Abstract
Transport of major histocompatibility complex (MHC) class II molecules to the endocytic route is directed by the associated invariant chain (Ii). In the endocytic pathway, Ii is proteolytically cleaved and, upon removal of residual Ii fragments, class II αβ dimers are charged with antigenic peptide and recognized by CD4+ T cells. Although distinct peptide-loading compartments such as MIIC (MHC class II loading compartment) and CIIV (MHC class II vesicles) have been characterized in different cells, there is growing evidence of a multitude of subcellular compartments in which antigenic peptide loading takes place. We employed a physiological cellular system in which surface Ii (CD74) and surface human leucocyte antigen (HLA)-DR were induced either alone or in combination. This was achieved by transient exposure of HT-29 cells to recombinant interferon-γ (rIFN-γ). Using distinct cellular variants, we showed that: (i) the majority of Ii molecules physically associate on the cell membrane with class II dimers to form DR αβ:Ii complexes; (ii) the presence of surface Ii is a prerequisite for the rapid uptake of HLA-DR-specific monoclonal antibodies into early endosomes because only the surface DR+/Ii+ phenotype, and not the DR+/Ii− variant, efficiently internalizes; and (iii) the HLA-DR:Ii complexes are targeted to early endosomes, as indicated by co-localization with the GTPase, Rab5, and endocytosed bovine serum albumin. Internalization of HLA-DR:Ii complexes, accommodation of peptides by DR αβ heterodimers in early endosomes and recycling to the cell surface may be a mechanism used to increase the peptide repertoire that antigen-presenting cells display to MHC class II-restricted T cells.
INTRODUCTION
There is ample evidence that multiple aspects in antigen presentation via MHC class II molecules are critically influenced by the invariant chain (Ii).1,2 Three main functions have been attributed to this molecule. First, by the formation of a trimeric structure following protein synthesis, Ii acts as a chaperone, stabilizing nascent human leucocyte antigen (HLA)-DR αβ-heterodimers. Second, by means of various sorting and internalization signals in its N-terminal cytoplasmic tail, Ii targets HLA-DR molecules to subcellular compartments. Third, by a stretch called CLIP (class II-associated invariant chain peptide), Ii prevents loading of antigenic peptides into the groove of HLA-DR molecules outside endosomes/lysosomes.
Recent data have indicated that at least two distinct pathways exist by which Ii:DR complexes reach these endocytic loading compartments. Along its classical pathway, Ii post-translationally associates with major histocompatibility complex (MHC) class II α and β chains in the lumen of the endoplasmic reticulum3 and directs the intracellular transport of class II molecules to late endocytic/lysosomal compartments4–8 where Ii is proteolytically degraded.9,10 Ii-freed class II αβ-dimers are then charged with antigenic peptides and appear on the cell surface to act as ligands for CD4-positive T lymphocytes. Co-localization of class II molecules and Ii on the cell surface11–14 and in early endosomes14–18 was observed and recent findings indicate that class II loading can take place throughout the endocytic pathway.19,20 This leads to the concept of an alternative pathway along which DR molecules are internalized from the cell surface and accumulate in early endosomes. Such an alternative route for class II molecules may be of immunological significance: owing to the remarkable difference in enzymatic equipment and pH displayed by early endosomes versus late endosomes/lysosomes, protein cleavage results in different sets of peptides,1,21–23 potentially broadening the peptide repertoire presented by class II molecules. Although two internalization motifs have been identified in human Ii,7,24–26 it is not yet clear whether Ii is required for internalization of HLA-DR molecules. This possibility has been recently questioned because class II internalization has been shown to function via an internalization sequence located to the class II β chain.22,27
In order to address the question of whether or not HLA-DR internalization requires Ii, we designed a physiological cellular system in which surface Ii (CD74) and surface HLA-DR could be studied alone and in combination. Based on the kinetics of sequential appearance and disappearance of these molecules induced by recombinant interferon-γ (rIFN-γ) exposure and withdrawal, respectively, we generated surface Ii+/DR−, Ii+/DR+ and Ii−/DR+ variants on Ii−/DR− HT-29 cells. Using these variants we were able to show that surface Ii is a prerequisite for the internalization of HLA-DR molecules.
MATERIALS AND METHODS
Cell lines and reagents
The HT-29 colon carcinoma cell line was obtained from the American Type Culture Collection (ATCC, Rockville, MD). The monoclonal antibodies (mAb) used and antigens detected in this study are listed in Table 1. CD74(BU43)11,12 and CD74(BU45)11,12 were kindly provided by I. C. M. MacLennan (Department of Immunology, University of Birmingham, Birmingham, UK); CD74(LN2)11,12 was obtained from Biotest (Dreieich, Germany); CD74(M-B741) was submitted by P. Rieber (Institute of Immunology, University of Munich, Munich, Germany) to the 5th International Workshop and Conference on Leucocyte Differentiation Antigens;28 VIC-Y111,28,29 was kindly supplied by W. Knapp (Institute for Immunology, University of Vienna, Vienna, Austria); L227 and L243 were obtained from the ATCC;30 DA6·23131 was kindly provided by K. Guy (Department of Immunology, University of Strathclyde, Glasgow, UK); TAL.1B532 and W6/3233 were supplied by DAKO (Hamburg, Germany) and anti-Rab5 was purchased from Transduction Laboratories (Lexington, KY). Biotin-labelled bovine serum albumin (BSA) was obtained from Sigma (Deisenhofen, Germany) and 7-amino-4-methylconmarin-3-acetic acid (AMCA)-streptavidin complex came from Vector Laboratories (Burlingame, CA). Monospecific, tetramethylrhodamine isothiocyanate (TRIC) or fluorescein isothiocyanate (FITC)-labelled goat-derived antisera to mouse immunoglobulin isotypes were obtained from Southern Biotechnology Association Inc. (Birmingham, AL).
Table 1.
Antibodies used and antigens detected in this study
These clones were used unlabelled or *biotinylated or †fluorescein isothiocyanate (FITC) labelled.
Cell culture
HT-29 cells were maintained in Iscove’s medium (Biochrom, Berlin, Germany) supplemented with 10% heat-inactivated fetal calf serum (FCS; GIBCO, Grand Island, NY), 2 mm l-glutamine, 100 U/ml penicillin, 100 mg/ml streptomycin and 2·5 mg/ml amphotericin B. Cells were incubated in 25-cm3 tissue culture flasks at 37° in a humified 5% CO2 atmosphere. To induce Ii and class II expression, HT-29 cells were cultured in the presence of 100 U/ml rIFN-γ (Boehringer Mannheim, Mannheim, Germany) for the indicated period of time. To modulate the surface expression of Ii and HLA-DR on rIFN- γ-stimulated HT-29 cells, brefeldin A (BFA; Boehringer Mannheim) was used at 1 μg/ml. Treated and untreated cells were harvested with 0·05% trypsin/0·02% EDTA (at 37° for 4 min) and were washed once in Iscove’s medium with supplements. This treatment had no detectable effect on surface Ii and HLA-DR staining.
Antibody internalization assay
HT-29 cells were harvested and washed once in prewarmed Iscove’s medium. Cells (1×105) were incubated in the presence of ≈25 μg/ml murine monoclonal antibody at 37° for 20 min to allow internalization and uptake into endosomal vesicles. Subsequently, cells were washed twice with ice-cold phosphate-buffered saline (PBS) to remove unbound mAb and cytospins were made using a cytocentrifuge (Shandon, Pittsburgh, PA). Cells were fixed in acetone for 10 min at room temperature, air-dried and subsequently stained by immunocytochemistry, as described below. For evaluation, cells were examined microscopically and judged positive according to the presence of a punctate pattern of reddish-stained intracellular vesicles. At least 1000 cells were analysed per sample and the result is represented as the percentage of positive cells.
