Abstract
Several studies have revealed that T lymphocytes and cytokines play a crucial role in determining the outcome of parasitic infections in terms of protective immunity. In this study we found that Rhipicephalus sanguineus tick saliva stimulates transforming growth factor-β (TGF-β), and reduces interleukin-12 (IL-12) secretion by cells from normal C3H/HeJ mice. Moreover, murine lymph node cells harvested 6 days after the fourth infestation with ticks presented an 82·4% decrease in their proliferative response to concanavalin A (Con A) compared with the response of control cells. In addition, lymph node cells cultured in the presence of Con A showed a T-helper 2-type (Th2-type) cytokine profile, represented by augmented IL-4 and IL-10 and TGF-β. On the other hand, the IL-2, interferon-γ (IFN-γ) and IL-12 synthesis was significantly inhibited. These results indicate that ticks may modulate the host’s immune response through saliva injection. Considering that C3H/HeJ mice develop no protective immunity to R. sanguineus infestation, our results suggest that tick-induced Th2-type cytokines and a decreased proliferative response probably lead the host to a susceptible state to both tick and tick-transmitted pathogens.
INTRODUCTION
The ‘brown dog tick’Rhipicephalus sanguineus, the most widely distributed tick species in the world, has been linked to many diseases, such as ehrlichiosis, babesiosis and haemobartonellosis,1 which are primarily canine, but may sporadically occur in other mammals, including man.2
Ticks inject saliva at the feeding site, containing a variety of pharmacologically active molecules possessing antihomeostatic, vasodilatory, anti-inflammatory and immunosuppressive properties.3,4 Interestingly, some hosts, such as guinea-pigs, can develop resistance to R. sanguineus tick feeding, while others, like dogs, develop no protective immunity to R. sanguineus challenge infestations.5,6 The understanding of the molecular basis of the strategies used by ticks to evade host resistance, and the immunological mechanisms that lead to host protection should open new possibilities for the design of vaccines or alternative products to control ectoparasites.
It has been suggested that the outcome of many parasitic infections with regards to resistance or susceptibility is determined by the pattern of response, involving subsets of CD4+ T-helper (Th) cells.7 Th1 cells produce interleukin-2 (IL-2) and interferon-γ (IFN-γ), while Th2 cells produce IL-4, IL-5, IL-6, IL-10 and IL-13, and these cytokine patterns may direct the immune response towards different effector mechanisms.8 For instance, Th1 cells lead to macrophage activation and have been associated with delayed-type hypersensitivity (DTH). On the other hand, Th2 cells increase eosinophils and mast cell numbers, and enhance antibody synthesis. Moreover, the products of Th1 and Th2 cells may negatively regulate the production and/or activity of each other.9 Macrophages also produce cytokines, like IL-12 and transforming growth factor-β (TGF-β), and prostaglandin E2 (PGE2) which can influence10 and be influenced by T-cell products.11 In this context, host resistance or susceptibility to tick infestations would most likely be explained by tick-induced modulation of the cytokine network.
Previous work has shown that R. sanguineus tick saliva inhibits in vitro IL-2 production, T-cell proliferation and impairs macrophage nitric oxide-dependent killing of intracellular parasites. Additionally, tick saliva stimulated IL-10 production, and decreased IFN-γ secretion by normal mice splenocytes.12 In the present work we show that R. sanguineus tick saliva also reduces IL-12 and increases TGF-β production by normal mice cells. Also, in an attempt to determine whether ticks modulate host immune response in vivo, we characterized the Th cytokine profile developed by mice infested with ticks. Data presented herein demonstrate that lymph node cells from tick-infested C3H/HeJ mice showed reduced concanavalin A (Con A)-induced proliferative response and developed a Th2-type cytokine profile, represented by augmented IL-4, IL-10 and TGF-β production, in detriment of IL-2, IFN-γ and IL-12 synthesis.
MATERIALS AND METHODS
Experimental animals
Female C3H/HeJ mice (6–8 weeks of age) were bred and maintained under standard conditions in the animal house of the Department of Immunology, University of São Paulo (USP), Ribeirão Preto-SP, Brazil. Female mongrel dogs were maintained and fed on a commercial diet in the central animal house, USP, Ribeirão Preto-SP, Brazil.
Tick infestations and saliva collection
Rhipicephalus sanguineus ticks were reared in our laboratory as previously described.13 C3H/HeJ mice were infested with two female and two male adult ticks (1–3 months old) in plastic feeding chambers (15 mm in diameter) glued to the animals backs’ (Britannia Adhesives P4104 Latex, Brentwood, UK). Four infestations were performed on each animal at 20–30-day intervals on different skin sites. The number of mice infested in each experimental group was five. Control groups consisted of mice on whose backs empty plastic feeding chambers were glued.
