Abstract
Aggregation of receptors for the constant region (Fc) of immunoglobulin G on myeloid cells results in endocytosis or phagocytosis and cellular activation. Previous work has shown, using the cell line U937, that the high-affinity immunoglobulin G receptor, FcγRI, activates alternate intracellular signalling pathways depending on the cell differentiation state, which results in a marked change in the nature of calcium transients within the cell. Here, we show that protein kinase C (PKC) is activated in both interferon-γ (IFN-γ) -primed and dibutyryl cyclic AMP (dbcAMP) -differentiated cells but that the nature of the particular isoenzymes recruited differs. Thus, in IFN-γ-primed U937 cells, FcγRI aggregation results in an increase of PKC activity which is essentially calcium independent resulting from the translocation to the membrane of the novel PKCs, δ and ε, together with the atypical PKC ζ. However, in cells differentiated to a more macrophage phenotype, all PKC enzyme activity after receptor aggregation is calcium dependent. Consistent with this finding, the isoenzymes translocated to the nuclear-free membrane fraction are the conventional PKCs α, β and γ; results consistent with our previous finding that FcγRI couples to phospholipase C in such dbcAMP-differentiated cells. Thus, the nature of PKC isoenzyme activated following FcγRI aggregation is defined by differentiation. The calcium dependence of the PKC isoenzyme is consistent with the duration of calcium transients previously reported in the two differentiation states.
INTRODUCTION
Binding of the constant region (Fc) of immunoglobulin G (IgG) to cell surface receptors (FcγR) on leucocytes provides a pivotal link between the humoral and cellular arms of the immune system (see reviews in ref. 1–3). Three different classes of Fcγ receptors have been defined; FcγRI (CD64), FcγRII (CD32) and FcγRIII (CD16) (see reviews in ref.4–6). Of these, the human high-affinity receptor, FcγRI, is an integral type I membrane glycoprotein7 constitutively expressed on monocyte and macrophage cell types. On myeloid cells, aggregation of these receptors triggers a number of different effector functions including endocytosis of immune complexes or phagocytosis of opsonized particles. Fcγ receptor aggregation activates a repertoire of responses including degranulation and release of proteases, activation of the respiratory burst and release of cytokines. These can ultimately lead to targeted cell killing through antibody-directed cellular cytotoxicity (ADCC)8,9 which is critically important for clearing virus-infected cells and in cancer surveillance.10
One feature of monocytes and macrophages is the heterogeneity of response to immune complex challenge using cells harvested under different conditions and different environments. Little is known about the signal transduction mechanisms underlying this or how they are modified as blood monocytes differentiate into tissue macrophages. Thus, to study early events in the FcγRI signalling pathway, we have used the human monocyte cell line, U937,11 which constitutively expresses FcγRI and FcγRII and which allows controlled differentiation into a more macrophage cell type by treatment with dibutyryl cAMP (dbcAMP).12 Previous work has shown that the nature of calcium transients markedly changes as the cells become differentiated.13 Thus, a single spike in calcium is observed in response to Fcγ receptor aggregation of cells treated with interferon-γ (IFN-γ) whereas calcium oscillations are generated in cells differentiated to a more macrophage state by dbcAMP.14 This switch in calcium signalling patterns is dictated by a switch in the intracellular signalling pathways activated by FcγRI in the two differentiation states.15 Thus, the calcium spike in IFN-γ-treated cells results from the sequential activation of phosphatidylcholine phospholipase D (PtdCho-PLD) and sphingosine kinase15,16 whereas the calcium oscillations observed in dbcAMP differentiated cells are associated with activation of phosphatidylinositol 4,5 bisphosphate phospholipase C (PtdInsP2-PLC) and subsequent generation of inositol 1,4,5 trisphosphate (InsP3). Both activation pathways generate diacylglycerol17,18 and thereby can activate protein kinase C (PKC)18,19 and this kinase has been shown to play an important role in mediating Fcγ receptor functions.20–23
PKC isoforms, depending on their structure and cofactor regulation, are divided into three groups:24,25 conventional (PKC α, βI, βII and γ) which are calcium- and diacylglycerol-activated isoenzymes, novel (PKC δ, ε, η and θ) which are calcium independent but diacylglycerol-activated isoenzymes, and the atypical ones (PKC ζ, λ/ι and μ) which do not require either calcium or diacylglycerol.24–28 Here, we show that the nature of the PKC isoenzymes activated by immune complexes differs in the two differentiation states. Thus, calcium-dependent typical PKCs (α, β and γ) were activated in dbcAMP-differentiated cells whereas the calcium-independent, novel PKCs, δ and ε, and the atypical isoenzyme ζ, were activated in the cells primed with IFN-γ.
MATERIALS AND METHODS
Cell culture
U937 cells were cultured in a humidified atmosphere at 37°, 6·8% CO2 in RPMI-1640 medium (GibcoBRL, Life Technologies Ltd, Paisley, UK) supplemented with fetal calf serum (10%), glutamine (2 mm), penicillin (10 U/ml) and streptomycin (10 mg/ml). The cells were treated with IFN-γ (a gift from Bender Wein Ltd, Vienna, Austria) (100 ng/ml) for 24 hr or dbcAMP (1 mm) for 48 hr.
Analysis of PKC isoform expression
U937 cells (107 cells), either IFN-γ primed or differentiated with dbcAMP, were harvested by centrifugation at 200 g for 5 min and the cell pellet was solubilized in 1 ml of lysis buffer [50 mm Tris, 150 mm sodium chloride, 2% Tween-40, 0·25% sodium deoxicholate, 1 mm ethylene glycol-bis(βaminoethyl ether)-tetraacetic acid (EGTA), 1 mm phenylmethylsulphonyl fluoride (PMSF), 10 mm sodium orthovanadate, 10 μg/ml chymostatin, 10 μg/ml leupeptin, 10 μg/ml antipain and 10 μg/ml pepstatin]. Cell lysates were frozen in liquid nitrogen, then thawed and homogenized (15–20 strokes) on ice in a precooled Dounce homogenizer. The cell lysates were centrifuged at 20 000 g for 15 min at 4° and the supernatants containing total solubilized cellular protein was harvested and stored at −20°. The amount of protein in supernatants was quantified using Bradford (Bio-Rad Laboratories Ltd, Hertfordshire, UK) assay.