Immunocytochemistry
The primary mouse anti-Ii and anti-HLA-DR mAbs internalized by HT-29 cells at 37° were detected by immunocytochemical staining after acetone fixation of cytospin preparations. For detection of cytoplasmic Ii and HLA-DR, HT-29 cells were first acetone fixed and then incubated with the primary mAb. A polyclonal biotinylated sheep antibody to mouse immunoglobulin, reactive with all mouse immunoglobulin isotypes, and a streptavidin-biotinylated horseradish peroxidase complex (Amersham, High Wycombe, UK), served as a detection system for the primary antibodies. Cells were incubated with these second- and third-step reagents, at a dilution of 1:100 for 30 min, respectively. All dilutions and washings were carried out in PBS. A substrate solution containing 0·4 mg/ml 3-amino-9-ethylcarbazole (AEC; Sigma), prepared in 0·1 m acetate buffer (pH 5·0) with 5%N,N-dimethylformamide (Sigma) and 0·01% H2O2 (Merck, Darmstadt, Germany), was applied for 10 min at room temperature. The peroxidase reaction caused an intense red precipitate. Stained cytospin preparations were rinsed in tap water, counterstained with Harris’ haematoxylin and mounted with glycerin gelatin.
Flow cytometry
Immunofluorescent staining was performed in polystyrene round-bottom tubes (Falcon, San Jose, CA). Dilutions and washings were performed throughout in D-PBS buffer (GIBCO) containing 2% heat-inactivated FCS, 0·1% sodium azide and 10 mm HEPES (Biochrom), referred to as FACS medium. Approximately 106 cells per sample, suspended in 50 μl of medium, were incubated on ice with an equal volume of the appropriate dilution of each mAb. After 45 min, cells were washed twice in 500 μl of cold FACS medium, and 2 μg F(ab′)2 goat antimouse immunoglobulin G (IgG) and immunoglobulin M (IgM) FITC conjugate (Jackson Immunoresearch Laboratories, West Grove, PA) were then added for 45 min on ice. Cells were rewashed twice and resuspended in 300 μl of FACS medium containing 1 μg/ml propidium iodide (Sigma). Alternatively, directly biotinylated mAbs were also used and detected by strepavidin-FITC (GIBCO). From each sample the green fluorescence of 104 viable cells was analysed. Dead cells were removed from analysis by selectively gating on propidium iodide fluorescence, forward-and side-scatter parameters. Flow cytometry was performed on a FACScan® cytometer using the cellquest® software (Becton-Dickinson, Immunocytometry Systems, Mountain View, CA). Results of FACS analysis are depicted as percentage positive cells above the cut-off fluorescence value set with a control mAb. Analysis of cells stained with a control mAb never exceeded 5% positive events.
Cell-surface iodination, immunoprecipitation and two-dimensional gel electrophoresis
HT-29 cells stimulated with 100 U/ml rIFN-γ for 72 hr were surface radioiodinated by the lactoperoxidase method, essentially as described previously.34 Briefly, 107 cells (viability >95%) were labelled with 18·5 MBq 125I (Amersham-Buchler, Braunschweig, Germany), in the presence of 10 μg lactoperoxidase (Sigma), by multiple increasing pulses with H2O2. Cells were solubilized in lysis buffer containing 1% Nonidet P-40 and proteinase inhibitors (100 μg/ml phenylmethylsulphonylfluoride and 1 μg/ml aprotinin; Sigma). Insoluble material was removed by centrifugation at 12 000 g for 30 min, the cell lysate-containing supernatant was precleared by four sequential cycles of non-specific precipitation using affinity-purified rabbit antimouse IgG+IgM antibody (own preparation; 15 μg/500 μl lysate) together with 100 μl/500 μl lysate protein A–Sepharose CL-4B (50% saturation; Pharmacia Fine Chemicals, Uppsala, Sweden). For immunoprecipitation, the lysate was mixed with 10 μg purified mAb antibody, 10 μg rabbit antimouse IgG+IgM and 20 μl of protein A–Sepharose CL-4B. After overnight incubation at 4° with gentle rotation, the adsorbent was washed extensively. A combination of isoelectric focusing (IEF) and SDS polyacrylamide gel electrophoresis (PAGE) was used to resolve precipitated immunocomplexes, essentially as described previously.35 For IEF, samples were solubilized in 9·8 m urea, 4% (wt/vol) NP-40, 2% (vol/vol), ampholines ph 7–12 (Pharmacia), pH 7–12, and 100 mm dithiothreitol. Tube gels used for the first dimension were run at 1200 V for 17 hr; the pH gradient after electrophoresis ranged from 4·57 to 8·03 and was linear between pH 4·6 and pH 7·2. For the second dimension, 15% SDS–PAGE (15% wt/vol acrylamide, 0·075%N,N′-methylene-bisacrylamide) was employed. Radioactive bands in dried gels were visualized by exposure to Kodak Biomax MR films (Sigma) using intensifying screens (Cronex Lightning Plus DuPont, Bad Hamburg, Germany).
Immunofluorescence microscopy
HT-29 cells grown on sterile glass slides were stimulated for 36 hr with 500 U/ml rIFNγ. After washing the cells three times with ice-cold PBS, the antibodies L227 (IgG1), L243 (IgG2a), BU43 (IgM) and anti-Rab5 (IgG1) [cf. Table 1] were added in various combinations. Absorption of antibodies was performed on ice for 30 min, after which time the cells were washed twice in ice-cold PBS and either warmed up at 37° to allow internalization for 10 min prior to acteone fixation or fixed in acetone immediately. These experiments were carried out in parallel, additionally exposing cells to biotin-labelled BSA. Absorbed and internalized antibodies and BSA were detected via FITC- or TRITC-conjugated isotype-specific goat antimouse antibodies and AMCA-conjugated streptavidin, respectively. Subsequently, slides were coverslipped using vectashield mounting medium (Vector Laboratories). Fluorescence microscopy was performed using a Zeiss-Axioskop (Zeiss, Wetzlar, Germany) with a UV/DAPI/FITC/TRITC (Cy3)-extinction filter set (AF Analysetechnik, Tübingen, Germany). In some experiments DAPI was used for staining of the nuclei. The image was transferred via a chilled CCD camera (Hamamatsu Photonics, Japan) to a personal computer and further processed using the isis 1·90 analysis software (Metasystems, Altlussheim, Germany).