To obtain engorged ticks for saliva collection, dogs were infested with 60 pairs of R. sanguineus ticks, contained in plastic feeding chambers (5 cm in diameter) fixed to their backs. The saliva collection procedure was performed using fully engorged female ticks by the inoculation of 10 μl of a 0·2% (w/v) solution of dopamine in phosphate-buffered saline (PBS), a technique modified from a previously described published method.14 Each saliva pool consisted of material harvested from 100–200 female ticks. Protein concentration was determined using a microbiurette assay.15
Trypanosoma cruzi-induced murine cell cultures
Peritoneal cells were obtained from C3H/HeJ mice 4 days after injection of 1 ml of 3% thioglycolate (Difco Laboratories, Detroit, MI) in PBS. The cells were centrifuged for 10 min at 150 g, washed three times and diluted in RPMI-1640 (Flow Laboratories, Inc., McLean, VA) supplemented with 5% fetal calf serum (Gibco-BRL Life Technologies, Grand Island, NY), 5×10−5 m 2-mercaptoethanol, 2 mm l-glutamine and antibiotics. Peritoneal cells were cultured in duplicate (2×106 cells per well) in 24-well culture plates (Corning, New York, NY), and the non-adherent cells were removed by exhaustive washing with Hanks’ balanced salt solution (Sigma Chemical Co., St Louis, MO) after a period of 14–16 hr at 37° in a humidified 5% CO2 incubator. Splenocytes were obtained from C3H/HeJ mice by teasing the spleen into Hanks’ medium. Next, cells were treated for 4 min with lysing buffer (9 parts 0·16 m ammonium chloride and 1 part 0·17 m Tris–HCl, pH 7·5), washed three times and suspended to 2×106 cells per ml in supplemented RPMI-1640 (Flow Laboratories). Erythrocyte-free cells were then cultured in duplicate (2×106 cells per well) in 24-well culture plates (Corning). Live T. cruzi trypomastigotes (one parasite per cell) were added to the macrophage and splenocyte cultures in the presence or absence of tick saliva (64 μg/ml). After 48 hr at 37° in a humidified 5% CO2 incubator, the supernatants were collected and stored at −20° until assayed for IL-12 and TGF-β.
Tick-infested cell cultures
Three groups of 15 female C3H/HeJ mice each were used for the tick-infestation experiments. Mice were killed 6 days after the beginning of each infestation. Axillary and brachial lymph nodes (tick-chamber-draining lymph nodes) and spleens were removed and the cells were obtained by teasing the organs into Hanks’ medium. Cells from each experimental group were pooled, centrifuged for 10 min at 150 g, washed three times and suspended in culture medium. Triplicate cultures were displayed in 96-well flat-bottom culture plates (Corning) at 5×105 cells per well and stimulated with Con A (2 μg/ml) (Sigma). After 64 hr at 37° in a humidified 5% CO2 incubator, the cultures were pulsed with methyl-[3H]thymidine (Amersham Life Science, Bukinghamshire, UK) at a concentration of 0·5 μCi per well. Eight hours later, the amount of label incorporated into cells was determined using a cell Harvester (Cambridge Technology, Inc. Watertown, MA) and a liquid scintillation counter (Beckman Instruments Inc., Fullerton, CA). Mean counts per minute (c.p.m. ± SD) were determined and the stimulation index was calculated for each experimental group as the ratio of counts in the Con A-stimulated cultures over the basal counts (cells in medium only).
Cytokine levels were determined in supernatants of lymph node cells cultured in 24-well culture plates (Corning) at (5×105 per well), for a period of 48 hr, at 37° in a humidified 5% CO2 incubator, with or without Con A (2 μg/ml). The supernatants were removed and stored at −20° until assayed for cytokines.
Cytokine assays
Measurements of IL-2, IL-4, IL-10, IFN-γ, IL-12 p40 and TGF-β in the supernatants were performed using specific solid-phase sandwich enzyme-linked immunosorbent assay (ELISA). Micro-ELISA plates (Corning) were coated overnight with a capture monoclonal antibody (mAb) in 0·06 m bicarbonate–carbonate buffer (pH 9·5), washed twice with PBS containing 0·05% Tween-20 (Sigma), and blocked with 5% skim-milk in PBS for 1 hr. Fifty microlitres of the cell culture supernatants were added to each well, incubated overnight and extensively washed with PBS–Tween. Then, plates were incubated for 1 hr with a specific biotin-labelled detection antibody, washed with PBS–Tween and incubated for 1 hr with an avidin–peroxidase conjugate (Vector Laboratories INC, Burlingame, CA). The assay was revealed with o-phenylenodiamine-2HCl substrate (Abbott Laboratories, Abbott Park, IL) for 10 min and the reaction was stopped by addition of 50 μl of sulphuric acid (1 M). The optical density at 492 nm was determined with an ELISA spectrophotometer (Emax, Molecular Devices Corporation, Sunnyvale, CA). ELISA capture and detection mAb pairs used in these studies were as follows: IL-2, MP6-XT3 (10 μg/ml) and JES6-1A12 (1 μg/ml); IL-4, BVD6 (10 μg/ml) and 11B11 (1 μg/ml); IL-10, JES5-2A5 (2 μg/ml) and SXC-1 (1 μg/ml); IL-12 p40, C17.15.10 (5 μg/ml) and C15.6.76 (1 μg/ml). For the detection of IFN-γ, we used 10 μg/ml of a capture mAb XMG1.2, and a rabbit polyclonal antibody (2 μg/ml) (Immunex Corporation, Seattle, WA). The cytokine concentration of each sample was determined with reference to a standard curve constructed with serially diluted recombinant proteins (Pharmigen, San Diego, CA). Each test sample was assayed in duplicate. TGF-β determination was performed using an ELISA kit (Promega Corporation, Madison, WI), following the manufacturer’s directions. Experiments were repeated at least three times.
Statistical analysis
Data were analysed using the Tukey–Kramer test for multiple comparisons.
