Abstract
Changes in lymphocyte subsets in the trachea, pulmonary tissue, bronchoalveolar lavage (BAL), peripheral blood and bronchial lymph node (BLN) of gnotobiotic calves infected with bovine respiratory syncytial virus (BRSV) were analysed by flow cytometry. Following BRSV infection, virus titres in the nasopharynx reached a peak between days 5 and 7 and infection was resolving from day 10. Although calves did not develop signs of clinical respiratory disease, there was evidence of gross pneumonia and histological changes typical of BRSV bronchiolitis, which were most extensive from day 7–10 of infection. Following BRSV infection there was a recruitment of CD8+ T cells into the trachea and lung, which peaked on day 10 after infection. Thus, there were approximately equal numbers of CD8+ and CD4+ T cells in the lung and trachea of uninfected calves, whereas by day 10 of infection, CD8+ cells outnumbered CD4+ cells by 3:1 in the lungs and 6:1 in the trachea of the infected calves. Although the increase in CD4+ T cells into the lungs was less marked than that of CD8+ T cells, changes in expression of CD45R, CD45RO, l-selectin and interleukin-2 receptors all suggested that CD4+ T cells were activated during BRSV infection. Changes in γδ T cells were not observed in BRSV-infected calves. There was a marked increase in B cells in the BLN after infection and BLN CD4+ T cells changed from the majority expressing l-selectin and CD45R in uninfected calves to a predominance of l-selectin− CD45R− CD45RO+ phenotype, 10 days after infection. In conclusion, CD8+ T cells constitute the major lymphocyte subpopulation in the respiratory tract of calves recovering from BRSV infection.
INTRODUCTION
The severe lower respiratory tract disease seen in children vaccinated with an inactivated human respiratory syncytial virus (HRSV) vaccine1 has highlighted the need to study the role of the immune response both in protection against and in the pathogenesis of RSV infection. Such studies, which have been carried out predominantly in small laboratory animals, have shown that although T cells are important in recovery from RSV infection, there is evidence that they also contribute to the pathogenesis of disease.2–4 However, whereas prolonged RSV infection in mice depleted of both CD4+ and CD8+ T cells is associated with the absence of lung lesions, prolonged RSV infections in immunosuppressed children and adults are severe and often fatal.5,6 This discrepancy illustrates the need to study the role of T cells in a natural host of RSV.
Bovine (B)RSV is a major cause of respiratory disease in young calves and the epidemiology and pathogenesis of RSV infection in calves resembles that in children.7 Studies in calves selectively depleted of T-cell subsets show that CD8+ T cells are important in clearance of BRSV from both the lungs and nasopharynx. Furthermore, delayed virus clearance is associated with an increase in the extent of pneumonic lesions, the histopathology of which is similar to that of HRSV-infected, immunocompromised children.8,9
A phenotypic analysis of the cells infiltrating the respiratory tract during RSV infection will contribute to our understanding of the pathogenesis of disease. In HRSV-infected BALB/c mice, the majority of lymphocytes recovered from bronchoalveolar lavage (BAL), early during infection, are CD4− CD8− surface immunoglobulin (sIg)−, but from day 5–6, CD8+ cells are the major population.10,11 This increase in CD8+ T cells correlates with the appearance of RSV-specific, major histocompatibility complex (MHC) class I-restricted, pulmonary CD8+ cytotoxic lymphocytes (CTL) and clearance of virus from the lungs.12,13 However, analysis of cells recovered from BAL of calves infected 8 days previously with BRSV failed to show any significant increase in lymphocytes, which accounted for less than 5% of all cells, whereas there was an influx of neutrophils.14 Neutrophils appear to be the predominant cell in BAL from infants infected with HRSV, and lymphocytes account for only 9% of cells, with CD4+ T cells outnumbering CD8+ T cells by 22:1.15
The present study was therefore undertaken to examine the development and distribution of lymphocyte subsets in the lung, trachea, bronchial lymph node and peripheral blood of calves infected with BRSV. In addition, changes in expression of the homing, activation and memory markers, l-selectin, interleukin-2 receptor (IL-2R), CD45R and CD45RO, on lymphocyte subsets were examined.
MATERIALS AND METHODS
Virus and cells
The Snook strain of BRSV16 was grown in primary calf kidney (CK) cells at a multiplicity of infection (MOI) of 0·1 plaque-forming units (PFU) per cell for 72–96 hr until a cytopathic effect was observed. The virus stock, which had a titre of 2×105 PFU/ml, was stored in liquid N2. The virus pool was free from mycoplasmas and bovine virus diarrhoea virus. Virus titres were determined by a plaque assay on secondary CK cells as described previously.16
Animals and experimental design
Gnotobiotic, BRSV seronegative calves were inoculated at 10 days of age with ≈4×105 PFU of BRSV in a volume of 20 ml, 10 ml administered intranasally (i.n.) and 10 ml intratracheally (i.t.). Nasopharyngeal swabs were obtained at intervals after infection to monitor virus excretion.16 Calves were killed at days 3 (n =3), 5 (n =4), 7 (n =5), 10 (n =4) and 15 (n =2) after infection by intravenous injection of sodium pentobarbitone (Euthatal; Merial Animal Health Ltd, Harlow, Essex, UK). A control group of four calves, inoculated i.n. and i.t. with 20 ml of tissue culture medium, was killed 10 days later and is referred to throughout as the day 0 group. Data were accumulated from a series of gnotobiotic calves, inoculated with the same stock of BRSV.