RESULTS
Ii and HLA-DR surface expression is induced on HT-29 cells upon treatment with rIFN-γ
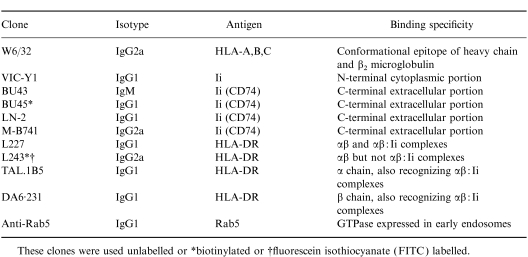
To study the transport of HLA-DR and Ii molecules to the cell surface, we used the class II- and Ii-negative colon carcinoma cell line, HT-29, which has been previously shown to express class II molecules after treatment with rIFN-γ.36 We confirmed that HT-29 cells not treated with rIFN-γ expressed neither class II antigens nor Ii, as determined by flow cytometry (see Fig. 1), Western blot analysis (data not shown) and immunocytochemistry (data not shown). We examined whether HT-29 cells can be also stimulated to express Ii on the cell surface after treatment with rIFN-γ. HT-29 cells were cultured in the presence of different concentrations of rIFN-γ for 72 hr and subsequently analysed by flow cytometry for surface expression of MHC class I molecules, HLA-DR and Ii (Fig. 1). rIFN-γ induced surface expression of both HLA-DR and Ii and increased, dose-dependently, the number of MHC class I molecules on HT-29 cells. After culture with 100 U/ml rIFN-γ for 72 hr, all cells expressed high levels of HLA-DR and Ii on the cell surface, whereas at lower concentrations of rIFN-γ only a subpopulation of cells stained positive. Surface expression of Ii was similarly demonstrated using three additional Ii-specific mAbs: BU43, LN2 and M-B741 (data not shown), all recognizing epitopes on the C-terminal (i.e. luminal or extracellular) domain of Ii.11,28 Thus, the rIFN-γ-driven induction of HLA-DR surface expression on HT-29 cells is accompanied by Ii surface expression.
Figure 1.
Surface expression of major histocompatibility complex (MHC) class I molecules, Ii and human leucocyte antigen (HLA)-DR on interferon-γ (IFN-γ)-treated HT-29 cells. HT-29 cells cultured in the presence of the indicated concentrations of rIFN-γ (0–500 U/ml) for 72 hr were analysed by flow cytometry using the following mAbs: W6/32 (anti-HLA-ABC framework), BU45 (reacting with a C-terminal/extracellular determinant of all Ii isoforms) and L243 (reacting with mature HLA-DR molecules), represented by filled histograms, respectively. As a negative control, HT-29 cells were stained with the mAb VIC-Y1 (reacting with a cytoplasmic determinant of Ii, outlined histograms).
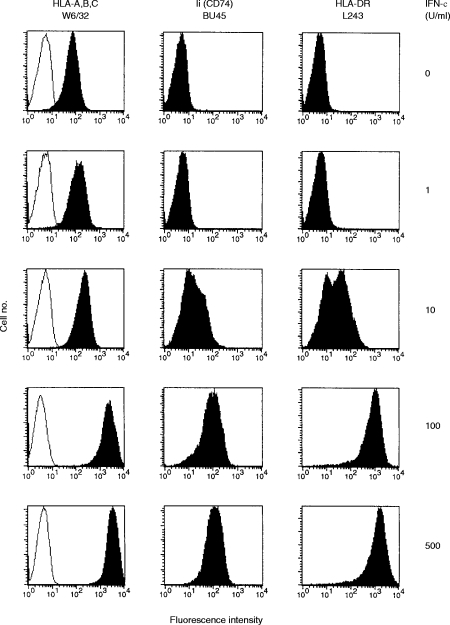
Kinetics of rIFN-γ-induced de novo MHC class II and Ii expression on HT-29 cells
Compared to class I molecules, the transport of newly synthesized class II molecules from the trans-Golgi reticulum to the cell surface showed a delay of 2–3 hr.37 Our previous results suggested a rapid pathway for Ii to the cell surface.12 However, these data did not allow for the distinction between free Ii and HLA-DR-associated Ii. We reasoned that, if newly synthesized class II:Ii complexes and excess Ii showed different behaviour in intracellular sorting, they might not appear simultaneously on the cell surface. To gain insight into the kinetics of de novo synthesis and subsequent surface appearance of class II molecules and Ii, HT-29 cells were incubated with 100 U/ml rIFN-γ for different periods of time. rIFN-γ was added sequentially to the cultures so that all probes could be analysed simultaneously. Immunocytochemistry and Western blotting revealed that Ii and HLA-DR appeared at the same time and rate within the cytoplasm (data not shown). However, there was a substantial delay in surface appearance. Flow cytometry revealed that Ii surface expression preceded HLA-DR surface expression by at least 2 hr (Fig. 2). Ii was first detectable 8 hr after the addition of rIFN-γ, while HLA-DR was first detected after 10 hr of rIFN-γ treatment. Following 8–24 hr of exposure to rIFN-γ, Ii-positive cells outnumbered HLA-DR-positive cells. Thus, Ii is transported to the plasma membrane independently of HLA-DR molecules.
Figure 2.
Kinetics of the interferon-γ (IFN-γ)-driven induction of Ii and human leucucyte antigen (HLA)-DR surface expression on HT-29 cells. HT-29 cells exposed for different lengths of time to 100 U/ml rIFN-γ were analysed by flow cytometry using saturating amounts of mAbs: BU45 (filled circles) and L243 (open circles). The results are depicted as percentage positive cells above the cut-off value of background fluorescence determined with the control mAb VIC-Y1. Results are the average of three independent experiments, the error bars representing the standard deviation.
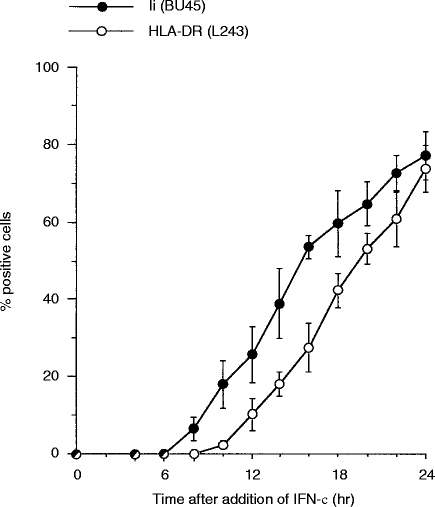
Sequential induction and decline of surface Ii and HLA-DR molecules generates four distinct phenotypes of HT-29 cells
Upon removal of rIFN-γ, synthesis of class II molecules and Ii decreased. To examine the kinetics of the decrease of induced HLA-DR and Ii surface levels on HT-29 cells, we followed the time course of HLA-DR and Ii disappearance after removal of rIFN-γ. HT-29 cells, maximally induced for HLA-DR and Ii by treatment with 100 U/ml rIFN-γ for 5 days, were thoroughly washed and recultured in the absence of rIFN-γ. Remaining surface expression of HLA-DR and Ii was analysed by flow cytometry after different lengths of time in culture. Twenty-four hours after removal of rIFN-γ, Ii surface expression had decreased by ≈80%, while the level of surface expression of HLA-DR proved stable (data not shown). In contrast to Ii, HLA-DR had a considerably longer resident time and was detectable on the cell surface for up to 6 days. As surface expression of Ii rapidly decreased after removal of rIFN-γ while that of HLA-DR remained for longer, HT-29 cells displayed an Ii−/HLA-DR+ phenotype between days 4 and 7, following removal of rIFN-γ. Exploiting these kinetic data, we chose different time-points in the addition and removal of rIFN-γ, to determine the sequence of the appearance and disappearance of Ii and HLA-DR molecules, in order to create three patterns of expression, i.e. Ii+/DR−, Ii+/DR+ and Ii−/DR+. Figure 3 shows the four distinct variants of HT-29 cells: Ii−/DR−, Ii+/DR−, Ii+/DR+ and Ii−/DR+.
Figure 3.
Four distinct phenotypes of HT-29 cells are generated by sequential induction and decline of DR and Ii. Surface expression of human leucocyte antigen (HLA)-DR and Ii molecules was measured by flow cytometry after the indicated time of culture in the presence of, or after removal of, recombinant interferon-γ (rIFN-γ) using mAbs W6/32, BU45, and L243(filled histograms). mAb VIC-Y1 (recognizing a cytoplasmic epitope of Ii) was used as a negative control (outlined histograms).
