+ cells are divided into three major subsets, T helper type 1 (Th1) cells produce IL-2 and IFN-γ, whereas Th2 cells generally secrete high levels of IL-4, IL-5, IL-10 and IL-13.12 Th0 cells produce both Th1 and Th2 cytokines. However, in humans, the production of IL-2, IL-10 and IL-13 is not restricted to the Th2 subset.13–15 The cytokine milieu during the initial presentation of antigen to naive T cells regulates the development of different subsets. IL-12 and IFN-γ favour the generation of Th1 responses, whereas IL-4 directs the development towards Th2 cells.16–18 IL-10 was recently shown to induce the generation of a new Th cell subset (T regulatory cells 1) producing high quantities of IL-10, but no IL-4, during chronic activation of human and murine CD4+ T cells.19 CD8+ T cells can also be divided into similar subsets, T cytotoxic type 1 (Tc1), Tc2 and Tc0, on the basis of cytokine production profiles, and the regulation of Tc1 or Tc2 differentiation by cytokines is similar to that observed with Th cells.12,20,21
Studies using animal models suggest that Th1 cytokines promote the development of several autoimmune disorders, such as experimental autoimmune encephalomyelitis and insulin-dependent diabetes mellitus, whereas the Th2 pattern may attenuate these diseases.22 The balance between Th1 and Th2 cytokines is also likely to play an important role in the initiation and outcome of synovial inflammation in RA, and cytokines inducing Th2 differentiation may be useful in the treatment of patients with RA. The goal of the present study was to compare the production of IFN-γ and IL-4 by synovial fluid (SF) and peripheral blood (PB) T cells from patients with RA using a three-colour immunofluorescence staining and flow cytometry, and, more importantly, to evaluate the efficacy of IL-4, IL-10 and IL-12 in modulating the cytokine synthesis profile of synovial T cells.
MATERIALS AND METHODS
Patients
SF samples were collected from 15 patients with RA, and paired PB samples were concurrently obtained from 10 patients. PB samples from five healthy volunteers were used as controls. SF samples were obtained by needle aspiration from inflamed knee joints into heparinized tubes.
Fifteen patients with RA (12 females and three males) were enrolled in this study. The median age of the patients was 58 years (range 33–68) and the median duration of disease 18 years (range 4–31). Mean (±SD) erythrocyte sedimentation rate was 52±16 mm/hr and mean C-reactive protein was 46±32 mg/l at the time SF samples were taken (n=13). RA was determined according to the criteria of the American College of Rheumatology.23 Three patients had seronegative RA. Eleven patients were treated with disease-modifying anti-rheumatic drugs, nine patients were receiving steroids and all patients were treated with non-steroidal anti-inflammatory drugs at the time of the study. This study was approved by the ethical committee of Turku University.
Reagents
Purified recombinant human (rHu) IL-4 and rHuIL-10 were obtained from Schering-Plough Research Institute (Kenilworth, NJ). The rHuIL-2, rHuIL-13 and neutralizing anti-human IFN-γ monoclonal antibody (mAb) A35 were kindly provided by Dr Jan de Vries (DNAX Research Institute, Palo Alto, CA). The rHu IL-12 and neutralizing anti-human IL-12 mAb were purchased from R & D Systems (Minneapolis, MN). Agonistic anti-CD3 and anti-CD28 mAb were kindly provided by Dr Gregorio Aversa (DNAX Research Insitute).
Fluorescein isothiocyanate (FITC)-conjugated anti-human IFN-γ and immunoglobulin G1 (IgG1) isotype control (MOPC-21) were purchased from Pharmingen (San Diego, CA). Phycoerythrin (PE)-conjugated anti-human IL-4 and IgG1 isotype control, and peridinin chlorophyll protein (PerCP)-conjugated anti-human CD4 and CD8 and IgG1 isotype controls were purchased from Becton Dickinson (San Jose, CA).