RESULTS
Effect of tick saliva on T. cruzi-induced IL-12 and TGF-β production by murine splenocytes and macrophages
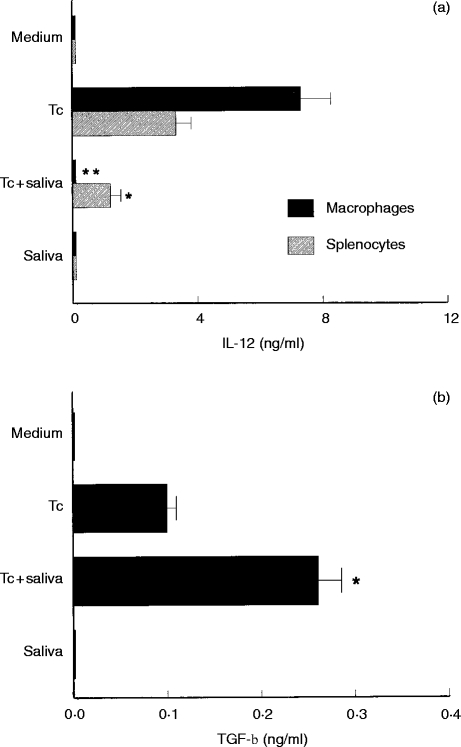
As shown in Fig. 1(a), addition of tick saliva (64 μg/ml) to cells cultured with T. cruzi led to decreases of 98·6% (P < 0·001) and 63·6% (P < 0·05) in IL-12 production by macrophages and splenocytes, respectively, when compared with cells cultured with T. cruzi alone. On the other hand, macrophages exposed to T. cruzi and treated with tick saliva showed an increase of 2·6 times (P < 0·05) in the TGF-β production compared to macrophages cultured only with T. cruzi (Fig. 1b). Normal splenocytes of mice exposed to T. cruzi in the presence or absence of tick saliva produced undetectable levels of TGF-β (data not shown). Saliva alone had no effect on IL-12 and TGF-β production by the cell cultures (Fig. 1).
Figure 1.
Effect of R. sanguineus saliva on Trypanosoma cruzi-induced IL-12 (a) and TGF-β (b) synthesis by normal mouse cells. Mouse macrophages or splenocytes were cultured in 24-well plates (2×106 cells per well) in the presence of T. cruzi (Tc) (one parasite per cell) and/or tick saliva (64 μg/ml). Supernatants were collected after 48 hr and assayed for IL-12 and TGF-β by ELISA. Results are shown as mean cytokine production ± SD. *P < 0·05 and **P < 0·001compared with cells cultured with T. cruzi parasites alone. The experiments were repeated three times.
Effect of tick infestations on murine T-cell proliferation stimulated with Con A
Lymph node cells harvested from mice infested once or four times with ticks showed a decrease of 77·9 and 82·4%, respectively, in the stimulation index compared to controls (Fig. 2). On the other hand, splenocytes from tick-infested mice showed no change in their proliferative response to Con A (stimulation indices of 16·9, 19·5 and 16·6 for control, one and four times tick-infested mice, respectively). Notably, lymph nodes removed from mice infested with ticks were three times increased in size compared to organs collected from non-infested controls.
Figure 2.
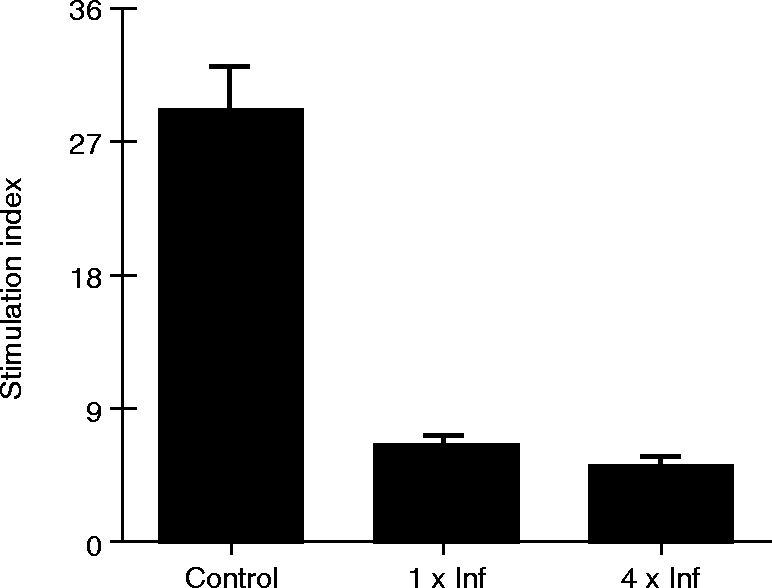
Effect of R. sanguineus tick infestations on Con A-induced cell proliferation in mice. Lymph node cells from mice infested once (1×Inf), four times (4×Inf) or non-infested (Control) with ticks were cultured (5×105 cells per well) with or without Con A (2 μg/ml) and assayed for cell proliferation by methyl-[3H]-thymidine incorporation. A stimulation index was calculated for each experimental group as described in the Material and Methods. Results are shown as mean of Stimulation Index ± SD from two independent experiments.
