Abstract
We examined the role of CD8+ T cells in a Brown–Norway rat model of asthma, using a monoclonal antibody to deplete CD8+ T cells. Ovalbumin (OA)-sensitized animals were given anti-CD8 antibody (0·5 mg/rat) intravenously 1 week prior to exposure to 1% OA aerosol and were studied 18–24 hr after aerosol exposure. Following administration of anti-CD8 antibody, CD8+ cells were reduced to <1% of total lymphocytes in whole blood and in spleen. In sensitized animals, OA exposure induced bronchial hyper-responsiveness (BHR), accumulation of eosinophils, lymphocytes and neutrophils in bronchoalveolar lavage (BAL) fluid, and also an increase in tissue eosinophils and CD2+, CD4+ and CD8+T cells in airways. Anti-CD8 antibody caused a further increase in allergen-induced BHR (P < 0·03, compared with sham-treated animals), together with a significant increase in eosinophil number in BAL fluid (P < 0·05). While CD2+ and CD4+ T cells in airways were not affected by anti-CD8 treatment, the level of CD8+ T cells was significantly reduced in sensitized, saline-exposed animals (P < 0·04, compared with sham-treated rats), and sensitized and OA-challenged rats (P < 0·002, compared with sham-treated rats). Using reverse transcription–polymerase chain reaction, an increase of T helper (Th)2 cytokine [interleukin (IL)-4 and IL-5], and also of Th1 cytokine [interferon-γ (IFN-γ) and IL-2], mRNA in the lung of sensitized and OA-exposed animals was found; after CD8+ T-cell depletion, Th1 cytokine expression was significantly reduced (P < 0·02), while Th2 cytokine expression was unchanged. CD8+ T cells have a protective role in allergen-induced BHR and eosinophilic inflammation, probably through activation of the Th1 cytokine response.
INTRODUCTION
There is increasing evidence of an important role for T lymphocytes in the orchestration of eosinophilic inflammation in asthma. CD4+ T lymphocytes have been localized to the airway mucosa of patients with asthma,1 and have been shown to express a T helper 2 (Th2) profile of cytokines such as interleukin (IL)-4 and IL-5,2 which are important for the regulation of immunoglobulin E (IgE) production and the differentiation and function of eosinophils, respectively.3,4 Adoptive transfer of allergen-specific CD4+ T cells can induce bronchial hyper-responsiveness (BHR) and airway eosinophilia in the rat.5 By contrast, less is known about CD8+ T cells, which are increased in numbers in the airway submucosa of sensitized Brown–Norway rats exposed to ovalbumin (OA).6,7 A protective role for CD8+ T cells in allergen-induced airway response has been suggested.8 Elimination of CD8+ T cells in OA-sensitized rats can result in an increased expression of the Th2-derived cytokines, IL-4, IL-5 and IL-10, from splenocytes in vitro,9 resulting in a persistently high IgE response in vivo.10 Adoptively transferred allergen-specific CD8+ T cells into sensitized mice prevented allergen-induced BHR.11 Thus, CD8+ T cells may have a protective role in allergen-induced bronchial responses.
The role of endogenous CD8+ T cells in allergen-induced responses has been previously examined in Sprague–Dawley rats.12,13 Depletion of CD8+ T cells using an anti-CD8 antibody enhanced late-phase response to allergen but no increase in BHR was observed. The Sprague–Dawley rat is a low IgE responder, with a low CD4+/CD8+ T-cell ratio.12,14 It is possible that the effect of depletion of CD8+ T cells may be different when examined in a different rat strain, such as the Brown–Norway rat, which is a high IgE producer and has a higher CD4+/CD8+ T-cell ratio.14 We therefore used a mouse antirat OX-8 monoclonal cytotoxic antibody to deplete CD8+ T cells in order to study the role of endogenously recruited CD8+ T cells in allergen-induced BHR and inflammation, and cytokine expression, in Brown–Norway rats. We postulated that, because of the intense CD8+ T-cell infiltration we have previously observed after allergen exposure in this model,6 CD8+ T cells may modulate allergen-induced BHR and eosinophilia.
MATERIALS AND METHODS
Animals, sensitization procedures and allergen exposure
Pathogen-free inbred male Brown–Norway rats (Harlan Olac Ltd., Bicester, UK) (200–250 g, 9–13 weeks old) were injected intraperitoneally (i.p.), on three consecutive days, with a suspension, in 0·9% (wt/vol) saline, of 1 ml of 1 mg OA (Grade V, salt-free; Sigma, Dorset, UK) in 100 mg Al(OH)3 (BDH, Dorset, UK). OA aerosol exposure to rats was performed in a 6·5 l Plexiglas chamber connected to a DeVilbiss Pulmon Sonic nebuliser (model no. 2512, DeVilbiss Health Care UK Ltd, Middlesex, UK) that generated an aerosol mist pumped into the exposure chamber by the airflow supplied by a small animal ventilator (Harvard Apparatus Ltd, Kent, UK) set at 60 strokes/min with a pumping volume of 10 ml. The exposure was 15 min to 1% (wt/vol) OA or 0·9% saline.