Analysis of cytokine production by flow cytometry
PB mononuclear cells (PBMC) and SF mononuclear cells (SFMC) were isolated by a Ficoll–Hypaque (Pharmacia, Uppsala, Sweden) density gradient centrifugation and washed twice with phosphate-buffered saline (PBS) at +4°. PBMC or SFMC were activated in Iscove’s modified Dulbecco’s medium (IMDM) supplemented with 10% heat-inactivated fetal calf serum (FCS; HyClone Laboratories, Logan, UT) and 50 μg/ml gentamicin for 5 hr in the presence of either phorbol 12-myristate 13-acetate (PMA 10 ng/ml; Sigma, St Louis, MO)+ ionomycin (0·5 μg/ml; Sigma); anti-CD3 mAb (1 μg/ml)+PMA; or anti-CD3 mAb+anti-CD28 mAb (10 μg/ml). The cells were activated at 1×106 cells/ml/well in 24-well plates and 10 μg/ml of Brefeldin A (Epicentre Technologies, Madison, WI) was added to the cultures to prevent cytokine secretion.
Cultured cells were harvested and washed twice with PBS supplemented with 0·5% bovine serum albumin (BSA) and 0·01% NaN3. The cells were stained with PerCP-conjugated anti-human CD4 or CD8 mAb and washed twice with PBS, after which the cells were fixed with 2% formaldehyde for 20 min at room temperature. After fixation, the cells were permeabilized in PBS supplemented with 0·5% BSA, 0·01% NaN3 and 0·1% saponin (permeabilization buffer) for 10 min at room temperature. The permeabilized cells were stained with FITC- and PE-conjugated anti-cytokine mAb diluted in permeabilization buffer for 30 min on ice, washed twice with permeabilization buffer and once with PBS–BSA. FITC-, PE- and PerCP-conjugated IgG1 isotype controls were used to differentiate between positive and negative cells. Labelled cells were analysed using a fluorescence-activated cell sorter (FACScan; Becton Dickinson) flow cytometer and CellQuest software.
Culture conditions and analysis of cytokine production by enzyme-linked immunosorbent assay (ELISA)
Lymphocytes were enriched from PBMC and SFMC by collecting the non-adherent cell population after incubating the cells on plastic Petri dishes for 1 hr at 37°. Non-adherent cells were cultured at 0·5×106–1×106 cells/ml/well in IMDM supplemented with 10% heat-inactivated FCS and 50 μg/ml gentamicin. Cells were cultured in 24-well plates at 37° in a humidified athmosphere containing 5% CO2. IL-2 (100 U/ml), IL-4 (100 U/ml), IL-10 (100 U/ml), IL-12 (10 ng/ml), IL-13 (100 U/ml), neutralizing anti-IL-12 mAb (10 μg/ml) and/or neutralizing anti-IFN-γ mAb (5 μg/ml) were added at the onset of the cultures. The cells were restimulated with cytokines after a culture period of 6 days, harvested after a total culture period of 12 days and washed twice with IMDM.
Cultured cells were activated at 0·5×106–1×106 cells/ml/well in IMDM for 24 hr in the presence of IL-2+anti-CD3 mAb+anti-CD28 mAb. A 24-hr stimulation was found to be optimal for the induction of IL-4 production in the initial experiments (data not shown). The supernatants were collected and stored at −70° until examination using ELISA. Cytokine levels in the culture supernatants were examined using specific ELISA, which were obtained commercially (CLB, Amsterdam, the Netherlands) and used according to the manufacturer’s instructions. The sensitivity of the IFN-γ ELISA was 5 pg/ml and that of the IL-10 ELISA was 2 pg/ml. The sensitivity of the IL-4 and IL-13 ELISA was 1 pg/ml.
Statistical analysis
Statistical analysis was performed using Wilcoxon signed-rank test.
RESULTS
The expression of IFN-γ and IL-4 in SF and PB T cells
We first compared the production of IFN-γ and IL-4 by paired SF and PB CD4+ and CD8+ cells from patients with RA. SFMC and PBMC were activated for 5 hr in the presence of different T-cell activation-inducing signals. Several activation stimuli were used to avoid possible skewing of the results due to preferential induction of either IFN-γ or IL-4 by one particular activator.