On Ii+/DR+ cells, surface Ii is physically associated with HLA-DR
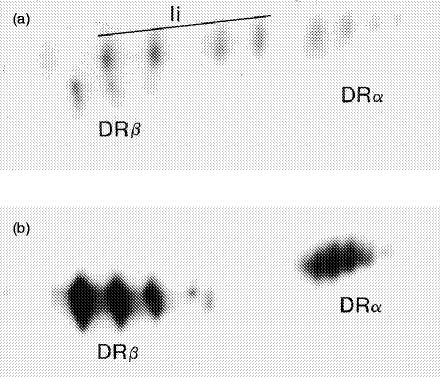
In order to address the question of whether or not Ii and HLA-DR molecules are physically associated on the Ii+/DR+ variant, 125I surface-labelled rIFN-γ-pretreated HT-29 cells were immunoprecipitated with BU45 and these precipitates were subjected to two-dimensional gel electrophoresis. Figure 4a displays a pattern of Ii and co-precipitated HLA-DRα and HLA-DRβ polypeptides. Four spots of Ii were present, which corresponded to increasingly sialylated forms of Ii. The pattern of this figure demonstrates that Ii co-precipitates a considerable amount of DR molecules, indicating that a large proportion of surface Ii is physically associated with DR. For comparison, the same lysate was precipitated with TAL.1B5, resulting in two series of spots representing DRα and DRβ chain polypeptides (Fig. 4b). Although DRα and DRβ spots were very prominent, no Ii spots were detectable, reflecting the fact that the surface expression of DR molecules is an order of magnitude higher than that of Ii (cf. Fig. 1). In conclusion, we provided a natural cellular system in which Ii and HLA-DR molecules were surface expressed, either separately or in combination. Once co-expressed on the cell surface, Ii was physically associated with HLA-DR-heterodimers, which, however, greatly outnumbered surface-exposed Ii molecules.
Figure 4.
Two-dimensional separation of human leucocyte antigen (HLA)-DR and Ii immunoprecipitates. HT-29 cells induced with 100 U/ml recombinant interferon-γ (rIFN-γ) for 72 hr were surface labelled with 125I. Invariant chains were immunoprecipitated with BU45 (a) and HLA-DR molecules with TAL.1B5 (b). Samples were separated in the first dimension by isoelectric focusing (acidic end to the right side) and in the second dimension by sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS–PAGE). Although DRα and DRβ spots are very prominent, no Ii spots were detectable, reflecting the fact that the surface expression of HLA-DR molecules is much higher than that of Ii.
Surface Ii is required for internalization of HLA-DR
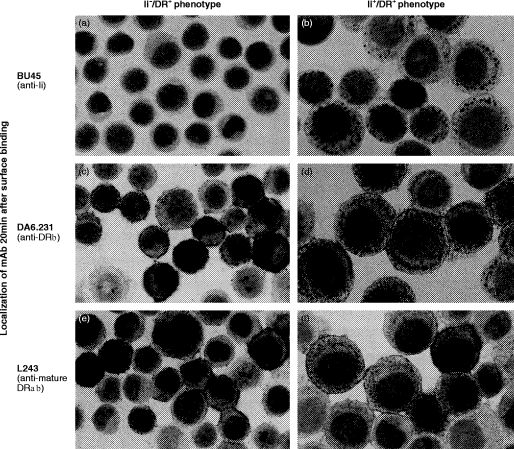
Antibody internalization experiments were performed with HT-29, expressing or lacking surface Ii, in the presence of HLA-DR. Upon exposure to antibody for 20 min at 37°, Ii+/DR+ cells internalized BU45, as shown by a punctate pattern of reddish-stained cytoplasmic vesicles (Fig. 5b). This result was not caused by non-specific uptake because internalization of BU45 by Ii+/DR+ cells was largely inhibited under hyperosmotic conditions (see below, Fig. 6). In addition to BU45, Ii+/HLA-DR+ cells also showed uptake of the mAbs DA6·231 (Fig. 5d) and L227 (data not shown), both reacting with HLA-DR αβ-heterodimers and αβ:Ii complexes.30,38 Ii−/DR+ cells stained negative with BU45 and did not show any internalization of this antibody (Fig. 5a). DA6·231 (and L227, data not shown) bound to the surface of Ii−/HLA-DR+ cells but were not internalized (Fig. 5c). L243, which fails to recognize HLA-DR αβ:Ii complexes,39 gave a clear-cut surface labelling on both Ii+/DR+ (Fig. 5f) and Ii−/DR+ cells (Fig. 5e) but was not internalized by either phenotype. Ii+/DR− cells internalized BU45, whereas uptake of the HLA-DRβ chain-specific antibody did not occur (data not shown) indicating that internalization of Ii was independent of the presence of HLA-DR molecules.
Figure 5.
Localization of mAb after exposure to living cells. HT-29 cells, either stimulated for 24 hr with 100 U/ml recombinant interferon-γ (rIFN-γ) (Ii+DR+ phenotype) or, alternatively, pretreated for 120 hr with 100 U/ml rIFN-γ followed by 4 days of culture without rIFN-γ (Ii−DR+ phenotype), were detached and incubated for 20 min at 37° in the presence of saturating amounts of the following mAbs: BU45, DA6·231 and L243. Subsequently, cells were placed on ice, non-bound mAb was removed by washing in cold medium, and internalized antibody was detected by immunocytochemical staining of acetone-fixed cytospin preparations. The difference in average cell size between Ii+/DR+ and Ii−/DR+ cells is a result of the effect of rIFN-γ.
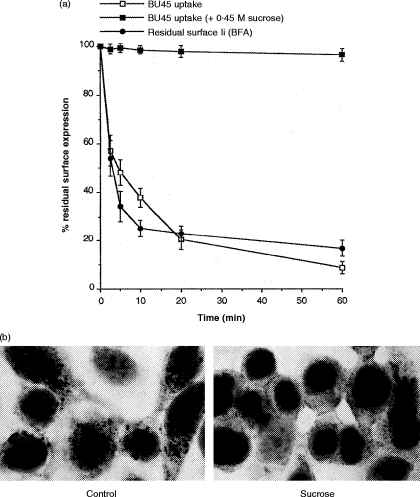
Figure 6.
(a) Rapid turnover of Ii on the surface of recombinant interferon-γ (rIFN-γ)-treated HT-29 cells. For internalization of surface-bound BU45, HT-29 cells pretreated for 72 hr with rIFN-γ were labelled with saturating amounts of BU45 on ice for 1 hr. After removing non-bound mAb, 50 μl of suspension containing 106 cells was added to 450 μl of medium, prewarmed to 37°, to allow endocytosis for different periods of time. Subsequently, cells were recooled by transfer to 4·5 ml of ice-cold medium and analysed for remaining surface-bound mAb by flow cytometry. Internalization of BU45 is depicted as the percentage of surface-bound mAb remaining after endocytosis at 37° relative to the total amount of mAb bound to cells that were kept on ice throughout. As a control, internalization of prebound BU45 was analysed under hyperosmotic conditions (0·45 m sucrose) known to inhibit coated pit-mediated endocytosis. The depletion of Ii from the plasma membrane at at steady state was measured after blocking transport of newly synthesized Ii by treatment with 1 μg/ml brefeldin A (BFA). Cells were preincubated with BFA for 15 min on ice followed by shifting the cells to 37° in the presence of BFA for different periods of time. The results are expressed as the percentage of Ii surface levels remaining after BFA treatment at 37° relative to the Ii level on cells not shifted to 37°. (b) HT-29 cells grown on slides were exposed to 100 U/ml IFN-γ for 24 hr. Control cells (Control) were incubated with saturating amounts of BU45 (50 μg/ml) in normal medium for 20 min at 37°. Alternatively, cells were preincubated for 10 min at 37° in hypertonic medium containing 0·45 m sucrose followed by further incubation for 20 min at 37° in the presence of 50 μg/ml BU45 diluted in the same sucrose-containing medium (0·45 m sucrose). Non-bound mAb was removed by rinsing in cold medium; subsequently, cells were acetone fixed and surface bound, and cytoplasmically located antibody was detected by immunocytochemical staining.