Aggregation of FcγRI
Cells were harvested by centrifugation (200 g for 5 min). After resuspension, the cells were incubated for 30 min on ice with 1 μm human monomeric, polyclonal IgG (Serotec Ltd, Oxford, UK) to occupy surface FcγRI. Excess unbound ligand was removed by dilution and centrifugation. Cells were resuspended and ligand occupied receptors were then aggregated by addition of 1:50 dilution of F(ab) goat anti-human IgG (Sigma-Aldrich Company Ltd, Dorset, UK) on ice. Cells were then warmed to 37° for the times specified in the assays.
Cell fractionation
Following receptor cross-linking, cells were harvested at the specified times and subcellular fractions were prepared by a modification of methods previously described.29–31 Briefly, cells were harvested and resuspended in cold nuclear preparation buffer (10 mm Tris–HCl, pH 7·4, 2 mm magnesium chloride, 0·14 m sodium chloride, 2% Tween-40, 0·25% sodium deoxycholate, 1 mm EGTA, 1 mm PMSF, 10 mm sodium orthovanadate, 10 μg/ml chymostatin, 10 μg/ml leupeptin, 10 μg/ml antipain and 10 μg/ml pepstatin). Cells were frozen in liquid nitrogen, then thawed under running hot water, the nuclei were released by 15–20 strokes of a precooled Dounce homogenizer and centrifuged at 15 000 g for 5 min. In order to examine the integrity of the nuclear membrane, the pellet was resuspended in 20 μg/ml of propidium iodide and viewed by fluorescence microscopy. The supernatant was centrifuged at 100 000 g and 4° for 30 min, the pellet containing the membrane fraction was resuspended in 200 μl of the nuclear preparation buffer and stored at −20°. The cytosol fraction, represented by the high-speed supernatant, was also stored at −20°. The amount of protein recovered in each fraction was quantified using the Bradford reagent system.
Gel electrophoresis and Western blots
Proteins (50 μg of whole cell lysate or 20 μg of the membrane fraction), were resolved on 10% polyacrylamide gels (SDS–PAGE) under denaturing conditions. The resolved proteins were transferred to nitrocellulose membranes. The membranes were blocked by incubating overnight in phosphate-buffered saline (PBS) with 5% non-fat milk, 0·1% Tween-20 buffer at 4°. The membrane then was washed in PBS, 0·1% Tween-20 and incubated individually with mouse monoclonal antibodies specific for each of human PKC isoforms (Transduction Laboratories, Lexington, KY) at dilutions as recommended by the manufacturer, in 5% non-fat milk/PBS/0·1% Tween-20 at room temperature for 4 hr. We have previously characterized the specificity of these PKC isoform-specific antibodies.31 Following washing of the membranes, bands were visualized using an enhanced chemiluminiscence (ECL) Western Blotting Detection System (Amersham International, Amersham, UK).
PKC enzyme activity assay
PKC assays were carried out using the Biotrak Protein Kinase C enzyme assay system (Amersham). Briefly, the system is based upon the PKC catalysed transfer of the γ-phosphate group of adenosine-5′-triphosphate to a peptide substrate specific for PKC. U937 cells treated with IFN-γ or differentiated with dbcAMP were stimulated by FcγRI aggregation at the indicated times. Following stimulation, proteins from whole cell lysates or fractionated, nuclear-free membrane samples, were partially purified by diethylaminoethyl (DEAE) cellulose chromatography (Whatman DE52). PKC enzyme activity, from partially purified samples, was measured from whole cell lysate, or fractionated, nuclear-free, membrane samples in the presence of 1·5 mm calcium, or substituting calcium with 1·5 mm EGTA-containing buffer.
RESULTS
Aggregation of FcγRI activates PKC in both IFN-γ-primed and dbcAMP-differentiated U937 cells
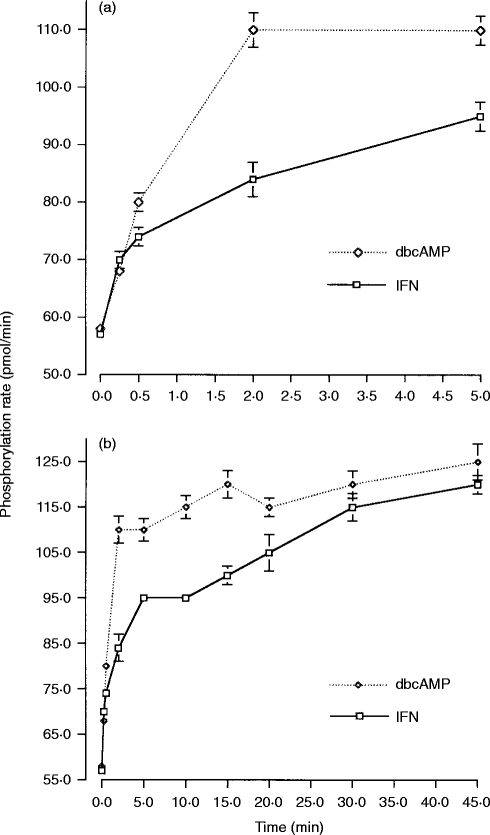
The kinetics of activation of PKC was measured in cells primed with IFN-γ and compared to that observed for cells differentiated by dbcAMP. Resting PKC activity was identical in the two cell types and aggregation of FcγRI resulted in a prompt increase in PKC activity. Thus, 30 seconds after receptor aggregation, PKC activity had increased by 50% in both cell types (Fig. 1a). In IFN-γ-treated cells, this initial rapid rise in PKC activity was followed by a sustained steady increase over the next 45 min after receptor aggregation (Fig. 1b). In contrast, in dbcAMP-differentiated cells, PKC activity increased very rapidly, such that peak PKC was achieved 2·5 min after receptor aggregation and this activity was then maintained over the subsequent 45 min (Fig. 1b). Although the time to reach a plateau of PKC activity differed for IFN-γ-primed and dbcAMP-differentiated cells, the plateau levels eventually reached in both cell types were very similar.