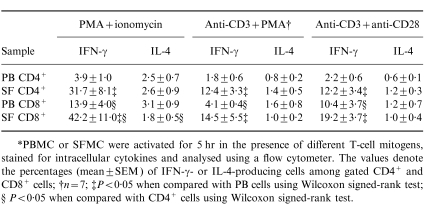
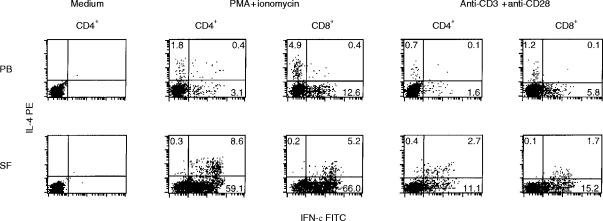
CD4+ and CD8+ T cells derived from SF expressed significantly more IFN-γ than those derived from PB (Table 1,Fig. 1). Similarly, the percentages of both Th1 and Tc1 cells were increased in SF when compared with PB (Table 2). T cells activated in the presence of PMA+ionomycin produced more IFN-γ than the cells activated in the presence of either anti-CD3 mAb+PMA or anti-CD3+anti-CD28 mAb, but the difference between SF and PB T cells was observed irrespective of the stimulus used. Both CD4+ and CD8+ cells in SF contributed to the high expression of IFN-γ, whereas the majority of IFN-γ-producing cells in PB were CD8+ (Table 1).
Table 1.
The expression of IFN-γ and IL-4 by PB and SF T cells from eight patients with RA*
*PBMC or SFMC were activated for 5 hr in the presence of different T-cell mitogens, stained for intracellular cytokines and analysed using a flow cytometer. The values denote the percentages (mean±SEM) of IFN-γ- or IL-4-producing cells among gated CD4+ and CD8+ cells; †n = 7; ‡P < 0·05 when compared with PB cells using Wilcoxon signed-rank test; §P < 0·05 when compared with CD4+ cells using Wilcoxon signed-rank test.
Figure 1.
The expression of IFN-γ and IL-4 by PB and SF CD4+ and CD8+ T cells. PBMC or SFMC were activated for 5 hr in the presence or absence of PMA (10 ng/ml)+ ionomycin (0·5 μg/ml), or anti-CD3 mAb (1 μg/ml)+ anti-CD28 mAb (10 μg/ml). Brefeldin A (10 μg/ml) was added to the cultures to prevent cytokine secretion. Activated cells were stained with PerCP-conjugated anti-CD4 or anti-CD8 mAb, fixed, permeabilized in 0·1% saponin and stained with FITC-conjugated anti-human IFN-γ and PE-conjugated anti-human IL-4 mAb. CD4+ or CD8+ cells were gated and quadrants were set on the basis of FITC- and PE-conjugated IgG1 isotype controls. The values denote the percentage of cells in each quadrant.
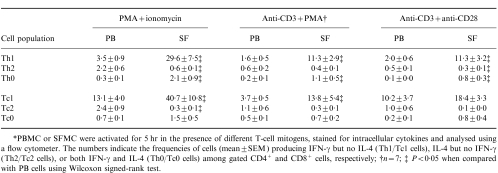
Table 2.
The percentages of different helper T cell (Th) and cytotoxic T cell (Tc) populations in PB and SF samples from eight patients with RA*
*PBMC or SFMC were activated for 5 hr in the presence of different T-cell mitogens, stained for intracellular cytokines and analysed using a flow cytometer. The numbers indicate the frequencies of cells (mean±SEM) producing IFN-γ but no IL-4 (Th1/Tc1 cells), IL-4 but no IFN-γ (Th2/Tc2 cells), or both IFN-γ and IL-4 (Th0/Tc0 cells) among gated CD4+ and CD8+ cells, respectively; †n =7; ‡P < 0·05 when compared with PB cells using Wilcoxon signed-rank test.
IL-4 production was lower than IFN-γ production in all cell populations studied (Table 1,Fig. 1). In contrast to IFN-γ expression, there was no significant difference in the frequence of IL-4-producing cells between SF and PB CD4+ or CD8+ T cells. However, the proportions of Th2 and Tc2 cells producing detectable levels of IL-4 but no IFN-γ were increased in PB when compared with SF (Table 2, Fig. 1). Accordingly, the percentages of Th0 and Tc0 cells, which produce both IFN-γ and IL-4, were higher in SF than in PB, and the majority of IL-4-producing cells in SF also produced IFN-γ.
The effects of IL-4, IL-10 and IL-12 on the cytokine production by SF T cells
A means to shift the cytokine production profile of synovial T cells towards type 2 cytokines could have therapeutic potential in the treatment of RA. Therefore, we investigated the capacity of IL-4, IL-10 and IL-12 to regulate the production of cytokines by differentiated SF T cells. Non-adherent SFMC and PBMC from patients with RA or healthy volunteers were cultured for 12 days in the presence or absence of different cytokines or neutralizing anti-cytokine mAb. IL-2 was added to all cultures to prolong the viability of the cells. After the culture, the cells were activated for 24 hr by anti-CD3+ anti-CD28 mAb, and the production of IFN-γ, IL-4, IL-10 and IL-13 was studied using specific ELISA.