Prior to euthanasia, 40 ml of heparinized blood was obtained from each calf by venepuncture. At post-mortem examination, macroscopic lung lesions were recorded on a standard lung diagram and expressed as percentage pneumonia. Tissues were taken from the bronchial lymph node, trachea and lung for isolation of mononuclear cells. In addition, BAL was performed on a single lung lobe. Tissue samples from pneumonic areas of lung were immersed in neutral-buffered formalin, processed into paraffin wax, stained with haematoxylin and eosin and examined by light microscopy.
Preparation of mononuclear cells from blood and tissues
Peripheral blood mononuclear cells were isolated from heparinized blood, diluted 1:1 in phosphate-buffered saline (PBS), and centrifuged over Histopaque 1083 (Sigma, St Louis, MO) as described previously.8 Mononuclear cells were prepared from bronchial lymph nodes (BLN) by teasing in RPMI-1640 medium containing 10% heat-inactivated fetal calf serum (FCS), 2 mm l-glutamine, 20 mm HEPES, 4·2 mg/ml sodium bicarbonate, 10 mg/ml streptomycin and 100 U/ml penicillin (RPMI-10).
BAL samples were obtained from a single lung lobe irrigated with 400 ml of PBS at 4°. Adherent cells were removed by incubation of the cell suspension in plastic tissue culture flasks at 37° in 5% CO2 in air for 3 hr. The non-adherent cells were pelleted at 800 g for 10 min, resuspended in RPMI-10 and mononuclear cells were separated over Histopaque 1083.
Pulmonary mononuclear cells were prepared from 3 g of finely chopped lung tissue incubated in 100 ml of RPMI-10 medium containing 200 units/ml Type 1 collagenase (Worthington Biochemical Corporation, Freehold, NJ) and 50 units/ml DNAase (Sigma) as described previously.17
Tracheal intraepithelial lymphocytes were prepared from 40 g of trachea in 180 ml of buffer consisting of 27 mm tri-sodium citrate, 5 mm Na2HPO4, 96 mm NaCl, 8 mm KH2PO4, 15 mm KCl, 0·5 mm dithiothreitol, 55 mm d-sorbitol, 44 mm sucrose.18 After 2 hr of incubation at 37° in an orbital shaker at 200 r.p.m., the cell suspension was filtered through sterile nylon gauze and muslin and the cells pelleted at 800 g for 10 min. The mononuclear cells were separated over Histopaque 1083.
Flow cytometry
The monoclonal antibodies (mAb) used for flow cytometry were as follows: CC8 (CD4), CC63 (CD8), CC42 (CD2), CC21 (CD21), CC84 (monocytes and granulocytes), CC15 (WC1 molecule expressed by the majority of CD4− CD8−γδ T cells in blood),19 MM1A (CD3),20 CACTB6A and CACT81A [γ/δ T-cell receptor (TCR); VMRD Inc., Pullmann, WA]. Blood, lung, trachea, BAL and BLN mononuclear cells were stained with mAb as described previously8 using fluorescein isothiocyanate (FITC)-conjugated anti-mouse immunoglobulin (Southern Biotechnology Associates Inc. Birmingham, AL) to detect bound mAb. For two-colour flow cytometry, cells were stained with the following mAb: CC32 (l-selectin),21 CC76 (CD45R), which recognizes high molecular weight isoforms of bovine CD45 and is most similar to human CD45RB,22 IL-A116, which recognizes the low molecular weight isoform of bovine CD45 (CD45RO),23 and IL-A111 (BoCD25),24 followed by FITC-conjugated anti-mouse immunoglobulin. After washing, cells were incubated with 25 μl of PBS containing 10% normal mouse serum (NMS) and 0·1% NaN3 for 15 min, before staining with biotinylated CC8, CC63 and CC15, followed by strepavidin-R–phycoerythrin (Southern Biotechnology Associates Inc.). Monoclonal antibodies to turkey rhinotracheitis virus (produced at IAH, Compton, Berks., UK) were used as isotype-matched controls. All the incubations were performed at room temperature and 0·1% NaN3 was included in all the staining solutions.
Cells stained by one- and two-colour immunofluorescence were analysed with a fluorescence-activated cell sorter (FACScan; Becton Dickinson, Mountain View, CA) as described previously.22
Statistical analysis
One-way analysis of variance (anova) was used to compare population means. If the significance level obtained with anova was less than or equal to 0·05, the Mann–Whitney test was employed to compare the means. P-values < 0·05 were considered significant. All calculations were performed using the Minitab Statistical Analysis Package.