Protocol
Four groups of animals were treated as follows:
Non-sensitized and OA-exposed animals (group NO, n =7)
Animals received a 0·9% saline (1 ml/kg) i.p. injection during the sensitization period, and were exposed 21 days later to 1% OA aerosol.
Sensitized, saline-exposed and anti-CD8 antibody-treated animals (SScd8, n =4)
Sensitized animals were injected with an anti-CD8 monoclonal cytotoxic antibody (MRC OX-8, mouse antirat CD8 immunoglobulin G (IgG); Serotec, Oxford, UK), 0·5 mg/rat intravenously (i.v.) via the caudal vein, on day 14 and were exposed, 7 days later, to 0·9% saline aerosol for 15 min. The anti-CD8 monoclonal antibody (mAb), MRC OX-8 has been shown to be a very potent depleting antibody in vivo.15
Sensitized, OA-exposed and sham-treated animals (SOsham, n =10)
Sensitized animals were injected with normal mouse IgG (Serotec), 0·5 mg/rat i.v., and were exposed to 1% OA aerosol 1 week later.
Sensitized, OA-exposed and anti-CD8 antibody-treated animals (SOcd8, n =10)
The procedures were similar as for the SOsham group, except that anti-CD8 antibody was injected (as described above for the SScd8 group) 1 week before exposure to OA aerosol.
All rats were studied 18–24 hr after exposure to either 1% OA or 0·9% NaCl aerosol.
Measurement of airway responsiveness to acetylcholine (ACh)
Airway responsiveness was measured as previously described.16 In brief, anaesthetized, tracheostomized and ventilated rats were monitored for airflow with a pneumotachograph (model F1l; Mercury Electronics Ltd, Glasgow, UK) connected to a transducer (model FCO40;±20 mm H2O; Furness Controls Ltd, Sussex, UK), transpulmonary pressure via a transpleural catheter connected to a transducer (model FCO40;±1000 mm H2O; Furness Controls Ltd) and blood pressure via carotid artery catheterization. Lung resistance (RL) was simultaneously calculated using software (LabView, National Instruments, Austin, TX) on a Macintosh II computer (Apple Computer Inc., Cupertino, CA). Aerosol generated from increasing half log10 concentrations of acetylcholine chloride (ACh) (Sigma) was administered by inhalation (45 breaths of 10 ml/kg stroke volume) with an initial concentration of 10−3·5 mol/l and a maximal concentration of 0·1 mol/l. The concentration of ACh needed to increase RL200% above baseline (PC200) was calculated by interpolation of the log concentration–lung resistance curve.
Bronchoalveolar lavage and cell counting
This is also described in detail elsewhere.16 Briefly, after an overdose of anaesthetic, rats were lavaged with 0·9% sterile saline (total volume 20 ml) via the endotracheal tube. Total cell counts, viability and differential cell counts from cytospin preparations stained by May–Grünwald stain were determined under an optical microscope (Olympus BH2, Olympus Optical Company Ltd, Tokyo, Japan). At least 500 cells were counted and identified as macrophages, eosinophils, lymphocytes and neutrophils, according to standard morphology, under ×400 magnification.
Flow cytometry of peripheral blood lymphocytes and splenocytes
Fifty microlitres of anticoagulated whole blood was deprived of red-blood cells by incubation with 2 ml of Becton-Dickinson fluorescence-activated cell sorter (FACS) lysis solution (1:10; Becton-Dickinson, Cowley, UK). Splenocytes were obtained by pressing spleen tissue through 70-μm nylon filters (Becton-Dickinson) into chilled phosphate-buffered saline (PBS). Approximately 5×105 cells in 50 μl were incubated with 5 μl of antibodies of choice at room temperature for 15 min, and then washed three times with PBS containing 0·1% bovine serum albumin (BSA) before fixation in 1% paraformaldehyde. Direct-conjugated mAb to fluorochrome fluorescein isothiocyanate (FITC) or phycoerythrin (PE) were used throughout. Mouse antirat CD3-FITC, CD4-FITC, CD4-PE, CD8-PE, and the relevant isotype controls (IgG1-FITC, IgG2a-PE) were obtained from Serotec Ltd. Flow cytometric analysis was performed with a FACScan (Becton-Dickinson). Lymphocytes were gated on forward scatter/side scatter (FSC/SSC) dot-plots and at least 5000 events were counted. Percentages of CD4+ and CD8+ cells were calculated relative to the total number of lymphocytes counted. The percentages of CD4− CD3+/CD3+ cells, which represent all CD4− T cells normally with a majority of CD8+ T cells, were also calculated in order to confirm depletion of CD8+ cells in peripheral blood and spleen instead of merely blocking CD8 surface antigen by anti-CD8 antibody.