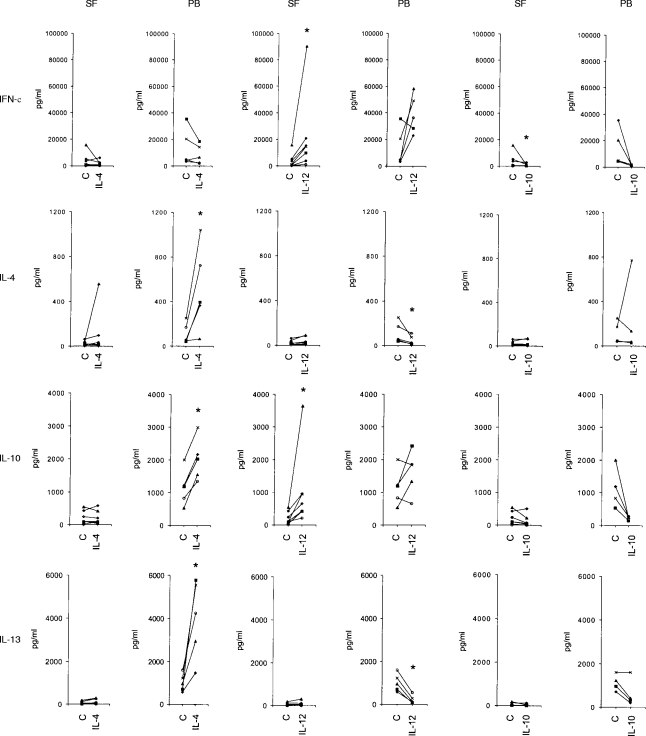
As shown in Fig. 2, the effects of IL-4 on the cytokine production profiles of SF and PB T cells were different. PBMC from healthy volunteers cultured in the presence of IL-2+IL-4 produced significantly higher levels of anti-inflammatory cytokines IL-4, IL-10 and IL-13 when compared with the cells cultured in the presence of IL-2 alone. These data are consistent with previous studies indicating that IL-4 directs differentiation of Th2 cells.16,18 In contrast, IL-4 treatment did not significantly increase IL-4 production by non-adherent SFMC (Fig. 2). Similarly, IL-4 had no significant effect on the production of IL-10 or IL-13 by SFMC, suggesting that SF T cells are unresponsive to the Th2-inducing effects of IL-4.
Figure 2.
The production of IFN-γ, IL-4, IL-10 and IL-13 by non-adherent SFMC from seven patients with RA and by non-adherent PBMC from five healthy volunteers after in vitro treatment with different cytokines. The cells were cultured for 12 days in the presence of IL-2 alone (control) or in the presence of IL-2+IL-4 (left two panels), IL-2+IL-12 (middle), or IL-2+IL-10 (right). The effects of IL-10 were studied in six patients with RA and in four healthy volunteers. Cultured cells were activated for 24 hr in the presence of IL-2 (100 U/ml), anti-CD3 mAb (1 μg/ml) and anti-CD28 mAb (10 μg/ml) and the production of cytokines was studied using specific ELISA. The dots represent individual samples and the lines connect cytokine production in the presence of IL-2 alone and in the presence of IL-2 + the cytokine tested. An asterisk denotes a significant difference (P < 0·05) from the control using Wilcoxon signed-rank test.
We next studied the possibility that the production of IFN-γ or IL-12 by SFMC inhibited the effects of IL-4 on SF T cells. This does not seem to be the case, because IL-4, IL-10, or IL-13 production could not be induced by the addition of neutralizing anti-IFN-γ or anti-IL-12 mAb together with IL-4 to SFMC cultures (n=2, data not shown). We also studied the effects of IL-13 on the production of cytokines by non-adherent SFMC. Consistent with the previous results using normal human T cells,24 IL-13 did not increase the production of type 2 cytokines by SFMC or PBMC (n=3, data not shown).
Similar difference in the responsiveness to IL-4 was observed when we compared the effects of IL-4 on the cytokine production by non-adherent paired SFMC and PBMC samples derived from three patients with RA. Culturing the cells in the presence of IL-2+IL-4 significantly increased the secretion of IL-4 (413±49% when compared with IL-2 alone; mean±SD), IL-10 (243±26%) and IL-13 (571±102%) by PBMC, whereas it had no, or only some, effect on the production of IL-4 (118±34%), IL-10 (104±30%) and IL-13 (251±203%) by SFMC.