Cytokine production by tick-infested C3H/HeJ mice
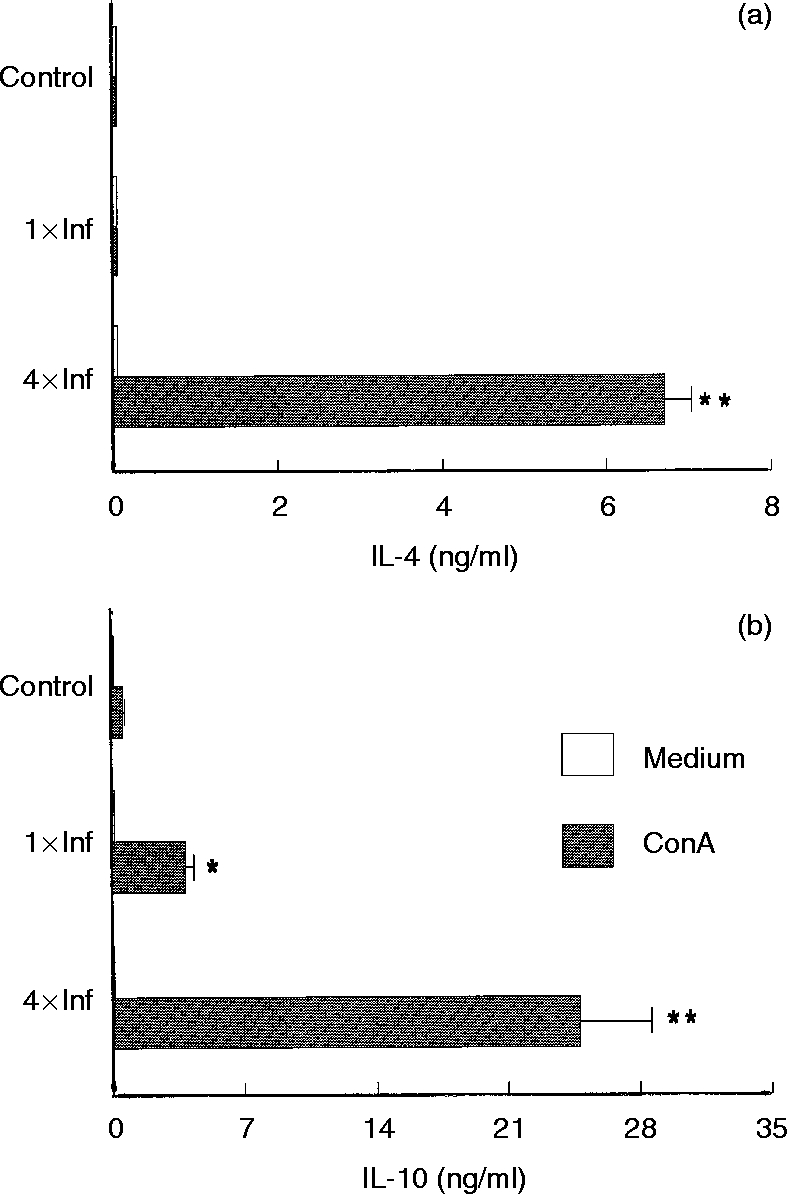
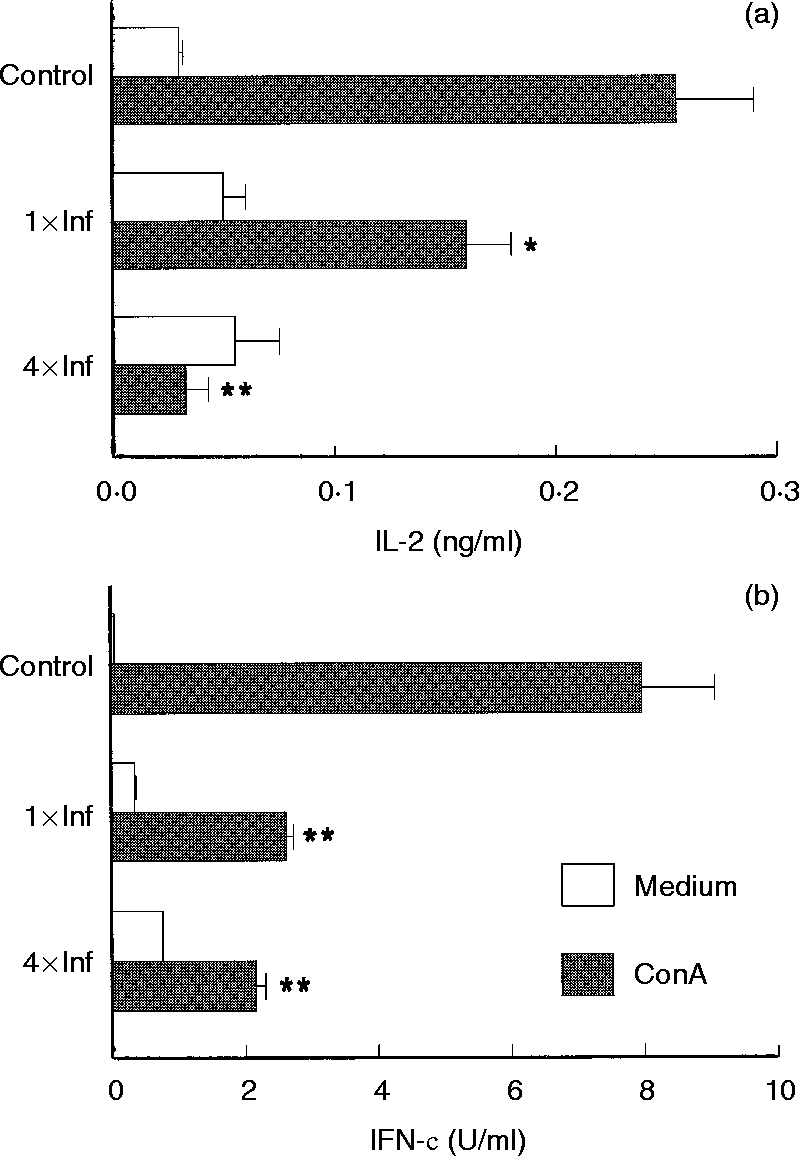
Within six days of tick infestation, mice lymph node cells were stimulated with Con A and culture supernatants were assayed for cytokines. Mice infested once with ticks showed decreased IL-2 and IFN-γ production (37·2% and 67·0% reduction, respectively), when compared to cells obtained from control mice (Fig. 3). The same cultured cells produced 6·5 times more IL-10 than cells from control mice (Fig. 4b), whereas IL-4 synthesis was unchanged (Fig. 4a). Con A-stimulated cells from mice infested four times were shown to be even more polarized towards the Th2-cytokine pattern. These cells displayed greatly enhanced IL-4 and IL-10 production (P < 0·001, Fig. 4), while IL-2 and IFN-γ production was strongly inhibited (P < 0·001, Fig. 3) as compared to the controls.