RESULTS
Infection of calves with BRSV
As seen previously,8 virus was first detected in the nasopharynx 2–3 days after infection, with peak titres evident at day 5, and was being cleared by day 10 (results not shown). Although clinical signs of respiratory disease were not observed, there was macroscopic evidence of pneumonia, with areas of red hepatization and catarrhal exudate in the small bronchioles in all infected calves. The extent of gross pneumonic lesions was maximum at day 7 and ranged from 2% to 15%. The minimal lesions observed in control uninfected calves were due to occasional scattered areas of atelactasis, often seen in normal young calves.25 Histological changes in the lungs of infected calves were typical of BRSV bronchiolitis and alveolitis, with infiltration by lymphoid cells into the lung parenchyma and peribronchiolar areas9 (Table 1). Immunoperoxidase labelling of lung tissue has shown that this lymphoid infiltration consists of CD8+, CD4+ and WC1+ T cells.9
Table 1.
Histological changes in the lung and trachea of gnotobiotic calves infected with the Snook strain of BRSV
Changes in mononuclear cells following BRSV infection
Preliminary investigations demonstrated that the conditions used for enzymatic digestion with collagenase and DNA-ase, when applied to peripheral blood lymphocytes, did not affect any of the cell-surface antigens examined in this study.
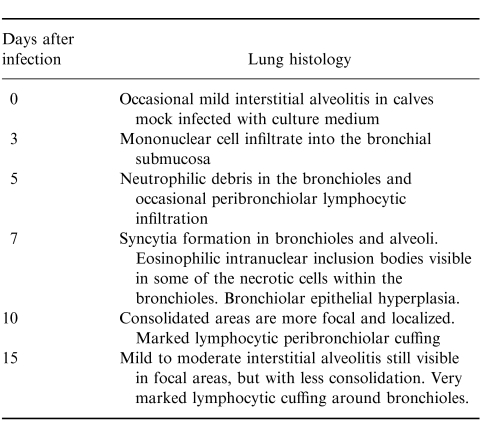
As seen previously,8 there was a decrease in the numbers of T cells in the peripheral blood of calves 7–10 days after infection (results not shown). Following infection, the BLN increased in size from day 3. This was due to an increase in B cells, which became the predominant cell by day 5 (Fig.1b). B-cell numbers in the BLN increased from 4·5±2·2×106 cells/g in controls to 26±6·6×106 cells/g at day 10 postinfection (P < 0·02). Although there was little change in B-cell numbers in the lung after BRSV infection, pulmonary T cells increased from a mean of 2·9±18×106 cells/g in controls to 11·2±49×106 cells/g at day 10. Lung macrophage numbers also increased, from 2±2×105 in uninfected controls to 24±15×105/g at day 10 (P < 0·04). In contrast, there was little change in lymphocyte numbers in BAL after BRSV infection (results not shown).
Figure 1.
Changes in T-cell subpopulations following BRSV infection. Values are the mean percentage staining (± standard deviations) of CD8+ (filled bars), CD4+ (open bars) and WC1+ T cells (hatched bars) and B cells (dotted bars) in (a) peripheral blood, (b) bronchial lymph node and (c) lung at intervals after BRSV infection.
Distribution of T-cell subsets following BRSV infection
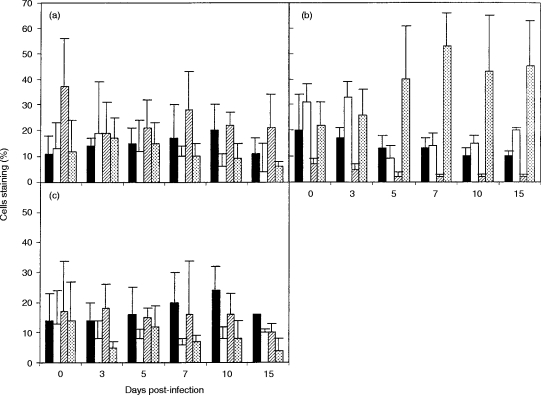
Following BRSV infection, there was a gradual increase in the proportion of CD8+ T cells in the peripheral blood, and the ratio of CD8+:CD4+ T cells changed from ≈1:1 in controls to 3:1, 10 days after infection (Fig. 1a). The ratio of CD8+:CD4+ T cells in the lung also shifted from about 1:1 in uninfected controls to 3:1, 10 days after infection (P =0·03) (Fig.1c). This was due to a rapid increase in the numbers of CD8+ T cells in the lung, which increased from 1·1±1·4×106/g in controls to 5·6±2·3×106/g at day 10 (P < 0·04). In contrast to the rapid increase in CD8+ cells in the lung, there was only a small increase in CD8+ cells in BAL, 10–15 days after infection (Fig. 2a). Similarly, approximately equal numbers of CD8+ and CD4+ cells were present in the trachea of uninfected calves (Fig. 2b). However, after BRSV infection, CD8+ T cells outnumbered CD4+ cells by 6:1, with CD8+ cells increasing from 2·3±1×103/g in controls to 22±24×103/g at day 10 postinfection (P < 0·002).