In contrast to the effects of IL-4, there were no major differences in the effects of IL-12 on the production of cytokines by non-adherent SFMC from patients with RA when compared with non-adherent PBMC from healthy volunteers. IL-12 treatment increased the production of IFN-γ by both cell populations (Fig. 2). It also consistently up-regulated the secretion of IL-10 by SFMC, and in PBMC the up-regulation of IL-10 production was observed in three of five samples. IL-12 significantly inhibited the production of IL-4 and IL-13 by PBMC, but no down-regulation could be observed in SFMC due to the low basal production of these cytokines by cultured SF T cells. IL-10 generally had suppressive effects on the production of all cytokines by both SFMC and PBMC, and therefore did not affect the balance between type 1 and type 2 cytokines (Fig. 2).
Taken together, the present results indicate that the cytokine production profile of SF T cells can be modulated by IL-12, but not by IL-4 or IL-10.
DISCUSSION
Previous studies investigating T-cell cytokine production at the protein level have mainly utilized T-cell clones derived from the synovial membranes or SF from RA patients.25–27 These studies demonstrated the presence of Th1 and Th0 clones in rheumatoid joints, but the clones derived from the PB also demonstrated a similar pattern.25–27 The most striking difference between T-cell clones derived from the joints or periphery of RA patients was the high production of IL-10 by synovial T cells when compared with PB T cells.27 The procedures used to clone T cells may favour the generation of Th1 cells because of IL-12 produced by the feeder cells. The recently developed staining method to detect intracellular cytokines requires only short in vitro activation and allows the estimation of the frequency of cells producing certain cytokines. Dolhain et al.28 used this method together with microscopic analysis to compare cytokine production by unfractionated PBMC and SFMC. Our results indicating increased production of IFN-γ by both CD4+ and CD8+ cells in SF when compared with PB are in line with those of Dolhain et al.28 who demonstrated that the percentage of IFN-γ-producing cells in SFMC was higher than in PBMC, whereas there was no significant difference in the expression of IL-4 between these two cell populations. Similarly, Morita et al.29 recently showed that CD4+ T cells derived from rheumatoid synovial tissue express higher levels of IFN-γ than PB CD4+ T cells, while the expression of IL-4 in these cell populations is comparable. In contrast, our results are different from those reported by Kusaba et al.30 because they demonstrated an increased production of both IFN-γ and IL-4 in SF CD4+ cells when compared with PB CD4+ cells, and higher expression of IL-4 than of IFN-γ by SF CD8+ cells from patients with RA.
The present results using freshly isolated synovial T cells suggest that the shift towards Th1/Tc1 responses in the joints of patients with RA is not due to defective production of IL-4 by these cells, but mainly to their increased capacity to produce IFN-γ. Although synovial T cells have the capacity to produce IFN-γ, only low levels of IFN-γ have been detected in the SF samples from patients with RA.31 It is possible that although T-cell cytokines, such as IFN-γ, are produced by synovial T cells, they are secreted in a polarized manner towards the responding cells and are rapidly bound by these cells, and therefore are not found in high quantities in a soluble form in SF. Alternatively, other cytokines present in the joints, such as IL-10,32 may suppress IFN-γ production by T cells.
Our results indicate that synovial T cells from chronically inflamed joints of patients with RA are unresponsive to the Th2-inducing effects of IL-4. One possible explanation for these results is that most SF T cells are already primed5 and are unable to change their cytokine production profile. This possibility is supported by the observation that IL-4 enhances IL-13 production to a lesser extent in human PB CD45RO+ than in CD45RA+ T cells.33 However, the finding that IL-12 increases IFN-γ production, but not IL-4 production, by SF T cells, indicates that the cytokine production profile of synovial T cells can be modulated by cytokines. Our data are also in line with those of Sornasse et al.18 who demonstrated that the phenotype of human Th1 cells, differentiated in the presence of IL-12 from neonatal CD4+ cells, is relatively stable and cannot be reversed by repeated stimulation with IL-4. The ability of IL-4 to modulate the phenotype of PB T cells towards Th2 may be due to the lower frequency of committed Th1 cells in PB samples. Therefore, the present data, together with previous studies, support the conclusion that terminally differentiated human Th1 cells cannot be reverted to Th2 cells by IL-4.