Figure 1.
PKC activity, measured as the phosphorylation rate (pmol/min), in samples from whole cell lysates. Short (up to 5 min) and Long (up to 45 min) time–courses. (a) PKC activity following FcγRI aggregation (short, up to 5 min, time–course) in dbcAMP-differentiated (dbcAMP) and IFN-γ-primed (IFN-γ) U937 cells. Data are the mean±SD of triplicate measurements for each time-point and are representative of four separate experiments. (b) PKC activity following FcγRI aggregation (long, up to 45 min, time course) in dbcAMP-differentiated (dbcAMP) and IFN-γ-primed (IFN-γ) U937 cells. Data is the mean±SD of triplicate measurements for each time-point and are representative of four separate experiments.
FcγRI aggregation results in calcium-dependent or -independent PKC enzyme activity depending on cell differentiation
Previous work has shown that the nature of calcium transients generated following FcγRI aggregation markedly change following differentiation of the cells.12,13 Since calcium is an important cofactor for PKC activity, we next investigated the calcium dependence of the observed PKC activity.
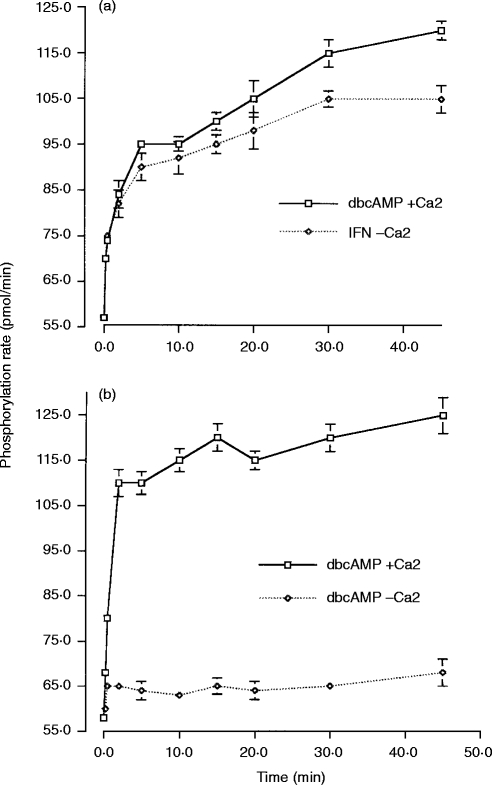
In IFN-γ-primed cells, the increase in PKC activity over the first 20 min after receptor aggregation was unaffected by the withdrawal of calcium from the assay (Fig. 2a). At the later time-points (30 min and 45 min), presence of calcium resulted in a small increase in measured PKC activity. These data indicate that over the first 20 min after receptor aggregation, calcium-independent novel or atypical PKCs account for the vast majority of the PKC activation observed in these cells. The calcium-dependent classical PKCs only contribute to the total PKC activation in the later stages following receptor aggregation, and even here they are only a minor component.
Figure 2.
Calcium dependence of PKC activity. PKC activity, measured as the phosphorylation rate (pmol/min), in samples from whole cell lysates and in the presence or absence of calcium in the assay buffer. (a) PKC activity following FcγRI aggregation in IFN-γ-primed U937 cells in the presence of 1·5 mm calcium (IFN+Ca2+) or substituting calcium with 1·5 mm EGTA (IFN – Ca2+) in the assay buffer. Data are the mean±SD of triplicate measurements for each time-point and are representative of three separate experiments. (b) PKC activity following FcγRI aggregation in dbcAMP-differentiated U937 cells in the presence of 1·5 mm calcium (dbcAMP+Ca2+) or substituting calcium with 1·5 mm EGTA (dbcAMP – Ca2+) in the assay buffer. Data are the mean±SD of triplicate measurements for each time-point and are representative of three separate experiments.
In contrast, in dbcAMP-differentiated cells, withdrawal of calcium from the assay completely abolished any increase in measurable PKC activity at all time-points. Thus, in these cells, aggregation of FcγRI results in the activation of calcium-dependent, typical PKCs and there is little contribution of calcium-independent novel or atypical PKCs (Fig. 2b).