Figure 2.
Changes in T-cell subpopulations following BRSV infection. Values are the mean percentage staining (±standard deviations) of CD8+ (filled bars) and CD4+ (open bars) T cells in (a) bronchoalveolar lavage and (b) trachea at intervals after BRSV infection.
The proportions of CD4+ and CD8+ T cells in the BLN decreased after BRSV infection as the numbers of B cells increased (Fig. 1b). However, the loss of CD8+ lymphocytes from the BLN after infection was gradual, whereas the loss of CD4+ lymphocytes was rapid between days 3 and 5 of infection, and was followed by an increase up to day 15 postinfection (Fig. 1b).
Changes in WC1+ T cells in the peripheral blood, lung, BAL, trachea and BLN during the course of BRSV infection were not observed (Fig. 1, results not shown). Alterations in WC1−γδ TCR T-cell populations during BRSV infection were studied using mAb, CACTB6A and CACT81A. The proportions of WC1− CACTB6A+ cells in the BLN, lung and blood of control calves were 3%±2, 5%±2 and 3%±1, respectively, and the proportions of WC1− CACT81A+ cells were 1%±1, 11%±8 and 4%±1, respectively. The relative frequencies of these cells did not change significantly during BRSV infection (results not shown).
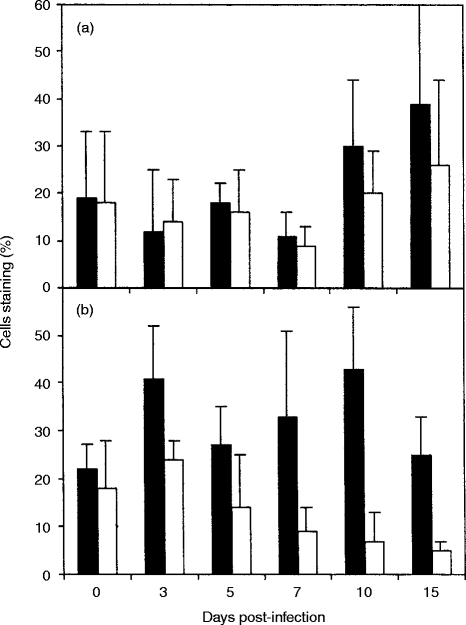
Expression of l-selectin, CD45R, CD45RO and IL-2R on CD4+ and CD8+ T cells in blood, lung and BLN after BRSV infection
In order to analyse changes in the activation/memory phenotype of CD4+ and CD8+ T cells during the course of BRSV infection, the proportions of CD4+ and CD8+ T cells expressing CD45R, CD45RO, l-selectin and CD25 in the blood, lung and BLN were assessed by two-colour flow cytometry. The numbers of T cells in BAL and trachea were insufficient to study by two-colour cytometry. It has been shown previously that the majority of bovine CD4+ and CD8+ T cells that express CD45RO do not express CD45R, although a small population of T cells do express both isoforms.23 The majority of CD4+ and CD8+ T cells in the peripheral blood of uninfected calves were CD45R+, but from day 5 of infection the proportion of cells expressing CD45R or CD45RO were similar (results not shown). The majority of CD4+ and CD8+ cells in the BLN of uninfected calves also expressed CD45R. However, the proportion of CD4+ cells expressing CD45R decreased and the proportion expressing CD45RO increased during the course of infection (Fig. 3b). So that by day 10 after infection, the proportion of CD4+ CD45RO+ BLN cells (49%±12) was significantly greater than that in BLN from uninfected calves (24%±15) (P =0·03). The number of CD8+ CD45RO+ BLN cells also increased during the course of RSV infection, although CD8+ CD45R+ were always the predominant subset (Fig. 3d). The majority of CD4+ T cells in the lungs of uninfected calves were CD45R+ CD45RO− until 7–10 days after infection, when equivalent numbers of CD4+ T cells expressing CD45R or CD45RO were present (Fig. 3a). This change in the pattern of CD45R and CD45RO expression was due to a significant increase in the proportion of CD4+ CD45RO+ T cells from a mean of 26%±7 in lungs of uninfected calves to 64%±6 on day 10 of infection (P =0·002). Changes in expression of CD45R and CD45RO were also observed in lung CD8+ cells (Fig. 3c), however, the changes were not as marked as those seen with lung CD4+ cells. Thus the lung contained similar numbers of CD45R+ and CD45RO+ CD8+ T cells until 15 days after BRSV infection, when the proportion of CD8+ T cells expressing CD45RO exceeded those expressing CD45R (Fig. 3c).
Figure 3.
Changes in expression of CD45R and CD45RO on CD4+ and CD8+ T cells after BRSV infection. Values are the mean percentage staining (±standard deviations) of CD4+ (a and b) and CD8+ (c and d) T cells expressing CD45R (filled bars) or CD45RO (open bars) in lung (a and c) and BLN (b and d) after BRSV infection.