The expression patterns of IFN-γ and IL-12 receptors differ on human and mouse Th1 and Th2 cells, which is likely to play a role in the capacity of these cytokines to direct Th-cell responses. Th1 cells do not express the IFN-γ receptor β-chain,34,35 and Th2 cells have lost their ability to express the IL-12 receptor β2-subunit.36,37 To our knowledge there are no reports indicating that Th1 cells lose IL-4 receptor expression during their differentiation, but one reason for the lack of Th2-inducing effects of IL-4 on SF T cells might be an abnormal expression or function of IL-4 receptor on these cells. However, we have previously shown that IL-4 is able to induce proliferation of SF T cells,11 indicating that synovial T cells have the capacity to respond to IL-4. Further studies on the effects of IL-4 and the function of IL-4 receptors on T cells derived from chronic inflammatory conditions are needed to clarify whether the inability of IL-4 to induce Th2 cells is a phenomenon unique to synovial T cells in RA or whether, and perhaps more likely, it is primarily related to the activation and differentiation status of SF T cells.
Taken together, we demonstrate an increased frequency of IFN-γ-producing T cells in rheumatoid joints, suggesting a pathogenetic role for Th1/Tc1 cells in RA. In addition, our results suggest that these cells have lost their capacity to differentiate towards Th2/Tc2 cells in response to IL-4. IL-4 has been previously shown to inhibit proinflammatory cytokine production by SF macrophages from patients with RA,11,38 suggesting that IL-4 may have therapeutic potential in the treatment of RA. It has been speculated that IL-4 treatment would also shift the pathogenetic T-cell response towards an anti-inflammatory type 2 response. However, on the basis of the present results, IL-4 is unable to modify the cytokine production profile of differentiated synovial T cells.
Acknowledgments
This work was supported in part by a grant from the Academy of Finland. We thank Satu Kling for excellent technical assistance. Dr Riitta Saario is acknowledged for providing patient samples and Jose Carballido for his assistance in the immunofiuorescence stainings.
Glossary
- RA
rheumatoid arthritis
- SF
synovial fluid
- SFMC
synovial fluid mononuclear cells.
REFERENCES
- 1.Feldmann M, Brennan FM, Maini RN. Rheumatoid arthritis. Cell. 1996;85:307. doi: 10.1016/s0092-8674(00)81109-5. [DOI] [PubMed] [Google Scholar]
- 2.Panayi GS, Lanchbury JS, Kingsley GH. The importance of the T cell in initiating and maintaining the chronic synovitis of rheumatoid arthritis. Arthritis Rheum. 1992;35:729. doi: 10.1002/art.1780350702. [DOI] [PubMed] [Google Scholar]
- 3.Cush JJ, Lipsky PE. Phenotypical analysis of synovial tissue and peripheral blood lymphocytes isolated from patients with rheumatoid arthritis. Arthritis Rheum. 1988;31:1230. doi: 10.1002/art.1780311003. [DOI] [PubMed] [Google Scholar]
- 4.Isomäki P, Aversa G, Cocks B, et al. Increased expression of SLAM in patients with rheumatoid arthritis and its role in the regulation of cytokine production in rheumatoid synovium. J Immunol. 1997;159:2986. [PubMed] [Google Scholar]
- 5.Thomas R, McIlraith M, Davis LS, Lipsky PE. Rheumatoid synovium is enriched in CD45RBdim mature memory T cells that are potent helpers for B cell differentiation. Arthritis Rheum. 1992;35:1455. doi: 10.1002/art.1780351209. [DOI] [PubMed] [Google Scholar]
- 6.Mima T, Saeki Y, Ohshima S, et al. Transfer of rheumatoid arthritis into severe combined immunodeficient mice. J Clin Invest. 1995;96:1746. doi: 10.1172/JCI118220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buchan G, Barrett K, Fujita T, Taniguchi T, Maini R, Feldmann M. Detection of activated T cell products in the rheumatoid joint using cDNA probes to interleukin-2 (IL-2), IL-2 receptor and IFN-γ. Clin Exp Immunol. 1988;71:295. [PMC free article] [PubMed] [Google Scholar]