Figure 3.
Effect of repeated tick infestations with R. sanguineus on the production of IL-2 (a) and IFN-γ (b) by mice. Lymph node cells from mice infested once (1×Inf) or four times (4×Inf) with ticks were cultured (2×106 cells per well) with or without Con A (2 μg/ml). After 48 hr the supernatants were collected and assayed by specific ELISA for IL-2 and IFN-γ. *P < 0·05 and **P < 0·001 compared with cells from control mice plus Con A. The experiments were performed in duplicate and repeated three times.
Figure 4.
Effect of repeated tick infestations with R. sanguineus ticks on the production of IL-4 (a) and IL-10 (b) by mice. Lymph node cells from mice infested once (1×Inf) or four times (4×Inf) with ticks were cultured (2×106 cells per well) with or without Con A (2 μg/ml). After 48 hr the supernatants were collected and assayed by specific ELISA for IL-4and IL-10. *P < 0·05 and **P < 0·001 compared with cells from control mice plus Con A. The experiments were performed in duplicate and repeated three times.
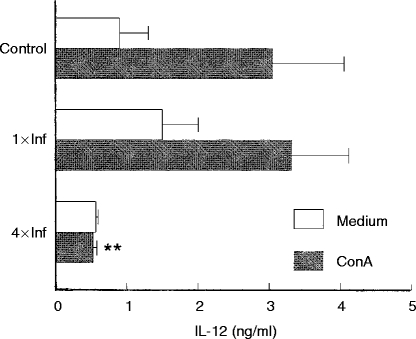
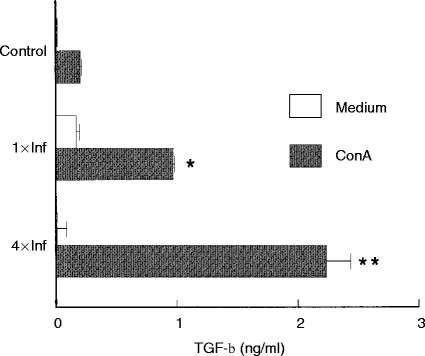
Figure 5 shows the IL-12 concentrations detected in the supernatants of lymph node cells from control or tick-infested mice. Non-stimulated cells from control mice and from mice infested once and four times produced similar levels of IL-12. When stimulated with Con A, cells from controls and from mice infested once also produced similar amounts of IL-12. However, mice infested four times had Con A-stimulated IL-12 production reduced to background levels (Fig. 5). On the other hand, Con A-stimulated cells from once and four times infested mice produced 4·8 and 11·2 times more TGF-β, respectively (P < 0·001), than the controls (Fig. 6).
Figure 5.
Effect of repeated tick infestations with R. sanguineus on the production of IL-12 by mice. Lymph node cells from mice infested once (1×Inf) or four times (4×Inf) with ticks were cultured (2×106 cells per well) with or without Con A (2 μg/ml). After 48 hr the supernatants were collected and were assayed by specific ELISA for IL-12. **P < 0·001 compared with cells from control mice plus Con A. The experiments were performed in duplicate and repeated three times.
Figure 6.
Effect of repeated tick infestations with R. sanguineus on the production of TGF-β by mice. Lymph node cells from mice infested once (1×Inf) or four times (4×Inf) with ticks were cultured (2×106 cells per well) with or without Con A (2 μg/ml). After 48 hr the supernatants were collected and assayed by specific ELISA for TGF-β. *P < 0·05 and **P < 0·001 compared with cells from control mice plus Con A. The experiments were performed in duplicate and repeated three times.
DISCUSSION
Considering that ticks remain attached to hosts skin for prolonged periods of time, and inoculate saliva as they feed, it is reasonable to suppose that ticks may modulate the host immune response. Only recently the issue of tick-induced immune modulation through cytokines has been addressed.16 However, this matter has not yet been studied in the specific care of the R. sanguineus tick–host association.