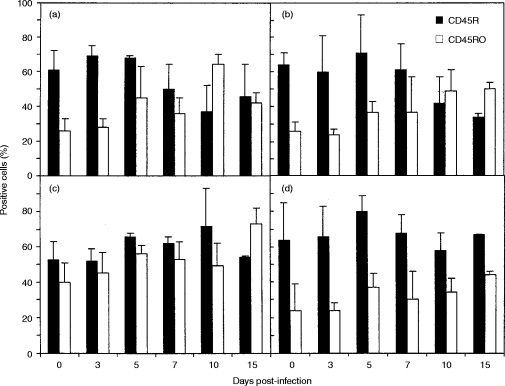
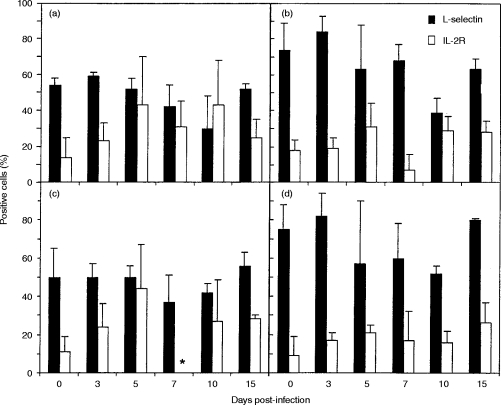
The majority of CD4+ and CD8+ T cells in the blood of uninfected calves were l-selectin+ and these cells did not change significantly during the course of RSV infection (results not shown). However, the proportion of BLN CD4+ l-selectin+ cells decreased from 74%±15 in uninfected calves to 39%±8 on day 10 postinfection (P < 0·02) (Fig. 4b), and the proportion of BLN CD8+ l-selectin+ cells decreased from 75%±13 in uninfected calves to 52%±4 on day 10 postinfection (P < 0·05). Approximately 50% of the CD4+ and CD8+ T cells in the lungs of uninfected calves were l-selectin+ and the proportion expressing l-selectin decreased after BRSV infection (Fig. 4a,c). However, differences in expression of l-selectin by CD8+ or CD4+ T cells from the lung were never statistically significantly different from those in control calves.
Figure 4.
Changes in expression of l-selectin and IL-2R on CD4+ and CD8+ T cells after BRSV infection. Values are the mean percentage staining (±standard deviations) of CD4+ (a and b) and CD8+ (c and d) T cells expressing l-selectin (filled bars) or IL-2R (open bars) in lung (a and c) and BLN (b and d) after BRSV infection. *not done.
Only 4% to 14% of CD4+ and CD8+ T cells in blood (results not shown), lung and BLN of uninfected calves expressed IL-2R. However, the proportion of cells expressing IL-2R increased after BRSV infection (Fig. 4) and was maximum at day 5.
DISCUSSION
Phenotypic analysis of lymphocytes from the blood, BLN, lung and trachea of calves infected with BRSV, showed that there was a major CD8+ T-cell response to the virus, with recruitment of these cells into sites of virus replication. The presence of increased numbers of CD8+ T cells in the blood, lung and trachea, after BRSV infection coincides with the presence of BRSV-specific CD8+ cytotoxic T lymphocyte (CTL) activity in the blood and lungs17 and correlates with the time of virus clearance.8 This study confirms and extends previous observations of BRSV infection in calves8,9 and lambs26,27 and is similar to studies in HRSV-infected BALB/c mice,10,11 but contrasts with studies in children with RSV bronchiolitis,15 in which CD4+ T cells appeared to outnumber CD8+ T cells in BAL. Differences in T-cell subsets in the lungs of BRSV-infected calves, in this study, and HRSV-infected children may reflect the severity of clinical illness. Further studies are needed to determine if CD8+ T cells still predominate in the lungs of calves with clinical BRSV respiratory disease.
Although CD8+ T cells increased in bovine lung tissue from days 3–5 after BRSV infection, increased CD8+ T cells were only observed in BAL on days 10–15 after infection. As seen previously, there was little change in total cell numbers in BAL from BRSV-infected calves14 and although the proportion of neutrophils increased, lymphocytes remained a minor population of BAL, at least until 10–15 days after infection. These results indicate that the cellular characteristics of the BAL do not fully reflect the changes in pulmonary lymphocyte subpopulations in calves infected with BRSV.
It has been suggested that γδ T cells, which are abundant at mucosal sites,28 may present a first line of defence against pathogens and alterations in γδ T-cell numbers occur during some respiratory virus infections.29,30 Large numbers of CD4− CD8−γδ T lymphocytes are found in the peripheral blood of cattle, although this population decreases with age,31 and the role of these cells in viral infections of cattle is not known. Previous studies have shown that the course of BRSV infection in calves is not affected by depletion of WC1+γδ T cells.8 Furthermore, changes in WC1+ and WC1−γδ T cells during the course of BRSV infection were not observed in this study, although an increase in intraepithelial WC1+γδ T cells has been observed by immunocytochemistry in the nose, trachea and lung of calves infected 10 days previously with BRSV9 (unpublished observations). Changes in γδ T-cell numbers were not detected in BAL from HRSV-infected mice.32 Taken together these observations indicate that γδ T cells do not have a major role in RSV infections.