- 8.Chen E, Keystone EC, Fish EN. Restricted cytokine expression in rheumatoid arthritis. Arthritis Rheum. 1993;36:901. doi: 10.1002/art.1780360706. [DOI] [PubMed] [Google Scholar]
- 9.Simon AK, Seipelt E, Sieper J. Divergent T-cell cytokine patterns in inflammatory arthritis. Proc Natl Acad Sci. 1994;91:8562. doi: 10.1073/pnas.91.18.8562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ulfgren A-K, Lindblad S, Klareskog L, Andersson J, Andersson U. Detection of cytokine producing cells in the synovial membrane from patients with rheumatoid arthritis. Ann Rheum Dis. 1995;54:654. doi: 10.1136/ard.54.8.654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Isomäki P, Luukkainen R, Toivanen P, Punnonen J. The presence of interleukin-13 in rheumatoid synovium and its anti-inflammatory effects on synovial fluid macrophages from patients with rheumatoid arthritis. Arthritis Rheum. 1996;39:1693. doi: 10.1002/art.1780391012. [DOI] [PubMed] [Google Scholar]
- 12.Mosmann TR, Sad S. The expanding universe of T-cell subsets: Th1, Th2 and more. Immunol Today. 1996;17:138. doi: 10.1016/0167-5699(96)80606-2. [DOI] [PubMed] [Google Scholar]
- 13.Yssel H, De Waal Malefyt R, Roncarolo M-G, et al. IL-10 is produced by subsets of human CD4+ T cell clones and peripheral blood T cells. J Immunol. 1992;149:2378. [PubMed] [Google Scholar]
- 14.De Waal Malefyt R, Abrams JS, Zurawski SM, et al. Differential regulation of IL-13 and IL-4 production by human CD8+ and CD4+ Th0, Th1 and Th2 T cell clones and EBV-transformed B cells. Int Immunol. 1995;7:1405. doi: 10.1093/intimm/7.9.1405. [DOI] [PubMed] [Google Scholar]
- 15.Mosmann TR, Coffman RL. Th1 and Th2 cells: Different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol. 1989;7:145. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- 16.Hsieh C-S, Heimberger AB, Gold JS, O’Garra A, Murphy KM. Differential regulation of T helper phenotype development by interleukins 4 and 10 in an αβ T-cell-receptor transgenic system. Proc Natl Acad Sci USA. 1992;89:6065. doi: 10.1073/pnas.89.13.6065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Seder RA, Gazzinelli R, Sher A, Paul WE. Interleukin 12 acts directly on CD4+ T cells to enhance priming for interferon γ production and diminishes interleukin 4 inhibition of such priming. Proc Natl Acad Sci USA. 1993;90:10188. doi: 10.1073/pnas.90.21.10188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sornasse T, Larenas PV, Davis KA, De Vries JE, Yssel H. Differentiation and stability of T helper 1 and 2 cells derived from naive human neonatal CD4+ T cells, analyzed at the single-cell level. J Exp Med. 1996;184:473. doi: 10.1084/jem.184.2.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Groux H, O’Garra A, Bigler M, et al. A CD4+ T-cell subset inhibits antigen-specific T-cell responses and prevents colitis. Nature. 1997;389:737. doi: 10.1038/39614. [DOI] [PubMed] [Google Scholar]
- 20.Sad S, Marcotte R, Mosmann TR. Cytokine-induced differentiation of precursor mouse CD8+ T cells into cytotoxic CD8+ T cells secreting Th1 or Th2 cytokines. Immunity. 1995;2:271. doi: 10.1016/1074-7613(95)90051-9. [DOI] [PubMed] [Google Scholar]
- 21.Croft M, Carter L, Swain SL, Dutton RW. Generation of polarized antigen-specific CD8 effector populations: reciprocal action of interleukin (IL) -4 and IL-12 in promoting type 2 versus type 1 cytokine profiles. J Exp Med. 1994;180:1715. doi: 10.1084/jem.180.5.1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liblau RS, Singer SM, McDevitt HO. Th1 and Th2 CD4+ T cells in the pathogenesis of organ-specific autoimmune diseases. Immunol Today. 1995;34:34. doi: 10.1016/0167-5699(95)80068-9. [DOI] [PubMed] [Google Scholar]
- 23.Arnett FC, Edworthy SM, Bloch DA, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31:315. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 24.De Vries JE, Carballido JM, Sornasse T, Yssel H. Antagonizing the differentiation and functions of human Th2 cells. Curr Opin Immunol. 1995;7:771. doi: 10.1016/0952-7915(95)80046-8. [DOI] [PubMed] [Google Scholar]