Several control experiments were performed to exclude non-specific uptake of antibody by fluid-phase endocytosis. First, cells exposed to antibody at 4° or at 37° in hypertonic medium (containing 0·45 m sucrose) failed to internalize antibody (see below). Hyperosmotic conditions are known to inhibit receptor-mediated endocytosis.40 Second, in contrast to BU45 and DA6·231, the control mAb, VIC-Y1, was not internalized by rIFN-γ-stimulated HT-29 cells (data not shown). Third, HT-29 cells not treated with rIFN-γ, or cells in the first 8 hr after addition of rIFN-γ, were never observed to internalize antibody. Fourth, HT-29 cells, exposed to rIFN-γ in the presence of 1 μg/ml BFA, blocking transport of newly synthesized molecules to the cell surface, also failed to internalize antibody (data not shown). Finally, internalization by antibody cross-linking of surface Ii was excluded by applying monovalent Fab fragments of BU45, which yielded identical results (data not shown). Thus, HLA-DR molecules are only rapidly internalized from the plasma membrane in the presence of Ii. Consequently, only the subset of Ii-associated HLA-DR molecules undergoes efficient endocytosis.
Rapid turnover of surface-expressed Ii on rIFN-γ-treated HT-29 cells
To examine the turnover of Ii on the cell surface, we labelled 72-h rIFN-γ-prestimulated HT-29 cells with BU45 on ice and then warmed them up to 37° to allow endocytosis of surface-bound antibody (Fig. 6a). Compared with cells kept on ice to inhibit endocytosis, the amount of surface-bound BU45 decreased rapidly with a half-life of less than 5 min. BU45 Fab fragments yielded essentially the same result (data not shown). This depletion of surface-bound antibody was completely inhibited by exposing cells to hypertonic medium containing 0·45 m sucrose (Fig. 6b), which is a condition that prevents internalization of surface molecules.
If some fraction of Ii is transported to the plasma membrane on a rapid route and subsequently directed to the endocytic pathway by rapid internalization, Ii surface expression is expected to be lost shortly after the transport of newly synthesized molecules is blocked. To examine this, we measured Ii surface expression after blocking the egress of newly synthesized Ii from the endoplasmic reticulum with BFA. rIFN-γ-treated HT-29 cells were cultured in the presence of 1 μg/ml BFA at 37° for different lengths of time and the remaining surface expression of Ii was determined by flow cytometric analysis (Fig. 6a). In the presence of BFA, Ii surface expression declined with a half-life of less than 5 min, very similar to the kinetics of internalization of Ii-specific antibody. These results suggest that surface Ii is mainly derived from the default secretory pathway and not from a recycling endosomal pool. Thus, the short half-life of Ii on the cell surface of rIFN-γ-stimulated HT-29 cells results from rapid and continous internalization of newly synthesized Ii molecules.
Internalized Ii:DR complexes are targeted to early endosomal compartments
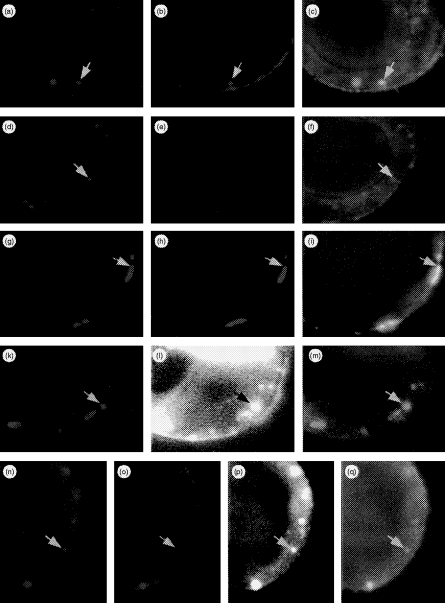
In order to address the question of whether or not internalization of HLA-DR is dependent on association with Ii, double- and triple-labelling experiments were performed using Ii+/DR+ HT-29 cells. Preincubation with BU43 antibody on ice and subsequent warming up to 37° resulted in vesicular cytoplasmic staining after 10 min (cf. Fig. 5, Fig. 7a, 7d, 7c, 7f). The same pattern of reactivity was obtained when antibody L227 was applied (Fig. 7b, 7c). Again, incubation with L243 yielded surface staining but no internalization (Fig. 7e, 7f). Internalization of BU43 and L227 was completely abolished under hyperosmolaric conditions (Fig. 7g, 7h, 7i). Next, we co-incubated cells with BU43 and L227. After 10 min at 37°, both antibodies were internalized and co-localized mainly in cytoplasmic vesicles (Fig. 7a, 7b, 7c). To characterize the vesicles to which both antibodies were targeted, we further introduced labelled BSA and anti-Rab5 antibody, specifically reacting with GTPase Rab5 that is specific for early endosomes.41 First, we demonstrated that, after endocytosis of BSA for 10 min at 37° (Fig. 7l), Rab5 antibody stained the same compartment in acetone-fixed, and thus permeabilized, cells (Fig. 7k, 7m). Therefore, we used 10-min internalization of BSA as a marker for early endosomes. The double labellings BU43/BSA and L227/BSA, respectively, revealed that both Ii and DR molecules were carried to the compartment containing BSA (data not shown). Triple stainings, BU43/L227/BSA, yielded evidence for co-localization of these three molecules (Fig. 7n, 7o, 7p, 7q). In conclusion, internalization of HLA-DR molecules requires their physical association with Ii which, in turn, targets the complex to early endosomes.
Figure 7.
Multicolour immunofluorescence microscopic analysis of antibody internalization. HT-29 cells grown on glass slides were stimulated for 36 hr with 500 U/ml recombinant interferon-γ (rIFN-γ). The antibodies were added simultaneously in various combinations. Absorption of antibodies was performed on ice for 30 min, after which the cells were washed and warmed at 37° to allow internalization for 10 min prior to acteone fixation. Bound and processed antibodies were then detected via isotype-specific goat antimouse antibodies carrying different fluorochromes. By combining filters, each spectral component of the image could be visualized individually or superimposed (rows 1–3). In an extension of these experiments, cells were additionally exposed to biotin-labelled bovine serum albumin (BSA; rows 4 and 5). Internalized BSA was detected via AMCA-conjugated streptavidin. Arrows mark identical areas of interest in different sets of filter combinations. First row: cells co-incubated with BU43 and L227 at 4° and then warmed to 37° show internalization of BU43 (a, red), L227 (b, green) in a co-localized manner (c: BU43/L227, yellow). Second row: cells co-incubated with BU43 and L243, and then treated as described above, show selective uptake of BU43 (d), while bound L243 antibodies remain at the cell surface (e) and therefore do not co-localize (f: BU43/L243). Third row: experimental setting as described for the first row except under hyperosmotic conditions. Antibody internalization of BU43 (g), L227 (h) is completely blocked resulting in their co-localization at the cell surface (i: BU43/L227). Fourth row: cells exposed to BSA at 37° for 10 min were acetone fixed to allow anti-Rab-5 antibody to penetrate the cells; Rab5 (k) and internalized BSA (l) are co-localized in early endosomes (m). Fifth row: in the presence of BSA, cells were exposed simultaneously to BU43 and L227. Co-internalized antibodies BU43 (n) and L227 (o) are targeted to the subcellular sites where BSA accumulates (p) resulting in co-localizion of BU43/L227/BSA (q). For enhancement of contrast, the AMCA blue in Fig. 1 and (p) has been changed to the pseudocolor white.