Expression of different isoforms of CD45 by T-cell populations identifies cells that have different functions, tissue distributions and recirculation pathways. The general consensus is that naïve CD4+ T cells express the high MW isoforms and not CD45RO. Upon activation, CD4+ T cells become CD45RO+ and lose expression of the high MW isoform. Resting memory cells are evident primarily in the CD45RO+ population, although some cells may revert back to a CD45RO− state.33 Therefore for CD4+ T cells, loss of expression of the high MW isoforms of CD45 and an increase in expression of the low MW isoform (CD45RO) is a feature of activated/memory T cells. However, expression of a high or low MW isoform is not necessarily mutually exclusive and expression of more than one isoform has been observed on a small population of T cells.23 For CD8+ T cells in cattle, as in other species, although naïve cells are predominantly CD45RO−, CD8+ memory CTL are evident equally in the CD45RO+ and CD45RO− population.23 In this study there was evidence for an increase in the activation/memory phenotype of both CD4+ and CD8+ T cells in the lung and BLN following BRSV infection, as indicated by a decrease in the proportion of cells expressing the high MW isoform, CD45R, and an increase in the proportion expressing the low MW isoform, CD45RO. A reduction in expression of the high MW isoform, CD45RB, has also been observed on CD4+ and CD8+ T cells in BAL from mice infected 9 days previously with HRSV.34 In both BRSV-infected calves and HRSV-infected mice, the change to the activation/memory phenotype appeared to be more pronounced for CD4+ than for CD8+ T cells. In contrast, loss of CD45RB expression was more dramatic for CD8+ than for CD4+ BAL cells from Sendai virus-infected mice, and the appearance of CD45RBlo CD8+ cells in BAL correlated with virus clearance.34 Despite the predominance of CD45RBlo CD8+ T cells in BAL from day 10 of Sendai virus infection, Sendai virus-specific effector CTL were found in both CD45RBhi and CD45RBlo CD8+ BAL populations.35 Similarly, Sendai virus-specific CD4+ Th memory cells were found in both CD45RBhi and CD45RBlo spleen cell populations.36
Loss of l-selectin expression and an increase in IL-2R expression are also indicators of T-cell activation. Studies in Sendai virus-infected mice have shown that there is a shift from approximately equal numbers of l-selectin+ and l-selectin− CD8+ BAL cells during the acute phase of infection to a predominance of l-selectin− cells during the recovery phase.35 Furthermore, less than 5% of CD4+ BAL cells from Sendai virus-infected mice were l-selectin+ at any stage of infection. In contrast, ≈50% of CD4+ and CD8+ T cells isolated from the lung of BRSV-infected calves expressed l-selectin, although the proportion of l-selectin+ CD4+ lung T cells decreased at about day 7. These differences in expression of l-selectin by CD4+ and CD8+ T cells in the respiratory tract of BRSV-infected calves and Sendai virus-infected mice may reflect the different sites sampled. Thus, the BAL population contains cells that are probably derived from the superficial layer of the bronchial epithelium and may therefore represent a different population from those residing within the pulmonary interstitium, which would be isolated by enzymatic digestion. The latter population of cells would also reflect lymphocytes present in the lung capillaries. Alternatively, differences in the phenotype of pulmonary CD4+ and CD8+ T cells induced by virus infection in mice and cattle, may be due to differences in expression of l-selectin by murine and bovine effector/memory T cells. Thus, murine CD4+ and CD8+ effector/memory T cells are predominantly l-selectin−35,36 whereas bovine CD4+ memory T cells are equally distributed within the l-selectin+ and l-selectin− populations.21 The phenotype of BRSV-specific bovine effector CD8+ CTL is not known. However, the observation that expression of l-selectin by bovine lung CD8+ T cells does not change dramatically at day 10 after BRSV infection, when BRSV-specific CD8+ CTLs can be demonstrated in the lung, suggests that, unlike mice, virus-specific bovine CD8+ effectors cannot be discriminated on the basis of l-selectin expression.
In conclusion, the onset of an acute BRSV infection in calves resulted in the recruitment of activated, CD8+ T cells into the trachea and lung, which peaked on day 10 after infection. Although the increase in CD4+ T cells into the lungs was less marked than that of CD8+ T cells, changes in expression of CD45R, CD45RO, l-selectin and IL-2R on lung CD4+ T cells all suggested that CD4+ cells were activated by BRSV infection. Since CD8+ T cells are responsible for BRSV clearance,8 failure to develop a rapid anti-viral CD8+ T-cell response may result in severe disease. BRSV-infected calves depleted of CD8+ T cells develop more severe lung histopathology, similar to that seen in immunocompromised children infected with HRSV.9 Since calves in this study resolved their RSV infection without showing signs of clinical respiratory disease, it will be important to analyse the pulmonary T-cell response in calves with clinical signs of disease. Such studies will help to determine if the balance of the T-cell response is important in determining the outcome of BRSV infection.