Collection of lung tissues
After opening of the thoracic cavity and removal of the lungs, the right lung without major vascular and connective tissues was cut into pieces and snap-frozen in liquid nitrogen (BOC, Luton, UK), and then stored at −80° for later assays for mRNA expression. The left lung was inflated with 3 ml of saline/optimal cutting temperature compound (OCT) (1:1). Two blocks of 0·5 cm3 were cut from the left lung around the major bronchus, embedded in OCT medium (Raymond A. Lamb, London, UK) and snap-frozen in melting isopentane (BDH) and liquid nitrogen. Cryostat sections (6 μm) of the tissues were cut, air-dried, fixed in acetone and then air-dried again, wrapped in aluminium foil and stored at −80° for later immunohistochemical studies.
Immunohistochemistry
For detection of eosinophils, we used a mouse IgG1 mAb against human major basic protein (MBP), BMK-13, which has been shown to be both sensitive and specific for staining rat eosinophils in frozen sections.6 The cryostat sections were incubated with BMK-13 at a dilution of 1:50 for 30 min at room temperature. After labelling with the second antibody, rabbit antimouse IgG, positively stained cells were visualized with alkaline phosphatase–antialkaline phosphatase.
For staining of CD2+, CD4+ and CD8+ T lymphocytes in tissues, sections were incubated with mouse antirat mAb (Pharmingen, Cambridge Bioscience, Cambridge, UK), antirat CD2 (pan T-cell marker), antirat CD4 and anti-CD8 antibodies at a dilution of 1:500 for 1 hr. Biotin goat antimouse antibody (Pharmingen) and avidin phosphatase (DAKO Ltd, High Wycome, UK), at a dilution of 1:200, were applied for 30 min in turn.
For all tissue sections, alkaline phosphatase was developed as a red stain after incubation with Naphthol AS-MX phosphate in 0·1 m trismethylamine-HCl buffer (pH 8·2), containing levamisole to inhibit endogenous alkaline phosphatase, and 1 mg/ml Fast Red-TR salt (Sigma). Then, sections were counterstained with Harris Hematoxylin (BDH) and mounted in Glycergel (DAKO). System and specificity controls were carried out for all staining. Slides were read in a coded, randomized, blind fashion, using an Olympus BH2 microscope. Cells within 175 μm beneath the basement membrane were counted. The submucosal area was quantified with the aid of a computer-assisted graphic tablet visualized by a sidearm attached to the microscope. Counts were expressed as cells/mm2 of cross-sectional subepithelial area.
Reverse transcription–polymerase chain reaction (RT–PCR)
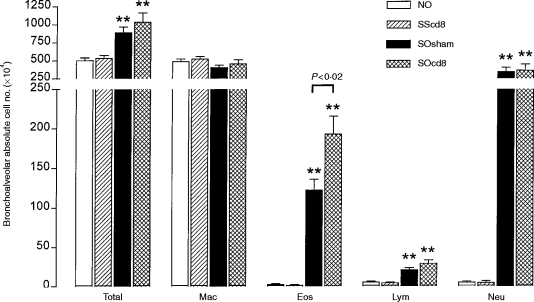
Total RNA from lung tissue was extracted according to the method of Chomczynski & Sacchi.17 The yield of RNA was measured by optical density at 260 nm in a spectrophotometer. The RNA was analysed on a 1·5% agarose/formaldehyde gel in order to check for degradation, and stored at −80° until later use. After denaturation at 70° for 5 min, 1 μg of total RNA was used for reverse transcription in a 20-μl reaction volume containing 1×avian myeloblastic virus (AMV) buffer (50 mm Tris-HCl, pH 8·3, 50 mm KCl, 10 mm MgCl2, 10 mm DTT, 0·5 mm spermidine), 1 mm of the four deoxynucleotide triphosphates (dNTP), comprising deoxyadenosine triphosphate (dATP), deoxycytidine triphosphate (dCTP), deoxyguanosine triphosphate (dGTP) and thymidine 5′-triphosphate (dTTP), ribonuclease inhibitor (32 U), 0·2 μg random primer pd(N)6 sodium salt (Pharmacia, Milton Keynes, UK) and 8 U AMV reverse transcriptase (all reagents apart from the random primer were obtained from Promega, Southampton, UK) at 42° for 60 min. The complementary DNA (cDNA) product was diluted to 100 μl in water. PCR was performed on 5 μl of diluted cDNA product in a total volume of 25 μl with a final