- 25.Miltenburg AM.M, Van Laar JM, De Kuiper R, Daha MR, Breedveld FC. T cells cloned from human rheumatoid synovial membrane functionally represent the Th1 subset. Scand J Immunol. 1992;35:603. doi: 10.1111/j.1365-3083.1992.tb03260.x. [DOI] [PubMed] [Google Scholar]
- 26.Quayle AJ, Chomarat P, Miossec P, Kjeldsen-Kragh J, Forre O, Natvig JB. Rheumatoid inflammatory T-cell clones express mostly Th1 but also Th2 and mixed (Th0-like) cytokine patterns. Scand J Immunol. 1993;38:75. doi: 10.1111/j.1365-3083.1993.tb01696.x. [DOI] [PubMed] [Google Scholar]
- 27.Cohen SB.A, Katsikis PD, Chu C-Q, et al. High level of interleukin-10 production by the activated T cell population within the rheumatoid synovial membrane. Arthritis Rheum. 1995;38:946. doi: 10.1002/art.1780380710. [DOI] [PubMed] [Google Scholar]
- 28.Dolhain RJ.E.M, Van Der Heiden AN, Ter Haar NT, Breedveld FC, Miltenburg AM.M. Shift toward T lymphocytes with a T helper 1 cytokine-secretion profile in the joints of patients with rheumatoid arthritis. Arthritis Rheum. 1996;39:1961. doi: 10.1002/art.1780391204. [DOI] [PubMed] [Google Scholar]
- 29.Morita Y, Yamamura M, Kawashima M, et al. Flow cytometric single-cell analysis of cytokine production by CD4+ T cells in synovial tissue and peripheral blood from patients with rheumatoid arthritis. Arthritis Rheum. 1998;41:1669. doi: 10.1002/1529-0131(199809)41:9<1669::AID-ART19>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 30.Kusaba M, Honda J, Fukuda T, Oizumi K. Analysis of type 1 and type 2 T cells in synovial fluid and peripheral blood of patients with rheumatoid arthritis. J Rheumatol. 1998;25:1466. [PubMed] [Google Scholar]
- 31.Firestein GS, Zvaifler NJ. Peripheral blood and synovial fluid monocyte activation in inflammatory arthritis. II. Low levels of synovial fluid and synovial tissue interferon suggest that γ-interferon is not the primary macrophage activating factor. Arthritis Rheum. 1987;30:864. doi: 10.1002/art.1780300804. [DOI] [PubMed] [Google Scholar]
- 32.Katsikis PD, Chu C-Q, Brennan FM, Maini RN, Feldmann M. Immunoregulatory role of interleukin 10 in rheumatoid arthritis. J Exp Med. 1994;179:1517. doi: 10.1084/jem.179.5.1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jung T, Wijdenes J, Neumann C, De Vries JE, Yssel H. Interleukin-13 is produced by activated human CD45RA+ and CD45RO+ T cells: modulation by interleukin-4 and interleukin-12. Eur J Immunol. 1996;26:571. doi: 10.1002/eji.1830260311. [DOI] [PubMed] [Google Scholar]
- 34.Pernis A, Gupta S, Gollob KJ, et al. Lack of interferon γ receptor β chain and the prevention of interferon γ signaling in Th1 cells. Science. 1995;269:245. doi: 10.1126/science.7618088. [DOI] [PubMed] [Google Scholar]
- 35.Groux H, Sornasse T, Cottrez F, et al. Induction of human T helper cell type 1 differentiation results in loss of IFN-gamma receptor beta-chain expression. J Immunol. 1997;158:5627. [PubMed] [Google Scholar]
- 36.Szabo SJ, Dighe AS, Gubler U, Murphy KM. Regulation of the interleukin (IL) -12R beta 2 subunit expression in developing T helper 1 (Th1) and Th2 cells. J Exp Med. 1997;185:817. doi: 10.1084/jem.185.5.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rogge L, Barberis-Maino L, Biffi M, et al. Selective expression of an interleukin-12 receptor component by human T helper 1 cells. J Exp Med. 1997;185:825. doi: 10.1084/jem.185.5.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Miossec P, Briolay J, Dechanet J, Wijdenes J, Martinez-Valdez H, Banchereau J. Inhibition of the production of proinflammatory cytokines and immunoglobulins by interleukin-4 in an ex vivo model of rheumatoid synovitis. Arthritis Rheum. 1992;35:874. doi: 10.1002/art.1780350805. [DOI] [PubMed] [Google Scholar]