FcγRI aggregation results in PKC translocation to membranes
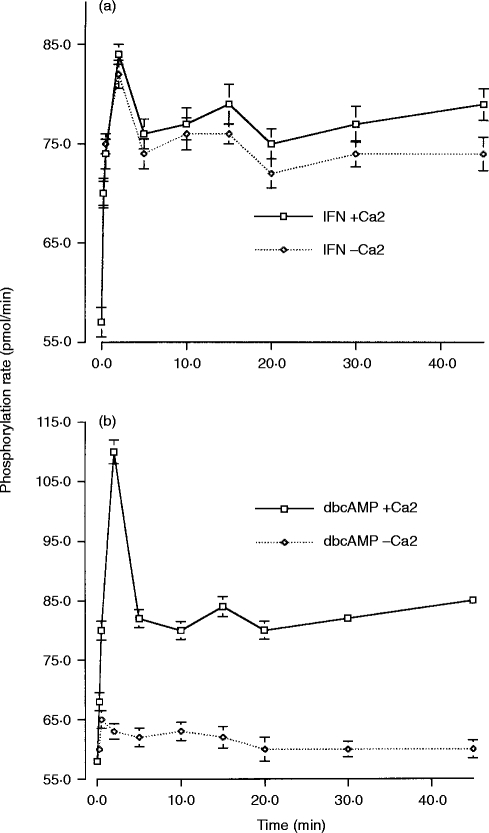
PKC activation is associated with its translocation to the plasma membrane.24,25,32 PKC activity in the nuclear-free membrane fraction was therefore measured together with the calcium dependence of this activity in IFN-γ-primed and dbcAMP-differentiated cells following aggregation of FcγRI. In IFN-γ-treated cells, membrane-associated PKC activity increased very rapidly after receptor aggregation. An increase was observed within the first 15 seconds and reached maximal activity at 2 min; thereafter being sustained for 45 min (Fig. 3a). Withdrawal of calcium from this assay had no effect on the PKC activity, indicating that the translocated PKCs were predominantly calcium independent. In contrast, in dbcAMP-differentiated cells, all the membrane-associated PKC activity was dependent on the presence of calcium in the assay, indicating that the PKCs translocated to the membrane are the conventional calcium-dependent PKCs. In these cells, receptor aggregation also resulted in a very rapid increase in PKC activity in the membrane fraction. However, in contrast to the cells primed with IFN-γ, this membrane-associated PKC activity peaked 2·5 min after receptor aggregation and then fell rapidly to achieve a new plateau level of activity that was maintained over the subsequent 45 min (Fig. 3b).
Figure 3.
PKC activity translocated to the nuclear-free membrane fraction and the calcium dependence of this PKC activity. PKC activity, measured as the phosphorylation rate (pmol/min), in samples from whole cell lysates and in the presence or absence of calcium in the assay buffer. (a) PKC activity following FcγRI aggregation in IFN-γ-primed U937 cells in the nuclear-free membrane fraction and in the presence of 1·5 mm calcium (IFN+Ca2+) or substituting calcium with 1·5 mm EGTA (IFN – Ca2+) in the assay buffer. Data are the mean±SD of triplicate measurements for each time-point and are representative of three separate experiments. (b) PKC activity following FcγRI aggregation in dbcAMP-differentiated U937 cells in the nuclear-free membrane fraction and in the presence of 1·5 mm calcium (dbcAMP+Ca2+) or substituting calcium with 1·5 mm EGTA (dbcAMP – Ca2+) in the assay buffer. Data are the mean±SD of triplicate measurements for each time-point and are representative of three separate experiments.
PKC isoenzyme expression is regulated by differentiation
Several PKC isoenzymes, from the three defined groups, have been shown to be expressed in monocytes and macrophages.21,33 However, changes following differentiation are still unclear. The findings reported here indicate that calcium-independent PKCs are preferentially activated in IFN-γ-treated cells compared to dbcAMP-differentiated cells, where calcium-dependent PKCs are activated. To determine whether this change in isoenzyme recruitment following FcγRI aggregation reflected a change in the relative expression of the various PKCs, cell lysates were probed with monoclonal antibodies specific for each enzyme for cells primed with IFN-γ or differentiated with dbcAMP and the patterns were compared to that for untreated, resting cells.
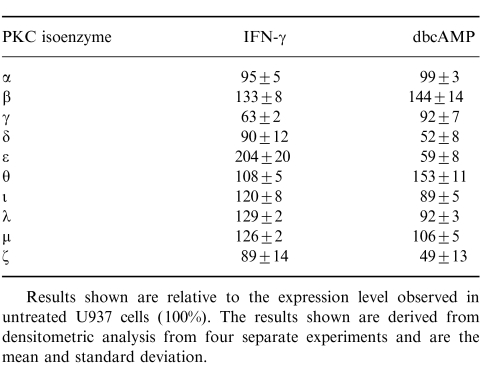
Western blot analysis showed that in untreated U937 cells PKC α, β, γ, δ, ε, θ, μ, ι/λ and ζ, are all expressed to varying degrees (Fig. 4 lane 1). Treatment of the cells with IFN-γ resulted in an increase in PKC δ, ε, μ, ι/λ and θ. PKC α and ζ remained unchanged and a reduction in expression of β and γ isoenzymes was observed (Fig. 4 lane 2; Table 1). In contrast, dbcAMP differentiation resulted in up-regulation of PKC α, β, and γ, and down-regulation of PKC δ, ε, ι/λ, and ζ. PKC μ and θ remained unchanged by dbcAMP treatment of U937 cells (Fig. 4 lane 3; Table 1).
Figure 4.