concentration of 1×KCl or NH4 buffer containing 1·5 mm MgCl2, 0·2 mm dNTP, 0·2 μg each of sense and antisense primers and 1 U Taq polymerase (Bioline, London, UK), in a thermal cycler. The primers (Table 1) were designed according to published sequences.18–23 The PCR reagents were overlaid with mineral oil and amplification was carried out using a multiwell thermal cycler through 20–40 cycles of denaturation at 94° for 30 seconds, annealing at individual temperatures for 30 seconds and extension at 72° for 30 seconds, followed by a final extension at 72° for 10 min. The optimal PCR conditions, in terms of suitable buffer, annealing temperature and number of cycles, were determined by PCR with pooled cDNA from all samples. Annealing temperatures were 62° for glyceraldehyde-3-phosphate dehydrogenase (GAPDH), IL-4 and interferon-γ (IFN-γ), 58° for IL-5, and 65° for IL-2 and IL-10. Serial sampling every two cycles through 20–42 cycles was used to determine the exponential phase of the product-amplification curve. The cycle numbers we used for PCR were 26 for GAPDH, 38 for IL-2, IL-4 and IL-5, and 34 for IL-10 and IFN-γ.
Table 1.
Sense and antisense primers used for reverse transcription–polymerase chain reaction (RT–PCR)
GAPDH, glyceraldehyde-3-phosphate dehydeogenase; IFN-γ, interferon-γ, IL, interleukin.
Southern blotting and Cerenkov counting
Ten microlitres of each PCR product was size-fractionated and visualized with ethidium bromide (Sigma) following 1·5% agarose-gel electrophoresis; this was followed by Southern blotting to Hybond-N membrane (Amersham, Bucks, UK)24 and hybridization to the appropriate cloned cDNA in order to confirm the identity of the product and, because all primer pairs cross at least one intron, to check for possible genomic contamination. Hybridizations were carried out at 65° overnight with the appropriate cloned cDNA, which had been 32P labelled, in 6×standard saline citrate (SSC), 10×Denhardt’s solution (0·2% wt/vol each of BSA, Ficoll and polyvinylpyrrolidone), 5 mm EDTA, 0·5% sodium dodecyl sulphate (SDS), 0·2% sodium pyrophosphate and 100 μg/ml sonicated salmon-sperm DNA. In addition, 5 μl of each PCR reaction was dot-blotted on to Hybond-N membrane and also hybridized to a cDNA probe.24 Dot-blots were excised and radioactivity measured by Cerenkov counting. All measurements were made below the saturation level of a Packard 1900CA liquid scintillation analyser (Packard Instrumentation BV, Groningen, the Netherlands). Results were generated from the counting of dot-blots and expressed as a ratio of cytokine to GAPDH count, the latter used as an internal control.
Data analysis
Data were presented as mean±SEM. For multiple comparison of different groups, the Kruskal–Wallis test for analysis of variance was used. If the Kruskal–Wallis test for analysis of variance was significant, we then used the Mann–Whitney U-test for comparison between two individual groups. Data analysis was performed utilizing SPSS for Windows statistical software package. A P-value of < 0·05 was considered to be significant.
RESULTS
Effect of anti-CD8 antibody on T-cell number
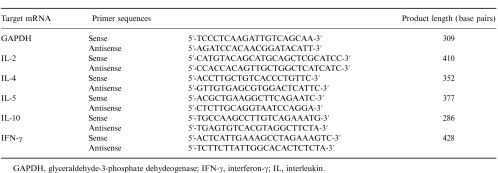
In peripheral blood, anti-CD8 antibody caused a marked reduction in the number of CD8+ T cells but had no effect on CD4+ T-cell counts. In the spleen, OA exposure of sensitized rats led to a significant decrease in the number of CD4+ T cells (P < 0·04 compared with sensitized and saline-exposed rats), but anti-CD8 antibody had no effect on the number of CD4+ T cells. By contrast, anti-CD8 antibody caused a significant reduction in the number of CD8+ T cells in both the sensitized and saline-exposed, and the sensitized and OA-exposed rats (Fig. 1).
Figure 1.