DISCUSSION
In a previous report, we have shown a rapid pathway of Ii to, and the association of Ii with, HLA-DR molecules on the cell surface.12 We now present evidence that surface Ii (CD74) plays an important role in the internalization and targeting of HLA-DR molecules to early endosomes.
We started by establishing a natural cellular system in which surface Ii and HLA-DR can be functionally probed, both in combination and separately. This was achieved in HT-29 cells (constitutively Ii−/DR−) by induction and depletion of Ii and DR by addition and removal of rIFN-γ. This transient treatment gave rise to Ii+/DR−, Ii+/DR+ and Ii−/DR+ variants. As is the case on B cells, Ii and HLA-DR molecules are physically associated on the surface of the Ii+/DR+ variant of HT-29. Using this system, we showed that, although HLA-DR molecules and Ii were simultaneously induced, Ii reached the plasma membrane ≈2 hr prior to HLA-DR. These results, obtained with antibodies BU45 and L243, were reproduced using different mAbs (M-B741 and DA6·231; data not shown). Therefore, differences in the affinity or specificity of mAbs are unlikely to account for the observed temporal difference in surface appearance between Ii and HLA-DR. The different kinetics of surface appearance of Ii compared with HLA-DR αβ and αβ:Ii may result from different transport properties or, alternatively, may be the consequence of some mis-sorting, which would be more pronounced for the more abundant Ii. In any event, trafficking of Ii to the endocytic pathway via the cell surface may occur independently of class II molecules. This view is further supported by our internalization experiments, which revealed the existence of a minority of Ii+/DR− endosomal vesicles.
An important finding was that the Ii−/DR+ variant did not internalize anti-DR antibodies under physiological conditions, whereas this was very efficiently achieved by the Ii+/DR+ variant. Ii+/DR+ cells, however, did not internalize anti-HLA-DR antibodies exclusively reactive with mature HLA-DR αβ dimers devoid of Ii. This implies that Ii is, in fact, a prerequisite for HLA-DR uptake. In critical consideration, several lines of evidence argue against non-specific uptake of mAbs by fluid-phase endocytosis or triggering of endocytosis by mAb binding. First, rapid internalization was blocked under hyperosmotic conditions known specifically to block formation of coated pits.40 Second, we found a striking connection between the ability of HLA-DR-specific mAbs to be efficiently internalized and their ability to react with HLA-DR αβ:Ii complexes. Third, rapid internalization of HLA-DR-specific mAbs required the presence of surface Ii. Taken together, these results demonstrate that binding of a mAb to surface HLA-DR per se does not trigger its internalization. Our finding, that only surface HLA-DR αβ:Ii complexes undergo efficient endocytosis whereas non-Ii-associated HLA-DR αβ dimers are more long-lived on the plasma membrane, is consistent with previous data showing that rapid internalization of surface HLA-DR αβ:Ii complexes is dependent on the cytoplasmic tail of Ii.13 However, this study failed to discriminate between newly synthesized HLA-DR αβ:Ii complexes and recycling complexes, which may form after binding of Ii to empty HLA-DR αβ dimers at the cell surface. Moreover, internalization of surface HLA-DR αβ:Ii complexes was analysed using an Ii-specific mAb, based on the observation that the majority (> 80%) of surface Ii is complexed with HLA-DR αβ dimers at steady state. Given that binding of peptide and Ii to class II αβ dimers is mutually exclusive,39,42–44 our finding that transport of HLA-DR and Ii to the plasma membrane is not linked suggests that Ii may associate with empty HLA-DR αβ dimers after reaching the cell surface.
Conflicting results have been reported concerning MHC class II endocytosis.13,16,45,46 In the light of our findings, some of these results may be explained by the different specificities of the anti-HLA-DR mAbs used. In two studies examining human B-lymphoblastoid cell lines, L243 was used to detect endocytosis of surface HLA-DR molecules.16,45 This antibody is known to react with mature HLA-DR αβ dimers but not with HLA-DR αβ:Ii complexes containing intact Ii.16,30,39,47 Therefore, lack of substantial endocytosis was demonstrated only for HLA-DR molecules not associated with Ii, which is in full accordance with our observations. Another important aspect was the kinetics of Ii uptake, which showed that more than 60% of surface-expressed material was internalized within only 10 min. During the same time period, BFA treatment depleted surface Ii. This means that every Ii molecule directed to the cytomembrane re-enters the cytoplasm with a remarkably short half-life on the cell surface.
We were further able to demonstrate that Ii:DR complexes are targeted to, and accumulate in, early endosomes. This observation gives further support to the concept that surface HLA-DR enters the early endosomal compartment via the cell membrane,13,14,18,19,22,27,48 and thus answers the question of whether Ii is required for this translocation. Recently, it was demonstrated by several groups that HLA-DR molecules can be loaded in an early endosomal compartment that is clearly distinct from the classical MHC class II loading compartment (MIIC). The biological significance may lie in the generation of an additional set of peptides that might be sensitive to the harsh conditions present in the MIIC. Furthermore, data suggest that peptides loaded in the early endosomal compartment display a weaker affinity to HLA-DR and may also contain self-peptides, e.g. derived from myelin basic protein.27,49
Whether ‘empty’ class II molecules naturally exist on the cell surface is still open to discussion. Some data argue in favour of the irreversibility of peptide association with class II molecules in living cells.50 Conversely, competition for antigen presentation through the exchange of peptides bound by class II molecules has also been observed.51,52 Soluble, recombinant Ii has been shown to compete effectively with peptide in binding to empty class II αβ dimers.43,44 If it is assumed that empty class II molecules do occur, which mechanisms might lead to this functional state? Under conditions where antigenic peptide is limiting, some proportion of class II αβ dimers reach the plasma membrane without peptide.44 Moreover, depending on the HLA-DR haplotype, cells carry significant amounts of DR:CLIP complexes on the cell surface and CLIP can be released from these complexes by an autocatalytic process.53,54 Alternatively, and perhaps more physiologically, class II molecules, e.g. loaded within the early endosomal compartment, might lose their peptide on the cell surface owing to low affinity.
Collectively, our data provide evidence that free Ii is exported via a direct and rapid route to the cell surface whereupon it physically associates with HLA-DR αβ chains, thereby mediating rapid internalization of the Ii:DR complex. Surface Ii may therefore act by depleting empty class II molecules from the plasma membrane, directing them for reutilization to the early endosomal compartment. This would explain why stable peptide–class II complexes arising from the MIIC compartment are long-lived on the cell surface, whereas class II molecules, which have weakly bound peptides are turned over more rapidly.50,55
Acknowledgments
We wish to thank Elvira Hallauer, Andrea Müller and Simone Westenfelder for their skilful technical assistance and Frank C. Kischkel for assisting with the two-dimensional gels. We also thank Dr Norbert Koch, Dr Anne Vogt and Dr Harald Kropshofer for useful comments and Caroline Higginson for help in preparing the manuscript. This study was supported by grants from the Tumorzentrum Heidelberg/Mannheim and the Deutsche Krebshilfe (Ko 810/4-3).