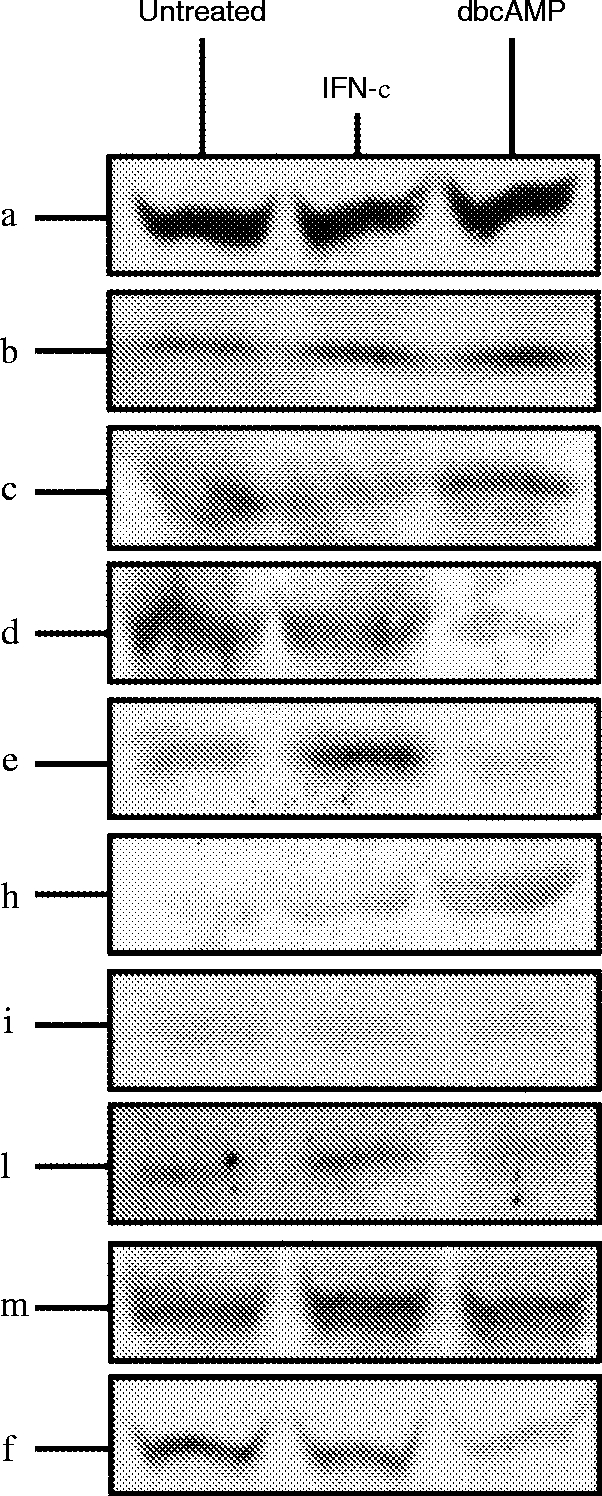
Western blot analysis of the different PKC isoenzymes expressed in whole cell lysates of U937 cells. Cells were cultured in their normal growth media (untreated), or in media containing IFN-γ (100 ng/ml) for 24 hr (IFN-γ), or in media containing dbcAMP (1 mm) for 48 hr (dbcAMP). Samples from whole cell lysates were subjected to SDS–PAGE and Western blotted. The blots were incubated individually with mouse monoclonal antibodies specific for each of the human PKC isoenzymes (α, β, γ, δ, ε, θ, ι/λ, μ, ζ). Bands were visualized by ECL (Amersham).
Table 1.
Densitometric analysis of the relative expression levels of PKC isoenzymes following treatment of cells with IFN-γ or differentiation with dbcAMP
Results shown are relative to the expression level observed in untreated U937 cells (100%). The results shown are derived from densitometric analysis from four separate experiments and are the mean and standard deviation.
Differentiation-dependent differential translocation of PKC isoenzymes to the membrane fraction following FcγRI cross-linking
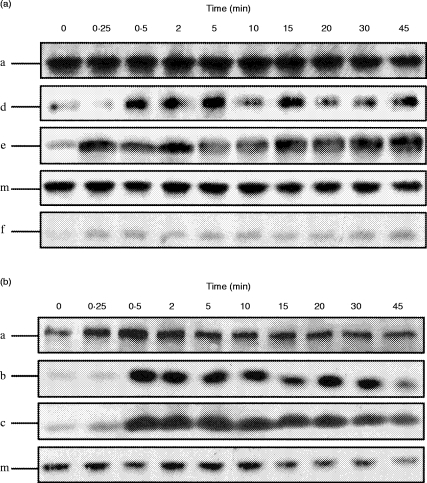
To determine whether aggregation of FcγRI results in differential activation of particular isoenzymes in IFN-γ-primed cells compared to dbcAMP differentiation, specific translocation of individual PKC was investigated. In IFN-γ-primed cells, the novel PKCs δ and ε and atypical ζ isoenzymes were translocated to the membrane fraction following FcγRI cross-linking. However, all of the conventional PKC α appeared to be membrane-bound in non-stimulated IFN-γ-primed cells and thus translocation could not be assessed (Fig. 5a). In contrast, in dbcAMP-differentiated cells, only the conventional PKCs α, β, γ were translocated to the membrane fraction in response to FcγRI cross-linking (Fig. 5b).
Figure 5.
Western blot analysis of the different PKC isoenzymes translocated to the nuclear-free membrane fraction following FcγRI aggregation in IFN-γ-primed and dbcAMP-differentiated U937 cells. (a) Western blot analysis of the different PKC isoenzymes translocated to the nuclear-free membrane fraction of IFN-γ-primed U937 cells following FcγRI aggregation time–course. The blots were incubated individually with mouse monoclonal antibodies specific for each of human PKC isoenzymes (α, β, γ, δ, ε, θ, ι/λ, μ, ζ). PKC isoenzymes found to translocate δ, ε,ζ. PKCs α and μ are membrane bound throughout the experiment. (b) Western blot analysis of the different PKC isoenzymes translocated to the nuclear-free membrane fraction of dbcAMP-differentiated U937 cells following FcγRI aggregation time–course. The blots were incubated individually with mouse monoclonal antibodies specific for each of the human PKC isoenzymes (α, β, γ, δ, ε, θ, ι/λ, μ, ζ). PKC isoenzymes were found to translocate α, β, γ. PKC μ is membrane bound throughout the experiment.
The novel PKC μ, which contains a putative transmembrane domain, is, as expected, membrane bound in both IFN-γ-primed and dbcAMP-differentiated cells even in the absence of receptor aggregation and thus translocation could not be assessed (Fig. 5b). No other PKC isoenzymes could be detected in the nuclear-free membrane fraction.
DISCUSSION
Endocytosis of immune complexes by FcγRI has been shown here to cause a prompt and sustained activation of PKC in both IFN-γ-primed U937 cells and cells differentiated to a more macrophage phenotype by dbcAMP. However, the precise nature of the PKCs activated by receptor aggregation differs. Calcium-independent PKC isoenzymes were activated in IFN-γ-primed cells whereas, following differentiation, calcium-dependent PKCs were the major activated form. Although IFN-γ and dbcAMP modified the relative expression levels of the various PKC isoenzymes, all the major species were still present in lysates of both cell types and thus, the changes in expression cannot account for the fundamental switch in PKC isoenzyme activation following receptor aggregation. This observation implies that FcγRI is able to recruit and specifically activate different isoenzymes depending on the differentiation state of the cell and likely results from the switch in signalling pathways activated by FcγRI following differentiation. This switch dictates the duration of calcium transients in these cells and it is noteworthy that the calcium dependence of the PKCs activated by FcγRI reflects this change.