Flow cytometric analysis of CD4+ and CD8+ T lymphocytes in peripheral blood (a) and spleen (b) for four different groups of rats. NO: non-sensitized and OA exposed, n =7; SScd8: sensitized, anti-CD8 antibody treated and saline exposed, n =4; SOsham: sensitized, mouse immunoglobulin G (IgG) treated and 1% ovalbumin (OA) challenged, n =10; and SOcd8: sensitized, anti-CD8 antibody treated and OA exposed, n =10. Cell number is expressed as percentage of total lymphocytes measured by expression of CD3+ T cells. There was a significant decrease in the proportion of splenic CD4+ T cells in the sensitized and OA-exposed rats. CD8+ cells were reduced to less than 1% by anti-CD8 treatment in both peripheral blood and spleen. †P < 0·03 compared with groups SScd8 and SOcd8; **P < 0·04 compared with groups NO and SScd8. Data are shown as mean±SEM.
In both peripheral blood and spleen, anti-CD8 antibody reduced the number of CD4− T cells, which consisted mostly of CD8+ cells, from 6 to 10% to 0·5–1·3%, indicating the absence of anti-CD8 antibody-conjugated CD4−CD8+ T cells. This indicates that there was a cytolytic depletion of CD8+ T cells.
Bronchial responsiveness to ACh
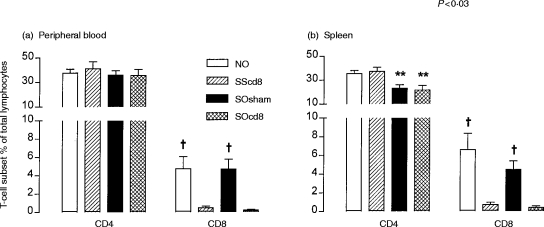
There was no significant difference in baseline RL among the four groups. Anti-CD8 antibody had no effect on the baseline responsiveness to ACh. Sensitised OA-exposed rats showed a significant increase in mean logPC200 compared with non-sensitized OA-exposed rats (2·19±0·05 versus 1·62±0·09, P < 0·001). However, there was a further increase in bronchial responsiveness to ACh in sensitized OA-exposed rats pretreated with anti-CD8 antibody (P < 0·03, compared with sensitized OA-exposed rats treated with IgG control antibody) (Fig. 2). This increase was reflected in the significantly greater responses of RL to ACh concentrations of 10−2·5 and 10−2 m (Fig. 2).
Figure 2.
(a) Mean percentage increase in lung resistance to increasing concentrations of acetylcholine (ACh) for four different groups of rats, described in the legend to Figure 1 (NO, SScd8, Sosham and SOcd8). The concentration–response curves are significantly shifted left for groups SOsham and SOcd8 by comparison with group NO. There was no effect of anti-CD8 antibody on non-sensitized ovalbumin (OA)-exposed animals, but the antibody further increased bronchial responsiveness of sensitized, allergen-exposed rats. *P < 0·05 for groups SOsham or SOcd8 compared with groups NO and SScd8; †P < 0·03 for SOsham compared with group SOcd8. (b) Mean −logPC200, which is the negative logarithm of the provocative concentration of ACh needed to increase baseline lung resistance by 200%, for the four groups of rats detailed in Figure 1. Anti-CD8 antibody treatment significantly enhanced allergen-induced increase in −logPC200 (P < 0·03). φP < 0·005 compared with groups NO and SScd8. Data are shown as mean±SEM.
Cell inflammatory response
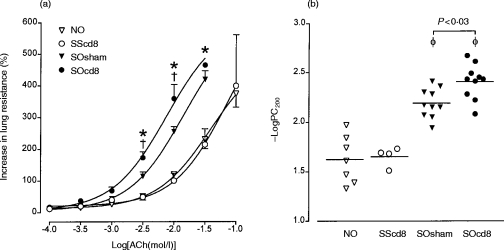
In bronchoalveolar lavage fluid, there was a significant increase in the numbers of eosinophils, lymphocytes and neutrophils recovered in bronchoalveolar (BAL) fluid of sensitized rats exposed to OA compared with sensitized rats exposed to saline (P < 0·005). Anti-CD8 antibody did not alter the cell profile of sensitized rats exposed to saline. However, antibody-treated, sensitized, OA-exposed rats showed a selective, significant, further increase in eosinophil count in BAL fluid (P < 0·04; Fig. 3).
Figure 3.
Mean numbers of total cells, macrophages (Mac), eosinophils (Eos), lymphocytes (Lym) and neutrophils (Neu) in bronchoalveolar (BAL) fluid from four different groups of rats (described in the legend to Figure 1). Anti-CD8 antibody treatment further enhanced the increase in eosinophil number induced by allergen exposure of sensitized rats in BAL fluid (P < 0·02), but had no effect on influx of lymphocytes and neutrophils. **P < 0·04 compared with groups NO and SScd8. Data are shown as mean±SEM.