REFERENCES
- 1.Nordeng TW, Bakke O. The biological role of invariant chain (Ii) in MHC class II antigen presentation. Immunol Lett. 1994;43:47. doi: 10.1016/0165-2478(94)00159-6. [DOI] [PubMed] [Google Scholar]
- 2.Cresswell P. Assembly, transport and function of MHC class II molecules. Annu Rev Immunol. 1994;12:259. doi: 10.1146/annurev.iy.12.040194.001355. [DOI] [PubMed] [Google Scholar]
- 3.Kvist S, Wiman K, Claesson L, Peterson PA, Dobberstein B. Membrane insertion and oligomeric assembly of HLA-DR histocompatibility antigens. Cell. 1982;29:61. doi: 10.1016/0092-8674(82)90090-3. [DOI] [PubMed] [Google Scholar]
- 4.Bakke O, Dobberstein B. MHC class II-associated invariant chain contains a sorting signal for endosomal compartments. Cell. 1990;63:707. doi: 10.1016/0092-8674(90)90137-4. [DOI] [PubMed] [Google Scholar]
- 5.Lotteau V, Teyton L, Peleraux A, et al. Intracellular transport of class II MHC molecules directed by invariant chain. Nature. 1990;348:600. doi: 10.1038/348600a0. [DOI] [PubMed] [Google Scholar]
- 6.Lamb CA, Yewdell JW, Bennink JR, Cresswell P. Invariant chain targets HLA class II molecules to acidic endosomes containing internalized influenza virus. Proc Natl Acad Sci USA. 1991;88:5998. doi: 10.1073/pnas.88.14.5998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Motta A, Bremnes B, Morelli MA, Frank RW, Saviano G, Bakke O. Structure–activity relationship of the leucine-based sorting motifs in the cytosolic tail of the major histocompatibility complex-associated invariant chain. J Biol Chem. 1995;270:27165. doi: 10.1074/jbc.270.45.27165. [DOI] [PubMed] [Google Scholar]
- 8.Lanzavecchia A. Mechanisms of antigen uptake for presentation. Curr Opin Immunol. 1996;8:348. doi: 10.1016/s0952-7915(96)80124-5. [DOI] [PubMed] [Google Scholar]
- 9.Blum JS, Cresswell P. Role for intracellular proteases in the processing and transport of class II HLA antigens. Proc Natl Acad Sci USA. 1988;85:3975. doi: 10.1073/pnas.85.11.3975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maric MA, Taylor MD, Blum JS. Endosomal aspartic proteinases are required for invariant chain processing. Proc Natl Acad Sci USA. 1994;91:2171. doi: 10.1073/pnas.91.6.2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wraight CJ, Van Endert P, Möller P, et al. Human major histocompatibility complex class II invariant chain is expressed on the cell surface. J Biol Chem. 1990;265:5787. [PubMed] [Google Scholar]
- 12.Koch N, Moldenhauer G, Hofmann WJ, Möller P. Rapid intracellular pathway gives rise to cell surface expression of the MHC class II-associated invariant chain (CD74) J Immunol. 1991;147:2643. [PubMed] [Google Scholar]
- 13.Roche PA, Teletski CL, Stang E, Bakke O, Long EO. Cell surface HLA-DR-invariant chain complexes are targeted to endosomes by rapid internalization. Proc Natl Acad Sci USA. 1993;90:8581. doi: 10.1073/pnas.90.18.8581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Warmerdam PA.M, Long EO, Roche PA. Isoforms of the invariant chain regulate transport of MHC class II molecules to antigen processing compartments. J Cell Biol. 1996;133:281. doi: 10.1083/jcb.133.2.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cresswell P. Intracellular class II HLA antigens are accessible to transferrin-neuraminidase conjugates internalized by receptor-mediated endocytosis. Proc Natl Acad Sci USA. 1985;82:8188. doi: 10.1073/pnas.82.23.8188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guagliardi LE, Koppelman B, Blum JS, Marks MS, Cresswell P, Brodsky FM. Co-localization of molecules involved in antigen processing and presentation in an early endocytic compartment. Nature. 1990;343:133. doi: 10.1038/343133a0. [DOI] [PubMed] [Google Scholar]
- 17.Romagnoli P, Layet C, Yewdell J, Bakke O, Germain RN. Relationship between invariant chain expression and major histocompatibility complex class II transport into early and late endocytic compartments. J Exp Med. 1993;177:583. doi: 10.1084/jem.177.3.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ma C, Blum JS. Receptor-mediated endocytosis of antigens overcomes the requirement for HLA-DM in class II-restricted antigen presentation. J Immunol. 1997;158:1. [PubMed] [Google Scholar]
- 19.Castellino F, Germain RN. Extensive trafficking of MHC class II-invariant chain complexes in the endocytic pathway and appearance of peptide-loaded class II in multiple compartments. Immunity. 1995;2:73. doi: 10.1016/1074-7613(95)90080-2. [DOI] [PubMed] [Google Scholar]
- 20.Kleijmeer MJ, Morkowski S, Griffith JM, Rudensky AY, Geuze HJ. Major histocompatibility complex class II compartments in human and mouse B lymphoblasts represent conventional endocytic compartments. J Cell Biol. 1997;139:639. doi: 10.1083/jcb.139.3.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sercarcz EE, Lehmann PV, Ametani A, Benichou G, Miller A, Moudgil K. Dominance and crypticity of T cell antigenic determinants. Annu Rev Immunol. 1993;11:729. doi: 10.1146/annurev.iy.11.040193.003501. [DOI] [PubMed] [Google Scholar]
- 22.Zhong G, Romagnoli P, Germain RN. Related leucine-based cytoplasmic targeting signals in invariant chain and major histocompatibility complex class II molecules control endocytic presentation of distinct determinants in a single protein. J Exp Med. 1997;185:429. doi: 10.1084/jem.185.3.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Griffin JP, Chu R, Harding CV. Early endosomes and a late endocytic compartment generate different peptide–class II MHC complexes via distinct processing mechanisms. J Immunol. 1997;158:1523. [PubMed] [Google Scholar]
- 24.Bremnes B, Madsen T, Gedde-Dahl M, Bakke O. An LI and ML motif in the cytoplasmic tail of the MHC-associated invariant chain mediate rapid internalization. J Cell Sci. 1994;107:2021. doi: 10.1242/jcs.107.7.2021. [DOI] [PubMed] [Google Scholar]
- 25.Odorizzi G, Trowbridge IS. Structural requirements for major histocompatibility complex class II invariant chain trafficking in polarized Madin-Darby canine kidney cells. J Biol Chem. 1997;272:11757. doi: 10.1074/jbc.272.18.11757. [DOI] [PubMed] [Google Scholar]
- 26.Simonsen A, Stang E, Bremnes B, Roe M, Prydz K, Bakke O. Sorting of MHC class II molecules and the associated invariant chain (Ii) in polarized MDCK cells. J Cell Sci. 1997;110:597. doi: 10.1242/jcs.110.5.597. [DOI] [PubMed] [Google Scholar]
- 27.Pinet V, Vergelli M, Martin R, Bakke O, Long EO. Antigen presentation mediated by recycling of surface HLA-DR molecules. Nature. 1995;375:603. doi: 10.1038/375603a0. [DOI] [PubMed] [Google Scholar]