Previous work has shown that, in these two differentiation states, FcγRI is coupled to the activation of distinct intracellular signalling pathways. FcγRI is able to switch between these two activation pathways as its cytoplasmic tail contains no signalling motif but, rather, the receptor must recruit an accessory molecule to activate tyrosine kinases. Thus, in IFN-γ-primed cells, FcγRI acts through the γ-chain and is coupled to the activation of PtdCho-PLD and sphingosine kinase. In these cells, changes in cytosolic calcium are transient as, following release from stores, calcium levels peak within 1 min and then fall to basal levels. Consistent with this, the PKC isoenzymes activated are calcium independent and the predominant PKCs translocated to membranes are the isoenzymes δ, ε and ζ.
Novel PKCs are activated by diacylglycerol which is generated in this pathway by phosphatidic acid-phosphohydrolase (PPH) converting phosphatidic acid, the immediate product of PtdCho-PLD, to diacylglycerol. However, the diacylglycerol species produced by this pathway differs from that generated by PtdInsP2-PLC and recent evidence suggests that the diacylglycerol produced by PtdCho-PLD and PPH is not able to activate PKC.34 Atypical PKCs are not activated by either calcium or diacylglycerol. However, phosphatidic acid itself has been shown to activate specifically PKC ζ; thus, providing a potential link between the novel intracellular signalling pathway we have previously defined in IFN-γ-primed U937 cells following FcγRI aggregation and the pattern of PKC activation observed. Our recent work has shown that aggregation of FcγRI also activates phosphatidyl inositol (PI3)-kinase in IFN-γ-treated cells35 and that PI3-kinase is upstream of phospholipase D activation. This finding provides an explanation for the observations reported here as both novel and atypical PKCs are activated by second messengers generated by PI3-kinase.36–39
In IFN-γ-primed cells, FcγRI recruits the γ-chain to act as its accessory molecule for signal transduction.15,40,41 The γ-chain acts as a signal transducing accessory molecule for a number of receptors, including the high-affinity IgE receptor, FcεRI in mast cells.42 In cells expressing FcεRI, endocytosis of this receptor has been correlated with threonine phosphorylation of the γ-chain and this phosphorylation was attributed specifically to PKC δ activation.43 Interestingly, PKC δ was physically associated with the FcεRI receptor complex, in particular with the β-chain of the receptor complex. Although similar phosphorylation of threonine residues of the γ-chain has been shown following aggregation of FcγRI,44 PKC δ immunoreactivity could not be demonstrated in FcγRI immunoprecipitates either before or after receptor aggregation by immune complexes (data not shown). This may result from the lack of expression of the FcεRI –β-chain in myeloid cells.
In contrast to IFN-γ-primed cells, differentiation of U937 cells by dbcAMP to a more macrophage phenotype results in a switch in the intracellular signalling pathways activated following FcγRI aggregation.15 Thus, PtdInsP2-PLC, and not PtdCho-PLD, is activated with the subsequent generation of diacylglycerol and InsP3. Associated with this, the calcium transients are prolonged as store dumping is followed by activation of calcium release activated current (ICRAC) and significant calcium entry.45,46 Consistent with this, the PKCs activated are the conventional type being calcium dependent and, specifically, the isoenzymes α, β and γ are translocated to membranes. The switch observed in activation pathway results from a switch in the accessory molecule used by FcγRI as in the dbcAMP-differentiated cells, signalling by FcγRI appears to be mediated by FcγRIIa.15
Activation of PKC is well established as essential in mediating phagocytosis in myeloid cells.20–23 The switch in calcium dependence of the activated PKC isoenzymes in the two cell types provides an explanation for the observation that phagocytosis mediated by FcγRI is calcium independent whereas phagocytosis mediated by FcγRII is calcium dependent.20 In dbcAMP, FcγRII is used as the signal transducing molecule.15 In these cells, only calcium-dependent PKCs are activated. Buffering of intracellular calcium would therefore abolish PKC activation and thereby prevent phagocytosis. In IFN-γ-primed cells, FcγRI uses the γ-chain for signal transduction and, under these circumstances, calcium-independent PKCs are activated. Buffering intracellular calcium will therefore have no effect on PKC activation.
Acknowledgments
We are grateful to Bender-Wein for the generous gift of IFN-γ. This work was supported by grants from the MacFeat Bequest, and the Scottish Hospitals Endowment Research Trust.