In an attempt to improve our knowledge on the immunology of host-tick relationships, we first studied the effects R. sanguineus tick saliva on the murine cytokine production. Based on our previous observations that T. cruzi induces IL-10, IFN-γ and IL-12 production,17,18 we added R. sanguineus tick saliva to normal murine cells cultured with trypomastigote forms of T. cruzi. We found that tick saliva reduced IL-12 and increased TGF-β production. These results extend our previous observations showing that R. sanguineus tick saliva increases IL-10 and diminishes IFN-γ production by T. cruzi-stimulated cells.12 Other work showed that salivary gland extracts from Dermacentor andersoni ticks inhibited IL-1 and tumor necrosis factor-α (TNF-α) synthesis by bovine macrophages, as well as decreased IL-2 and IFN-γ production by lipopolysaccharide-stimulated murine splenocytes.19,20 Taken together, these results support the view that ticks may modulate the host’s cytokine network. To test this hypothesis, we assayed the proliferative response and cytokine production by lymphocytes from R. sanguineus tick-infested mice. The results showed that lymph node cells of R. sanguineus tick-infested mice presented impaired IL-2 production and reduced the proliferative response to Con A since the first infestation. Impaired IL-2 production may lead to decreased T-helper and effector functions, including reduction of DTH reactions, cytotoxic T-cell responses, as well as antibody production. Such an immunosuppressive state could hinder the host’s resistance to ticks and to tick-transmitted pathogens. Other studies have observed reduced proliferative responses to mitogens on different tick–host associations.4 Considering that C3H/HeJ mice develop no resistance to R. sanguineus ticks,12 and that their proliferative response to Con A was suppressed by the first to fourth tick infestation, it is reasonable to argue that the tick-susceptible state results from the lack of efficient response to antigens introduced with tick saliva. Differently, in tick–host interactions that result in host resistance, the magnitude of suppression of responsiveness to Con A is progressively reduced from the first to the fourth infestations,21 suggesting acquired resistance to tick-introduced molecule(s).4
Our results showed that IL-2 and IFN-γ production (Th1 cytokines) were largely down-regulated by lymphocytes from lymph nodes draining the tick attachment sites, whereas IL-4 and IL-10 (Th2 cytokines) were up-regulated, mainly on the fourth infestation. These findings indicate a polarization of the immune response toward Th2-lymphocytes. Ixodes ricinus tick-infested BALB/c mice also displayed a Th2-cytokine profile in response to Con A.22,23 In contrast, IL-2 and IFN-γ mRNA was shown to be expressed in much more cells of lymph node sections from BALB/c mice infested with I. ricinus than IL-4.24 The contrasting results may be because transcription of cytokine mRNA can be increased in the absence of cell protein release.
Since the first infestation, lymph node cells from tick-infested mice presented enhanced IL-10 and decreased IFN-γ synthesis. The IL-10 produced by tick-infested mice can interfere with the functional maturation of dendritic cells, and can selectively inhibit their capacity to induce the development of IFN-γ-producing T cells in vivo, favouring Th2 differentiation.25 Lymph node cells from mice infested four times with ticks also exhibited reduced IL-12 production, probably due to an up-regulation of IL-10 and IL-4, since IL-10 can suppress IL-12 synthesis,26,27 and IL-4 can work in an additive fashion with IL-10, inhibiting the IL-12 production even more.27 Considering that IL-12 is essential for the cell-mediated control of several intracellular parasites,28 its decrease could impair the host’s ability to control tick-borne pathogens. Additionally, data presented herein showed that tick saliva was able to inhibit IL-12 production by T. cruzi-stimulated mouse cells, strongly suggesting that tick saliva, in fact may play a major role in the host’s cytokine modulation.
The observation that tick infestations and tick saliva increased production of TGF-β by mouse cells, added to previous results that demonstrated that R. sanguineus saliva impairs in vitro macrophage microbicidal activity,12 suggest that tick saliva may induce TGF-β production during tick infestations, thus compromising the host’s capacity to mount a protective immune response to ticks and tick-transmitted pathogens. This hypothesis is based on the observation that TGF-β mediates immunosuppression by decreasing Th1-associated cytokines,29 and inhibits IFN-γ-induced intracellular parasite killing.30,31 This is the first study that correlates decreased IL-12 and augmented TGF-β production with host susceptibility to tick infestations.
The Th2-cytokine response observed on R. sanguineus tick-infested mice could be advantageous for the ticks, considering that Th2-cytokines have been intrinsically connected to anti-inflammatory responses32 and inflammation at the tick attachment site can impair tick feeding.3 Interestingly, it was reported that repeatedly tick-infested guinea-pigs that had developed resistance to ticks, presented both an immediate and a strong DTH reaction to a tick extract. Conversely, dogs, which are naturally susceptible to ticks, developed a much more intense immediate but, not a DTH, reaction after repeated infestations with ticks.33 These results suggest that resistance to ticks is due to a DTH reaction, which has been largely associated with Th1-cytokine responses.34 This hypothesis is supported by the observation that dogs immunized with a tick gut extract in Freund’s adjuvant, but not saponin, develop resistance to this tick species.35
The knowledge of which host T-cell subpopulations are relevant to develop acquired resistance to ticks could help vaccine development, considering that this may suggest the most adequate adjuvants (such as IL-12), in order to stimulate specific T-cell subsets associated with protective long-lasting immune responses. The benefits of such approach may be twofold, since it may not only eliminate the ectoparasites, but also increase the host’s resistance to tick-borne pathogens controlled by a Th1-type response. Further studies should be performed to identify tick molecules that lead to Th2 response, as they could possibly be used to modulate diseases in which a Th1-cytokine response may be deleterious for patients.