Acknowledgments
We thank the staff of the gnotobiotic unit for their care of the experimental calves. This work was funded by the Ministry of Agriculture, Fisheries and Food, UK.
REFERENCES
- 1.Kim HW, Canchola JG, Brandt CD, et al. Respiratory syncytial virus disease in infants despite prior administration of antigenic inactivated vaccine. Am J Epidemiol. 1969;89:422. doi: 10.1093/oxfordjournals.aje.a120955. [DOI] [PubMed] [Google Scholar]
- 2.Cannon MJ, Openshaw PJ.M, Askonas BA. Cytotoxic T cells clear virus but augment lung pathology in mice infected with respiratory syncytial virus. J Exp Med. 1988;168:1163. doi: 10.1084/jem.168.3.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alwan WH, Record FM, Openshaw PJ.M. CD4 T cells clear virus but augment disease in mice infected with respiratory syncytial virus. Comparison with effects of CD8 T cells. Clin Exp Immunol. 1992;88:527. doi: 10.1111/j.1365-2249.1992.tb06482.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Graham BS, Bunton LA, Wright PF, Karzon DT. Role of T lymphocytes subsets in the pathogenesis of primary infection and rechallenge with respiratory syncytial virus in mice. J Clin Invest. 1991;88:1026. doi: 10.1172/JCI115362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fishaut M, Turbergeb D, Mcintosh K. Cellular response to respiratory viruses with particular reference to children with disorders of cell-mediated immunity. J Pediatr. 1980;96:179. doi: 10.1016/s0022-3476(80)80799-2. [DOI] [PubMed] [Google Scholar]
- 6.Hall CB, Powell KR, Macdonald NE, et al. Respiratory syncytial viral infection in children with compromised immune function. N Engl J Med. 1986;315:77. doi: 10.1056/NEJM198607103150201. [DOI] [PubMed] [Google Scholar]
- 7.Stott EJ, Taylor G. Respiratory Syncytial Virus. Brief Review. Arch Virol. 1985;84:1. doi: 10.1007/BF01310552. [DOI] [PubMed] [Google Scholar]
- 8.Taylor G, Thomas LH, Wyld SG, Furze J, Sopp P, Howard CJ. Role of T-lymphocytes subsets in recovery from respiratory syncytial virus infection in calves. J Virol. 1995;69:6658. doi: 10.1128/jvi.69.11.6658-6664.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thomas LH, Cook RS, Howard CJ, Gaddum RM, Taylor G. Influence of selective T-lymphocyte depletion on the lung pathology of gnotobiotic calves and the distribution of different T-lymphocyte subsets following challenge with bovine respiratory syncytial virus. Res Vet Sci. 1996;61:38. doi: 10.1016/s0034-5288(96)90108-3. [DOI] [PubMed] [Google Scholar]
- 10.Openshaw PJ.M. Flow cytometric analysis of pulmonary lymphocytes from mice infected with respiratory syncytial virus. Clin Exp Immunol. 1989;75:324. [PMC free article] [PubMed] [Google Scholar]
- 11.Kimpen JL.L, Rich GA, Mohar CK, Ogra PL. Mucosal T cell distribution during infection with respiratory syncytial virus. J Med Virol. 1992;36:172. doi: 10.1002/jmv.1890360305. [DOI] [PubMed] [Google Scholar]
- 12.Taylor G, Stott EJ, Hayle AJ. Cytotoxic lymphocytes in the lungs of mice infected with respiratory syncytial virus. J Gen Virol. 1985;66:2533. doi: 10.1099/0022-1317-66-12-2533. [DOI] [PubMed] [Google Scholar]
- 13.Anderson JJ, Norden J, Saunders D, Toms GL, Scott R. Analysis of the local and systemic immune responses induced in BALB/c mice by experimental respiratory syncytial virus infection. J Gen Virol. 1990;71:1561. doi: 10.1099/0022-1317-71-7-1561. [DOI] [PubMed] [Google Scholar]
- 14.Taylor G, Thomas LH, Stott EJ. Effect of vaccination on cell populations in lung washes from calves after infection with RSV. Res Vet Sci. 1989;47:231. [PubMed] [Google Scholar]
- 15.Everard ML, Swarbrick A, Wrightham M, et al. Analysis of cells obtained by bronchial lavage of infants with respiratory syncytial virus infection. Arch Dis Child. 1994;71:428. doi: 10.1136/adc.71.5.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thomas LH, Gourlay RN, Stott EJ, Howard CJ, Bridger JC. A search for new microorganisms in calf pneumonia by inoculation of gnotobiotic calves. Res Vet Sci. 1982;33:170. doi: 10.1016/S0034-5288(18)32331-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gaddum RM, Cook RS, Thomas LH, Taylor G. Primary cytotoxic T-cell responses to bovine respiratory syncytial virus in calves. Immunology. 1996;88:421. doi: 10.1046/j.1365-2567.1996.d01-667.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Flint N, Cove FL, Evans GS. A low temperature method for the isolation of small intestinal epithelium along the crypt–villus axis. Biochem J. 1991;280:331. doi: 10.1042/bj2800331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Howard CJ, Morrison WI. Leukocyte antigens in cattle, sheep and goats. Vet Immunol Immunopathol. 1991;27:1. [PubMed] [Google Scholar]