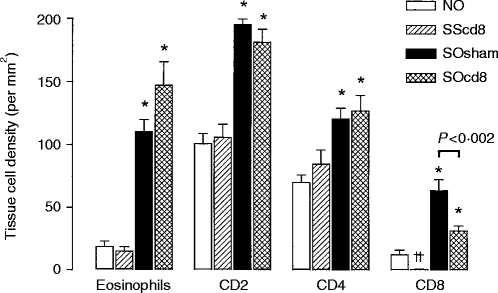
In the airways, the anti-CD8 antibody caused a near-complete suppression of the number of CD8+ T cells in sensitized, saline-exposed rats. Allergen exposure of sensitized rats resulted in a significant increase in eosinophil counts, and in the number of CD2+T cells, CD4+T cells and CD8+ T cells. The CD8+ T-cell count increase induced by allergen exposure was significantly decreased from 63·9±8·8 to 31·7±4·0/mm2 by the anti-CD8 antibody (P < 0·002; Fig. 4). However, there was a non-significant increase in eosinophil count in airways from 110·3±9·6/mm2 to 147·1±18·7/mm2 after anti-CD8 antibody treatment of sensitized OA-exposed rats.
Figure 4.
Mean eosinophil and T-lymphocyte subset (CD2+, CD4+ and CD8+) counts in airway submucosa (expressed per mm2) of the four groups of rats described in the legend to Figure 1. Allergen exposure of sensitized rats increased the infiltration of eosinophils, as well as of T-cell subsets (CD2+, CD4+ and CD8+). There was a reduction in CD8+ cell counts after anti-CD8 antibody treatment in both saline and ovalbumin (OA)-exposed animals; however, in the sensitized OA-exposed rats, anti-CD8 antibody did not completely suppress CD8+ T cells in tissue. CD2+ and CD4+ T-cell counts were not significantly affected, and eosinophil counts were non-significantly increased after anti-CD8 antibody treatment of sensitized and OA-exposed rats. *P < 0·05 as SOsham or SOcd8 compared with NO and SScd8 groups; ††P < 0·02 compared with the NO group. Data are shown as mean±SEM.
Cytokine expression in lungs
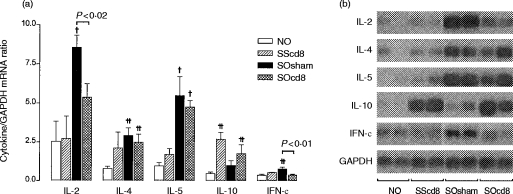
In sensitized saline-exposed rats (compared with non-sensitized OA-exposed rats) the anti-CD8 antibody caused a significant increase in IL-10 mRNA expression and a non-significant increase in IL-4 and IL-5 mRNA expression, without affecting the expression of IL-2 and IFN-γ mRNA (Fig. 5). OA exposure of sensitized rats induced a significant increase in IL-2, IL-4, IL-5 and IFN-γ mRNA expression, and a non-significant increase in IL-10 mRNA expression. The anti-CD8 antibody significantly inhibited the increase in IL-2 and IFN-γ mRNA expression following allergen exposure of sensitized rats, while having no effect on IL-4 and IL-5 mRNA expression; IL-10 mRNA expression, by contrast, showed a non-significant increase.
Figure 5.
Mean interleukin (IL)-2, IL-4, IL-5, IL-10 and interferon-γ (IFN-γ) mRNA expression in rat lung, expressed as a ratio to glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA (a), as determined by reverse transcription–polymerase chain reaction (RT–PCR), followed by Southern blot analysis. Representative bands from Southern blot analysis are shown in (b). The expression was obtained on a radioactive probe-hybridized dot-blot of the PCR products. There was a significant increase in the expression of IL-2, IL-4, IL-5 and IFN-γ following allergen exposure of sensitized rats (group SOsham). Anti-CD8 antibody significantly reduced the increased expression of IL-2 and IFN-γ mRNA, but was without effect on IL-4 and IL-5 mRNA expression. The IL-10 mRNA expression was increased following both saline and OA exposure of sensitized rats after anti-CD8 antibody treatment. †P < 0·03 compared with groups NO and SScd8; ††P < 0·02 compared with group NO. Data are shown as mean±SEM.