Acknowledgments
This work was supported by grants from CNPq and FINEP, and by fellowships to B. R. Ferreira and J. S. Silva from CNPq. We wish to thank M. M. Rossi and W.C.R. da Silva for excellent technical assistance.
Glossary
Abbreviations
- Con A
concanavalin A
- DTH
delayed-type hypersensitivity
- ELISA
enzyme-linked immunosorbent assay
- IFN-γ
interferon-γ
- IL
interleukin
- PGE2
prostaglandin E2
- TGF-β
transforming growth factor-β
- Th
T helper
- TNF-α
tumour necrosis factor α
REFERENCES
- 1.Cupp EW. Biology of ticks. Vet Clin North Am Small Anim Prac. 1991;21:1. doi: 10.1016/s0195-5616(91)50001-2. [DOI] [PubMed] [Google Scholar]
- 2.Goddard J. Focus of human parasitism by the brown dog tick, Rhipicephalus sanguineus(Acari: Ixodidae) J Med Entomol. 1989;26:628. doi: 10.1093/jmedent/26.6.628. [DOI] [PubMed] [Google Scholar]
- 3.Ribeiro JM.C, Makoul GT, Levine J, Robinson DR, Spielman A. Antihemostatic, anti-inflammatory and immunosuppressive properties of the saliva of a tick, Ixodes dammini. J Exp Med. 1985;161:332. doi: 10.1084/jem.161.2.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wikel SK. Host immunity to ticks. Annu Rev Entomol. 1996;41:1. doi: 10.1146/annurev.en.41.010196.000245. [DOI] [PubMed] [Google Scholar]
- 5.Garin NS, Grabarev PA. Protective reactions in rabbits and guinea-pigs upon repeated feeding on them of ixodid ticks Rhipicephalus sanguineus (Latr, 1806) Meditsinskaia Parazitologiia I Parazitarnye Bolezni. 1972;41:274. [PubMed] [Google Scholar]
- 6.Szabó MP.J, Mukai LS, Rosa PC.S, Bechara GH. Differences in the acquired resistance of dogs, hamsters and guinea pigs to repeated infestations with adult ticks R. sanguineus (Acari: Isodidae) Braz J Vet Res An Sc. 1995;32:43. [Google Scholar]
- 7.Sher A, Coffman RL. Regulation of immunity to parasites by T cells and T cell-derived cytokines. Annu Rev Immunol. 1992;10:385. doi: 10.1146/annurev.iy.10.040192.002125. [DOI] [PubMed] [Google Scholar]
- 8.Mosmann TR, Sad S. The expanding universe of T-cell subsets: Th1, Th2 and more. Immunol Today. 1996;17:138. doi: 10.1016/0167-5699(96)80606-2. [DOI] [PubMed] [Google Scholar]
- 9.Mosmann TR, Moore KW. The role of interleukin-10 in cross regulation of Th1 and Th2 responses. Immunol Today. 1991;12:49. doi: 10.1016/S0167-5699(05)80015-5. [DOI] [PubMed] [Google Scholar]
- 10.Hsieh CS, Macatonia SE, O’Garra A, Murphy KM. Pathogen-induced Th1 phenotype development in CD4+ αβ-TCR transgenic T cells is macrophage dependent. Int Immunol. 1993;5:371. doi: 10.1093/intimm/5.4.371. [DOI] [PubMed] [Google Scholar]
- 11.Narumi S, Finke JH, Hamilton TA. Interferon-γ and interleukin-2 synergize to induce selective monokine expression in murine peritoneal macrophages. J Biol Chem. 1990;265:7036. [PubMed] [Google Scholar]
- 12.Ferreira BR, Silva JS. Saliva of Rhipicephalus sanguineus tick impairs T cell proliferation and IFN-γ-induced macrophage microbicidal activity. Vet Immunol Immunopathol. 1998;64:267. doi: 10.1016/s0165-2427(98)00135-4. [DOI] [PubMed] [Google Scholar]
- 13.Ferreira BR, Machado RZ, Bechara GH. Western blot analysis of tick antigens from a Rhipicephalus sanguineus unfed larval extract and identification of antigenic sites in tick sections using immunohistochemistry. A comparative study between resistant and susceptible host species. Vet Parasitol. 1996;62:161. doi: 10.1016/0304-4017(95)00838-1. [DOI] [PubMed] [Google Scholar]
- 14.Bechara GH, Szabó MP.J, Machado RZ, Rocha UF. A technique for collecting saliva from the cattle tick Boophilus microplus (Canestrini) using chemical stimulation. Environmental and temporal influences on secretion yield. Braz J Med Biol. 1988;21:478. [PubMed] [Google Scholar]
- 15.Itzhaki FR, Gill DM. A micro-biuret method for estimating proteins. Anal Biochem. 1964;9:401. doi: 10.1016/0003-2697(64)90200-3. [DOI] [PubMed] [Google Scholar]
- 16.Wikel SK. Tick modulation of host cytokines. Exp Parasitol. 1996;84:304. doi: 10.1006/expr.1996.0118. [DOI] [PubMed] [Google Scholar]