Glossary
Abbreviations
- dbcAMP
dibutyryl cyclic AMP
- FcγRI
high-affinity immunoglobulin G receptor
- IFN-γ
interferon-γ
- PKC
protein kinase C
REFERENCES
- 1.Ravetch JV, Kinet J-P. Fc Receptors. Annu Rev Immunol. 1991;9:457. doi: 10.1146/annurev.iy.09.040191.002325. [DOI] [PubMed] [Google Scholar]
- 2.Van De Winkel JG.J, Anderson CL. Biology of human IgG Fc receptors. J Leu Biol. 1991;49:511. doi: 10.1002/jlb.49.5.511. [DOI] [PubMed] [Google Scholar]
- 3.Hulett MD, Hogarth PM. Molecular basis of Fc receptor function. Adv Immunol. 1994;57:1. doi: 10.1016/s0065-2776(08)60671-9. [DOI] [PubMed] [Google Scholar]
- 4.Van De Winkel JG.J, Capel PJ.A. Human IgG Fc receptor heterogeneity: molecular aspects and clinical implications. Immunol Today. 1993;14:215. doi: 10.1016/0167-5699(93)90166-I. [DOI] [PubMed] [Google Scholar]
- 5.Ravetch JV. Fc Receptor: Rubor Redux. Cell. 1994;78:553. doi: 10.1016/0092-8674(94)90521-5. [DOI] [PubMed] [Google Scholar]
- 6.Raghavan M, Bjorkman PJ. Fc receptors and their interactions with immunoglobulins. Annu Rev Cell Dev Biol. 1996;12:181. doi: 10.1146/annurev.cellbio.12.1.181. [DOI] [PubMed] [Google Scholar]
- 7.Allen JM, Seed B. Isolation and expression of functional high-affinity receptor complementary DNAs. Science. 1989;243:378. doi: 10.1126/science.2911749. [DOI] [PubMed] [Google Scholar]
- 8.Graziano RF, Fanger MW. FcγRI and FcγRII on monocytes and granulocytes are cytotoxic trigger molecules for tumor cells. J Immunol. 1987;138:3536. [PubMed] [Google Scholar]
- 9.Fanger MW, Shen L, Graziano RF, Guyre PM. Cytotoxicity mediated by human Fc receptors for IgG. Immunol Today. 1989;10:92. doi: 10.1016/0167-5699(89)90234-X. [DOI] [PubMed] [Google Scholar]
- 10.Ely P, Wallace PK, Givan AL, Guyre PM, Fanger MW. Bispecific-armed, interferon gamma-primed macrophage-mediated phagocytosis of malignant non-Hodgkin’s lymphoma. Blood. 1996;87:3813. [PubMed] [Google Scholar]
- 11.Harris P, Ralphp Human leukemic models of myelomonocytic development: a review of the HL-60 and U937 cell lines. J Leukocyte Biol. 1985;37:407. doi: 10.1002/jlb.37.4.407. [DOI] [PubMed] [Google Scholar]
- 12.Sheth B, Dransfield I, Partridge LJ, Barker MD, Burton DR. Dibutyryl cyclic AMP stimulation of a monocytic cell line, U937: a model for monocyte chemotaxis and Fc receptor-related functions. Immunology. 1988;63:483. [PMC free article] [PubMed] [Google Scholar]
- 13.Davis W, Sage SO, Allen JM. Cytosolic calcium elevation in response to Fc receptor cross-linking in undifferentiated and differentiated U937 cells. Cell Calcium. 1994;16:29. doi: 10.1016/s0143-4160(05)80005-3. [DOI] [PubMed] [Google Scholar]
- 14.Floto RA, Somasundram B, Mahaut-Smith MP, Allen JM. IgG-induced Ca2+ oscillations in differentiated U937 cells: a study using laser scanning confocal microscopy and co-loaded Fluo3 and Fura-Red. Cell Calcium. 1995;18:377. doi: 10.1016/0143-4160(95)90053-5. [DOI] [PubMed] [Google Scholar]
- 15.Melendez A, Floto RA, Cameron AJ, Gillooly DJ, Harnett MM, Allen JM. A molecular switch changes the signalling pathway used by the FcγRI antibody receptor to mobilise calcium. Current Biology. 1998;8:210. doi: 10.1016/s0960-9822(98)70085-5. [DOI] [PubMed] [Google Scholar]
- 16.Melendez A, Floto RA, Gillooly DJ, Harnett MM, Allen JM. FcγRI coupling to phospholipase D initiates sphingosine kinase mediated calcium mobilization and vesicular trafficking. J Biol Chem. 1998;273:9393. doi: 10.1074/jbc.273.16.9393. [DOI] [PubMed] [Google Scholar]
- 17.Briscoe CP, Plevin R, Wakelam MJ.O. Rapid desensitization and resensitization of bombesin stimulated phospholipase D activity in Swiss 3T3 cell. Biochem J. 1994;298:61. doi: 10.1042/bj2980061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Plevin R, Cook SJ, Palmer S, Wakelam MJ.O. Multiple sources of sn-1,2-diacylglycerol in platelet-derived-growth-factor-stimulated Swiss 3T3 fibroblasts. Evidence for activation of phosphoinositidase C and phosphatidylcholine-specific phospholipase D. Biochem J. 1991;279:559. doi: 10.1042/bj2790559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nishizuka Y. Intracellular signaling by hydrolis of phospholipids and activation of protein Kinase C. Science. 1992;258:607. doi: 10.1126/science.1411571. [DOI] [PubMed] [Google Scholar]
- 20.Edberg JC, Lin C-T, Lau D, Unkeless JC, Kimberly RP. The Ca2+ Dependence of Human Fcγ Receptor-initiated Phagocytosis. J Biol Chem. 1995;270:22301. doi: 10.1074/jbc.270.38.22301. [DOI] [PubMed] [Google Scholar]
- 21.Zheng L, Zomerdjijk TP.L, Aarnoudse C, Van Furth R, Nibbering PH. Role of protein kKinase C isozymes in Fcγ receptor-mediated intracellular killing of Staphylococcus aureus by human monocytes. J Immunol. 1995;155:776. [PubMed] [Google Scholar]
- 22.Karimi K, Lennartz MR. Protein kinase C activation precedes arachidonic acid release during IgG-mediated phagocytosis. J Immunol. 1995;155:5786. [PubMed] [Google Scholar]
- 23.Zhelesnyak A, Brown E. Immunoglobulin-mediated phagocytosis by human monocytes requires protein kinase C activation. J Biol Chem. 1992;267:12042. [PubMed] [Google Scholar]