DISCUSSION
In order to elucidate the contribution of CD8+ T cells to the regulation of allergen-induced airway responses, we studied the effect of a mouse antirat OX-8 mAb, which is cytotoxic to CD8+ T cells, in the high IgE-responder Brown–Norway rat strain. Depletion of CD8+ T cells with this antibody enhanced allergen-induced BHR and eosinophil numbers in BAL fluid in the sensitized rats. These observations indicate that CD8+ T cells have a major role in the regulation of allergen-induced bronchial hyper-responsiveness and eosinophilic inflammation. The profile of expression of cytokines in the lungs shows a reduction in the gene expression of Th1 cytokines (IL-2 and IFN-γ), while the level of Th2 cytokines, IL-4 and IL-5, remain unchanged following CD8+ T-cell depletion. The enhanced allergen-induced bronchial hyper-responsiveness and eosinophilia may result from a reduction in the expression of Th1 cytokines induced by CD8+ T-cell depletion.
The technique of in vivo cell depletion was used in order to investigate the role of CD8+ T cells. Using the mouse antirat anti-OX8 mAb (MRC OX-8), the numbers of CD8+ T cells were reduced to very low levels in the circulating blood and spleen of Brown–Norway rats, as measured by immunofluorescence staining. The anti-CD8 antibody did not completely suppress the increase in CD8+ T cells accumulating in the airways following allergen challenge, but caused a significant inhibition of this increase. Similar depletion of CD8+ T cells has been achieved by other investigators.13 In Sprague–Dawley rats, such depletion led to an increase in the late response after allergen exposure, associated with an increase in BAL macrophages, neutrophils and lymphocytes.12 However, despite the enhancement of airway inflammation, no change in airway responsiveness was observed.13 It is possible that the contrasting results observed between the Sprague–Dawley and Brown–Norway rats is related to the relatively low CD4+/CD8+ ratios found in the former strain, emphasizing the relative contribution of CD8+ T cells to bronchial hyper-responsiveness. Another reason was related to the regulation of IgE responses by CD8+ T cells,25 such that in the high-IgE Brown–Norway rat responder, a greater IgE response may be found following CD8+ T-cell depletion than in the low-IgE Sprague–Dawley rat.
Recently, rat alveolar macrophages were shown to express CD8, using the anti-CD8 antibody MRC OX-8, to detect the CD8α hinge region in over 60% of alveolar macrophages.26 Depletion of alveolar macrophages may lead to an increased immune response to inhaled antigens.27,28 However, in our study, depletion of alveolar CD8+ macrophages is unlikely because there was no change of their counts in BAL fluid. We cannot exclude the possibility that CD8+ macrophages comprised a proportion of tissue CD8+ cells in airways after allergen exposure of sensitized animals and following anti-CD8 treatment. Cross-linking CD8α with OX-8 up-regulated inducible NO synthase and NO production from alveolar macrophages.29 Whether such an effect could underlie the increase in eosinophil numbers and in bronchial hyper-responsiveness is not clear, but could be tested for by inhibition of inducible NO synthase.
There are a number of mechanisms by which CD8+ T cells may regulate airway eosinophilia and BHR. Transfer of purified spleen CD8+ T cells from ovalbumin-sensitized mice to sensitized recipients prevents the increase in airway responsiveness to electrical field stimulation that usually occurs in sensitized mice.11 It is possible that IFN-γ production by CD8+ T cells may have mediated this inhibitory effect. Such a mechanism would be supported by our current observations in the Brown–Norway rat. However, elimination of CD8+ T cells by ricin in rats resulted in increased airway eosinophilia in ricin-treated rats compared with control rats, but this was not accompanied by further increase in bronchial hyper-responsiveness.30 While ricin-induced CD8+ T-cell depletion was associated with a decreased capacity for splenic T cells to produce IFN-γ, there was also an increased ability to express IL-4 and IL-5 mRNA in the splenic T cells.9,31 In our current studies, depletion of CD8+ T cells in sensitized, saline-challenged rats resulted in an increased expression of IL-4, IL-5 and IL-10 mRNA in the lung, although this only achieved statistical significance with IL-10 mRNA. By contrast, following ovalbumin-challenge of sensitized rats, mRNA levels of IL-2, IL-4, IL-5 and IFN-γ showed a significant increase, while IL-10 mRNA did not. Depletion of CD8+ T cells prior to ovalbumin challenge led to a significant reduction of Th1-cytokine IL-2 and IFN-γ mRNAs, while increasing IL-10 mRNA expression but not that of IL-4 or IL-5 mRNA. Overall, these observations indicate that the reduction in mRNA levels of IL-2 and IFN-γ may be the most important changes specific to CD8+ T-cell depletion following allergen challenge, with the increase in IL-10 mRNA occurring with CD8+ T-cell depletion and allergen or saline exposure, indicating an inhibitory effect of CD8+ T cells on IL-10 expression.