- 17.Cardillo F, Voltarelli JC, Reed SG, Silva JS. Regulation of Trypanosoma cruzi infection in mice by IFN-γ and IL-10: the role of NK cells. Infect Immun. 1996;64:128. doi: 10.1128/iai.64.1.128-134.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aliberti JC, Cardoso MA.G, Martins GA, Gazzinelli RT, Vieira LQ, Silva JS. Interleukin-12 mediates resistance to Trypanosoma cruzi in mice and is produced by murine macrophages in response to live trypomastigotes. Infect Immun. 1996;64:1961. doi: 10.1128/iai.64.6.1961-1967.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ramachandra RN, Wikel SK. Modulation of host-immune responses by ticks (Acari: Ixodidae): Effect of salivary gland extracts on host macrophages and lymphocyte cytokine production. J Med Entomol. 1992;29:818. doi: 10.1093/jmedent/29.5.818. [DOI] [PubMed] [Google Scholar]
- 20.Ramachandra RN, Wikel SK. Effects of Dermacentor andersoni(Acari: Ixodidae) salivary gland extracts on Bos indicus and B. taurus lymphocytes and macrophages: in vitro cytokine elaboration and lymphocyte blastogenesis. J Med Entomol. 1995;32:338. doi: 10.1093/jmedent/32.3.338. [DOI] [PubMed] [Google Scholar]
- 21.Wikel SK. Influence of Dermacentor andersoni infestation on lymphocyte responsiveness to mitogens. Ann Trop Med Parasitol. 1982;76:627. doi: 10.1080/00034983.1982.11687593. [DOI] [PubMed] [Google Scholar]
- 22.Ganapamo F, Rutti B, Brossard M. In vitro production of interleukin-4 and interferon-γ by lymph node cells from BALB/c mice infested with nymphal Ixodes ricinus ticks. Immunology. 1995;85:120. [PMC free article] [PubMed] [Google Scholar]
- 23.Ganapamo F, Rutti B, Brossard M. Immunosuppression and cytokine production in mice infested with Ixodes ricinus ticks: a possible role of laminin and interleukin-10 on the in vitro responsiveness of lymphocytes to mitogens. Immunology. 1996;87:259. doi: 10.1046/j.1365-2567.1996.450512.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mbow ML, Rutti B, Brossard M. IFN-gamma, IL-2 and IL-4 mRNA expression in the skin and draining lymph nodes of BALB/c mice repeatedly infested with nymphal Ixodes ricinus ticks. Cell Immunol. 1994;156:254. doi: 10.1006/cimm.1994.1170. [DOI] [PubMed] [Google Scholar]
- 25.De Smedt T, Van Mechelen M, De Becker G, Urbain J, Leo O, Moser M. Effect of interleukin 10 on dendritic cell maturation and function. Eur J Immunol. 1997;27:1229. doi: 10.1002/eji.1830270526. [DOI] [PubMed] [Google Scholar]
- 26.D’Andrea A, Aste-Amezaga M, Valiante NM, Ma X, Kubin M, Trinchieri G. Interleukin 10 (IL-10) inhibits human lymphocyte interferon gamma-production by suppressing natural killer cell stimulatory factor/IL-12 synthesis in accessory cells. J Exp Med. 1993;178:1041. doi: 10.1084/jem.178.3.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Koch F, Stanzl U, Jennewein P, et al. High level IL-12 production by murine dendritic cells: upregulation via MHC class II and CD40 molecules and downregulation by IL-4 and IL-10. J Exp Med. 1996;184:741. doi: 10.1084/jem.184.2.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Trinchieri G. Interleukin-12: a proinflammatory cytokine with immunoregulatory functions that bridge innate resistance and antigen-specific adaptive immunity. Annu Rev Immunol. 1995;13:251. doi: 10.1146/annurev.iy.13.040195.001343. [DOI] [PubMed] [Google Scholar]
- 29.Chantry D, Turner M, Abney E, Feldmann M. Modulation of cytokine production by transforming growth factor-β. J Immunol. 1989;142:4295. [PubMed] [Google Scholar]
- 30.Silva JS, Twardzik DR, Reed SG. Regulation of Trypanosoma cruzi infections in vitro and in vivo by TGF-β. J Exp Med. 1991;174:539. doi: 10.1084/jem.174.3.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Barral A, Barral-Neto M, Yong EC, Brownell CE, Twardzik DR, Reed SG. Transforming growth factor-β as a virulence mechanism for Leishmania braziliensis. Proc Natl Acad Sci USA. 1993;90:3442. doi: 10.1073/pnas.90.8.3442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Abbas A, Murphy KM, Sher A. Functional diversity of helper T lymphocytes. Nature. 1996;383:787. doi: 10.1038/383787a0. [DOI] [PubMed] [Google Scholar]
- 33.Szabó MP.J, Morelli J, Jr, Bechara GH. Cutaneous hypersensitivity induced in dogs and guinea-pigs by extracts of the tick Rhipicephalus sanguineus (Acari: Ixodidae) Exp App Acarol. 1995;19:723. doi: 10.1007/BF00052083. [DOI] [PubMed] [Google Scholar]
- 34.Cher DJ, Mosmann TR. Two types of murine helper T cell clone. II. Delayed-type hypersensitivity is mediated by Th1 clones. J Immunol. 1987;138:3688. [PubMed] [Google Scholar]
- 35.Szabó MP.J, Bechara GH. Immunisation of dogs and guinea pigs against Rhipicephalus sanguineus ticks using gut extract. Vet Parasitol. 1997;68:283. doi: 10.1016/s0304-4017(96)01079-5. [DOI] [PubMed] [Google Scholar]