- 28.Möller P, Henne C, Moldenhauer G. CD74 workshop panel report. In: Schlossman SF, Boumsell L, et al., editors. Leucocyte Typing V: White Cell Differentiation Antigens. Vol. 1. Oxford: Oxford University Press; 1995. p. 568. [Google Scholar]
- 29.Quaranta V, Majdic O, Stingl G, Liszka K, Honigsmann H, Knapp W. A human Ia cytoplasmic determinant located on multiple forms of invariant chain (′, ′2, ′3) J Immunol. 1984;132:1900. [PubMed] [Google Scholar]
- 30.Shackelford DA, Lampson LA, Strominger JL. Analysis of HLA-DR antigens by using monoclonal antibodies: recognition of conformational differences in biosynthetic intermediates. J Immunol. 1981;127:1403. [PubMed] [Google Scholar]
- 31.Guy K, Van Heyningen V, Cohen BB, Deane DL, Steel CM. Differential expression and serologically distinct subpopulations of human Ia antigens detected with monoclonal antibodies to Ia alpha and beta chains. Eur J Immunol. 1982;12:942. doi: 10.1002/eji.1830121109. [DOI] [PubMed] [Google Scholar]
- 32.Adams TE, Bodmer JG, Bodmer WF. Production and characterization of monoclonal antibodies recognizing the α-chain subunits of human Ia alloantigens. Immunology. 1983;50:613. [PMC free article] [PubMed] [Google Scholar]
- 33.Barnstable CJ, Bodmer WF, Brown G, et al. Production of monoclonal antibodies to group A erythrocytes, HLA and other human surface antigens – new tools for genetic analysis. Cell. 1978;14:9. doi: 10.1016/0092-8674(78)90296-9. [DOI] [PubMed] [Google Scholar]
- 34.Goding JW. Structural studies of murine lymphocyte surface IgD. J Immunol. 1980;124:2082. [PubMed] [Google Scholar]
- 35.Peter M, Hellbrandt S, Schwartz-Albiez R, et al. Cell surface sialylation plays a role in modulating sensitivity towards APO-1-mediated cell death. Cell Death Diff. 1995;2:163. [PubMed] [Google Scholar]
- 36.Sollid LM, Gaudernack G, Markussen G, Kvale D, Brandtzaeg P, Thorsby E. Induction of various HLA class II molecules in a human colonic adenocarcinoma cell line. Scand J Immunol. 1987;25:175. doi: 10.1111/j.1365-3083.1987.tb01061.x. [DOI] [PubMed] [Google Scholar]
- 37.Neefjes JJ, Stollorz V, Peters PJ, Geuze HJ, Ploegh HL. The biosynthetic pathway of MHC class II but not class I molecules intersects the endocytic route. Cell. 1990;61:171. doi: 10.1016/0092-8674(90)90224-3. [DOI] [PubMed] [Google Scholar]
- 38.Demotz S. DR αβ dimers released from complexes with invariant chain fail to stimulate alloreactive T cell clones. Eur J Immunol. 1993;23:2100. doi: 10.1002/eji.1830230909. [DOI] [PubMed] [Google Scholar]
- 39.Bijlmakers MJ.E, Benaroch P, Ploegh HL. Assembly of HLA-DR1 molecules translated in vitro: binding of peptide in the endoplasmic reticulum precludes association with invariant chain. EMBO J. 1994;13:2699. doi: 10.1002/j.1460-2075.1994.tb06560.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Heuser JE, Anderson RG. Hypertonic media inhibit receptor-mediated endocytosis by blocking clathrin-coated pit formation. J Cell Biol. 1989;108:389. doi: 10.1083/jcb.108.2.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chavrier P, Parton RG, Hauri HP, Simons K, Zerial M. Localization of low molecular weight GTP binding proteins to exocytic and endocytic compartments. Cell. 1990;62:317. doi: 10.1016/0092-8674(90)90369-p. [DOI] [PubMed] [Google Scholar]
- 42.Roche PA, Cresswell P. Invariant chain association with HLA-DR molecules inhibits immunogenic peptide binding. Nature. 1990;345:615. doi: 10.1038/345615a0. [DOI] [PubMed] [Google Scholar]
- 43.Teyton L, O’Sullivan D, Dickson PW, et al. Invariant chain distinguishes between the exogenous and endogenous antigen presentation pathways. Nature. 1990;348:39. doi: 10.1038/348039a0. [DOI] [PubMed] [Google Scholar]
- 44.Ericson ML, Sundström M, Sansom DM, Charron DJ. Mutually exclusive binding of peptide and invariant chain to major histocompatibility complex class II antigens. J Biol Chem. 1994;269:26531. [PubMed] [Google Scholar]
- 45.Davis JE, Cresswell P. Lack of detectable endocytosis of B lymphocyte MHC class II antigens using an antibody-independent technique. J Immunol. 1990;144:990. [PubMed] [Google Scholar]
- 46.Reid PA, Watts C. Constitutive endocytosis of major histocompatibility complex class II glycoproteins in human B-lymphoblastoid cells. Immunology. 1992;77:539. [PMC free article] [PubMed] [Google Scholar]
- 47.Benaroch P, Yilla M, Raposo G, et al. How MHC class II molecules reach the endocytic pathway. EMBO J. 1995;14:3749. doi: 10.1002/j.1460-2075.1995.tb06973.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang K, Peterson PA, Karlsson L. Decreased endosomal delivery of major histocompatibility complex class II-invariant chain complexes in dynamin-deficient cells. J Biol Chem. 1997;272:17055. doi: 10.1074/jbc.272.27.17055. [DOI] [PubMed] [Google Scholar]
- 49.Vergelli M, Pinet V, Vogt AB, et al. HLA-DR-restricted presentation of purified myelin basic protein is independent of intracellular processing. Eur J Immunol. 1997;27:941. doi: 10.1002/eji.1830270421. [DOI] [PubMed] [Google Scholar]
- 50.Lanzavecchia A, Reid PA, Watts C. Irreversible association of peptides with class II molecules in living cells. Nature. 1992;357:249. doi: 10.1038/357249a0. [DOI] [PubMed] [Google Scholar]
- 51.Adorini L, Appella E, Doria G, Cardinaux F, Nagy ZA. Competition for antigen presentation in living cells involves exchange of peptides bound by class II MHC molecules. Nature. 1989;342:800. doi: 10.1038/342800a0. [DOI] [PubMed] [Google Scholar]
- 52.Reay PA, Wettstein DA, Davis MM. pH dependence and exchange of high and low responder peptides binding to a class II MHC molecule. EMBO J. 1992;11:2829. doi: 10.1002/j.1460-2075.1992.tb05350.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kropshofer H, Vogt AB, Hämmerling GJ. Structural features of the invariant chain fragment CLIP controlling rapid release from HLA-DR molecules and inhibition of peptide binding. Proc Natl Acad Sci USA. 1995;92:8313. doi: 10.1073/pnas.92.18.8313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kropshofer H, Vogt AB, Stern LJ, Hämmerling GJ. Self release of CLIP in peptide loading of HLA-DR molecules. Science. 1995;270:1357. doi: 10.1126/science.270.5240.1357. [DOI] [PubMed] [Google Scholar]
- 55.Germain RN, Hendrix LR. MHC class II structure, occupancy and surface expression determined by post-endoplasmic reticulum antigen binding. Nature. 1991;353:134. doi: 10.1038/353134a0. [DOI] [PubMed] [Google Scholar]