- 20.Davis WC, MacHugh ND, Park YH, Hamilton MJ, Wyatt CR. Identification of a monoclonal antibody reactive with the bovine orthologue of CD3 (BoCD3) Vet Immunol Immunopathol. 1993;39:85. doi: 10.1016/0165-2427(93)90167-3. [DOI] [PubMed] [Google Scholar]
- 21.Howard CJ, Sopp P, Parsons KR. l-selectin expression differentiates T cells isolated from different lymphoid tissues in cattle but does not correlate with memory. Immunology. 1992;77:228. [PMC free article] [PubMed] [Google Scholar]
- 22.Howard CJ, Sopp P, Parsons KR, et al. Distinction of naive and memory BoCD4 lymphocytes in calves with a monoclonal antibody, CC76, to a restricted determinant of the bovine leukocyte common antigen, CD45. Eur J Immunol. 1991;21:2219. doi: 10.1002/eji.1830210933. [DOI] [PubMed] [Google Scholar]
- 23.Bembridge GP, MacHugh ND, Mckeever D, et al. CD45RO expression on bovine T cells: relation to biological function. Immunology. 1995;86:537. [PMC free article] [PubMed] [Google Scholar]
- 24.Bujdoso R, Lund BT, Evans CW, McConnell I. Different rates of interleukin 2 receptor expression by ovine gamma/delta and alpha/beta T cells. Vet Immunol Immunopathol. 1993;39:109. doi: 10.1016/0165-2427(93)90170-9. [DOI] [PubMed] [Google Scholar]
- 25.Castleman WL, Lay JC, Dubovi EJ, Slauson DO. Experimental bovine respiratory syncytial virus infection in conventional calves: Light microscopic lesions, microbiology, and studies on lavaged lung cells. Am J Vet Res. 1985;46:547. [PubMed] [Google Scholar]
- 26.Sharma R, Woldehiwet Z, Spiller DG, Warenius HM. Lymphocyte subpopulations in the peripheral blood of lambs experimentally infected with bovine respiratory syncytial virus. Vet Immunol Immunopathol. 1990;24:383. doi: 10.1016/0165-2427(90)90008-g. [DOI] [PubMed] [Google Scholar]
- 27.Sharma R, Woldehiwet Z. Pathogenesis of bovine respiratory syncytial virus in experimentally infected lambs. Vet Microbiol. 1990;23:267. doi: 10.1016/0378-1135(90)90157-q. [DOI] [PubMed] [Google Scholar]
- 28.Augustin A, Kubo R, Sim GK. Resident pulmonary lymphocytes expressing the gamma delta T cell receptor. Nature. 1989;340:239. doi: 10.1038/340239a0. [DOI] [PubMed] [Google Scholar]
- 29.Carding SR, Allan W, Kyes S, Hayday A, Bottomly K, Doherty PC. Late dominance of the inflammatory process in murine influenza by gamma delta T cells. J Exp Med. 1990;172:1225. doi: 10.1084/jem.172.4.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ogasawara T, Emoto M, Kiyotani K, et al. Sendai virus pneumonia: Evidence for the early recruitment of γδ T cells during the disease course. J Virol. 1994;68:4022. doi: 10.1128/jvi.68.6.4022-4027.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hein WR, MacKay CR. Prominence of γ/δ T cells in the ruminant immune system. Immunol Today. 1991;12:30. doi: 10.1016/0167-5699(91)90109-7. [DOI] [PubMed] [Google Scholar]
- 32.Openshaw PJ.M. Pulmonary epithelial T cells induced by viral infection express T cell receptors α/β. Eur J Immunol. 1991;21:803. doi: 10.1002/eji.1830210338. [DOI] [PubMed] [Google Scholar]
- 33.Bell EB, Sparshott SM. Interconversion of CD45R subsets of CD4+ T cells in vivo. Nature. 1990;348:163. doi: 10.1038/348163a0. [DOI] [PubMed] [Google Scholar]
- 34.Openshaw PJ.M, Clarke SL, Record FM. Pulmonary eosinophilic response to respiratory syncytial virus infection in mice sensitised to the major surface glycoprotein G. Int Immunol. 1992;4:493. doi: 10.1093/intimm/4.4.493. [DOI] [PubMed] [Google Scholar]
- 35.Hou S, Doherty PC. Partitioning of responder CD8 T cells in lymph node and lung of mice with Sendai virus pneumonia by LECAM-1 and CD4RB phenotype. J Immunol. 1993;150:5494. [PubMed] [Google Scholar]
- 36.Ewing C, Topham DJ, Doherty PC. Prevalence and activation phenotype of Sendai virus-specific CD4+ T cells. Virology. 1995;210:179. doi: 10.1006/viro.1995.1329. [DOI] [PubMed] [Google Scholar]