- 24.Hug H, Sarre TF. Protein kinase C isoenzymes: divergence in signal transduction? Biochem J. 1993;291:329. doi: 10.1042/bj2910329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dekker LV, Parker PJ. Protein kinase C – a question of specificity. TIBS. 1994;19:73. doi: 10.1016/0968-0004(94)90038-8. [DOI] [PubMed] [Google Scholar]
- 26.Nishizuka Y. Studies and perspectives of protein kinase C. Science. 1986;233:305. doi: 10.1126/science.3014651. [DOI] [PubMed] [Google Scholar]
- 27.Nishizuka Y. The molecular heterogeneity of protein kinase C and its implications for cellular regulation. Nature. 1988;334:661. doi: 10.1038/334661a0. [DOI] [PubMed] [Google Scholar]
- 28.Kazanietz MG, Areces LB, Bahador A, et al. Characterization of ligand and substrate specificity for the calcium-dependent and calcium-independent protein kinase C isozymes. Mol Pharmacol. 1993;44:298. [PubMed] [Google Scholar]
- 29.Bunce CM, Thick JA, Lord JM, Mills D, Brown G. A rapid procedure for isolating hemopoietic cell nuclei. Anal Biochem. 1988;175:67. doi: 10.1016/0003-2697(88)90362-4. [DOI] [PubMed] [Google Scholar]
- 30.Divecha N, Banfic H, Irvine RF. The polyphosphoinositide cycle exists in the nuclei of Swiss 3T3 cells under the control of a receptor (for IGF-I) in the plasma membrane, and stimulation of the cycle increases nuclear diacylglycerol and apparently induces translocation of protein kinase C to the nucleous. EMBO J. 1991;10:3207. doi: 10.1002/j.1460-2075.1991.tb04883.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Deehan MR, Harnett MM, Harnett W. A filarial nematode secreted product differentially modulates expression and activation of protein kinase C isoforms in B lymphocytes. J Immunol. 1997;159:6105. [PubMed] [Google Scholar]
- 32.Berridge MJ, Irvine RF. Inositol phospholipids and cell signaling. Nature. 1989;341:197. doi: 10.1038/341197a0. [DOI] [PubMed] [Google Scholar]
- 33.Chang Z-L, Beezhold DH. Protein kinase C activation in human monocytes: regulation of PKC isoforms. Immunology. 1993;80:360. [PMC free article] [PubMed] [Google Scholar]
- 34.Pettitt TR, Martin A, Horton T, Liosis C, Lord JM, Wakelam MJ.O. Diacylglycerol and phosphayidate generated by phospholipases C and D, respectively, have distinct fatty acid Compositions and Functions. J Biol Chem. 1997;272:17354. doi: 10.1074/jbc.272.28.17354. [DOI] [PubMed] [Google Scholar]
- 35.Melendez AJ, Gillooly DJ, Harnett MM, Allen JM. Aggregation of the human high affinity immunoglobulin G receptor (Fc-gamma-RI) activates both tyrosine kinase and G-protein coupled phosphoinositide 3-kinase isoforms. Proc Natl Acad Sci USA. 1998;95:2169. doi: 10.1073/pnas.95.5.2169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nakanishi H, Brewer KA, Exton JH. Activation of the ζ isozyme of protein kinase C by phosphatidylinositol 3,4,5–trisphosphate. J Biol Chem. 1993;268:13. [PubMed] [Google Scholar]
- 37.Toker A, Meyer M, Reddy KK, et al. ACTIVATION of protein kinase C family members by the novel polyphosphoinositides PtdIns,4–P2 and PtdIns,4,5–P3. J Biol Chem. 1994;269:32358. [PubMed] [Google Scholar]
- 38.Akimoto K, Takahashi R, Moriya S, et al. EGF or PDGF receptors activate atypical PKCλ through phosphatidylinositol 3–kinase. EMBO J. 1996;15:788. [PMC free article] [PubMed] [Google Scholar]
- 39.Standaert ML, Galloway L, Karnam P, Bandyopadhyay G, Moscat J, Farese RV. Protein kinase C–ζ as a downstream effector Phosphatidylinositol 3–Kinase during Insulin Stimulation in Rat Adipocytes. J Biol Chem. 1997;272:30075. doi: 10.1074/jbc.272.48.30075. [DOI] [PubMed] [Google Scholar]
- 40.Ernst LK, Duchemin A-M, Anderson CL. Association of the high-affinity receptor for IgG (FcγRI) with the γ subunit of the IgE receptor. Proc Natl Acad Sci USA. 1993;90:6023. doi: 10.1073/pnas.90.13.6023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Scholl PR, Geha RS. Physical association between the high-affinity receptor for IgG (FcγRI) and the γ subunit of the high-affinity IgE receptor (FcεRIγ) Proc Natl Acad Sci USA. 1993;90:8847. doi: 10.1073/pnas.90.19.8847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ra C, Blank M-H, Kinet J-P. A macrophage Fcγ receptor and the mast cell receptor for immunoglobulin E share an identical subunit. Nature. 1989;341:752. doi: 10.1038/341752a0. [DOI] [PubMed] [Google Scholar]
- 43.Germano P, Gomez J, Kazanietz MG, Blumberg PM, Rivera J. Phosphorylation of the γ chain of the high affinity receptor for immunoglobulin E by receptor-associated protein kinase C-δ. J Biol Chem. 1994;269:23102. [PubMed] [Google Scholar]
- 44.Durden DL, Rosen H, Cooper JA. Serine/threonine phosphorylation of the γ-subunit after activation of the high-affinity Fc receptor for immunoglobulin G. Biochem J. 1994;299:569. doi: 10.1042/bj2990569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Davis W, Halliwell EL, Sage SO, Allen JM. Increased capacity for store regulated calcium influx in U937 cells differentiated by treatment with dibutyryl cAMP. Cell Calcium. 1995;17:345. doi: 10.1016/0143-4160(95)90108-6. [DOI] [PubMed] [Google Scholar]
- 46.Floto RA, Mahaut–smith MP, Allen JM, Somasundaram B. Differentiation of the human monocytic cell line U937 results in an upregulation of the calcium release–activated current, ICRAC. J Physiol 495. 1996;2:331. doi: 10.1113/jphysiol.1996.sp021597. [DOI] [PMC free article] [PubMed] [Google Scholar]