The contributory role of CD8+ T cells to allergen-induced bronchial hyper-responsiveness and eosinophilia has also been demonstrated in studies where allergen-specific CD4+ and CD8+ T cells were transferred to recipient animals. While allergen-specific CD4+ T cells transferred bronchial hyper-responsiveness and airway eosinophilia to the Brown–Norway rat, CD8+ T cells did not.5,7 Transfer of purified spleen CD8+ T cells from ovalbumin-sensitized mice to sensitized recipients prevented the development of airway hyper-responsiveness following ovalbumin exposure, indicating a negative regulation of allergen-specific CD8+ T cells.11 In this model, IFN-γ was found to mediate the inhibitory function of CD8+ T cells. IFN-γ has been shown to prevent allergen-induced eosinophil infiltration in the mouse trachea, while anti-IFN-γ antibody increased eosinophil and CD4+ T-cell infiltration in the mouse trachea.32 Antigen-specific CD8+ T cells are known to produce IFN-γ,33 and circulating CD8+ T cells are a major source of IFN-γ in the rat.34 Thus, it is possible that CD8+ T cells may contribute to both allergen-induced airway eosinophilia and hyper-responsiveness through the production of IFN-γ.
There was a significant increase in the expression of IL-2 mRNA following allergen exposure, an effect that was inhibited by CD8+ T-cell depletion. Activated Th1 cells are a major source of IL-2,35 and IL-2 stimulates the growth and differentiation of T cells together with monocytes/macrophages.36 IL-2 is also able to induce eosinophilia, and administration of IL-2 to rats induces bronchial hyper-responsiveness and an increase in late-phase response.37–39 IL-2 caused an inflammatory response around the airways with a significant increase in eosinophils, lymphocytes and mast cells in the Brown–Norway rat.38 Therefore, the effects of CD8+ T-cell depletion could have been partly mediated through an inhibition of IL-2 expression.
In summary, CD8+ T cells contribute some protection against allergen-induced bronchial hyper-responsiveness and eosinophilia, probably by increasing the expression of Th1-derived cytokines such as IFN-γ and IL-2.
Glossary
Abbreviations
- ACh
acetylcholine
- BAL
bronchoalveolar lavage
- BHR
bronchial hyper-responsiveness
- FITC
fluorescein isothiocyanate
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- IFN
interferon
- IL
interleukin
- i.p
intraperitoneal
- OA
ovalbumin
- PBS
phosphate-buffered saline
- PC200
provocative concentration of acetylcholine needed to increase lung resistance by 200% above baseline
- PE
phycoerythrin
- RL
lung resistance
- RT–PCR
reverse transcription–polymerase chain reaction
- Th
T helper
REFERENCES
- 1.Azzawi M, Bradley B, Jeffery PK, et al. Identification of activated T lymphocytes and eosinophils in bronchial biopsies in stable atopic asthma. Am Rev Respir Dis. 1990;142:1407. doi: 10.1164/ajrccm/142.6_Pt_1.1407. [DOI] [PubMed] [Google Scholar]
- 2.Robinson DS, Hamid Q, Ying S, et al. Predominant Th2-like bronchoalveolar T-lymphocyte populations in atopic asthma. N Engl J Med. 1992;326:298. doi: 10.1056/NEJM199201303260504. [DOI] [PubMed] [Google Scholar]
- 3.Romagniani S. Regulation and deregulation of human IgE synthesis. Immunol Today. 1990;11:316. doi: 10.1016/s0167-5699(10)80004-0. [DOI] [PubMed] [Google Scholar]
- 4.Sanderson CJ. Interleukin-5, eosinophils and disease. Blood. 1992;79:3101. [PubMed] [Google Scholar]
- 5.Haczku A, MacAry P, Huang T-J, et al. Adoptive transfer of allergen-specific CD4+ T cells induces airway inflammation and hyperresponsiveness in Brown–Norway rats. Immunology. 1997;91:176. doi: 10.1046/j.1365-2567.1997.d01-2221.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Haczku A, Moqbel R, Jacobson M, Kay AB, Barnes PJ, Chung KF. T-cell subsets and activation in bronchial mucosa of sensitised Brown–Norway rats after single allergen exposure. Immunology. 1995;85:591. [PMC free article] [PubMed] [Google Scholar]
- 7.Watanabe A, Mishima H, Renzi PM, Xu L-J, Hamid Q, Martin JG. Transfer of allergic airway responses with antigen-primed CD4+ but not CD8+ T-cells in Brown–Norway rats. J Clin Invest. 1995;96:1303. doi: 10.1172/JCI118165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gonzalez MC, Diaz P, Galeguillos FR, Ancic P, Cromwell O, Kay AB. Allergen-induced recruitment of bronchoalveolar helper (OX-4) and suppressor (OX-8) T cells in asthma. Am Rev Respir Dis. 1987;136:600. doi: 10.1164/ajrccm/136.3.600. [DOI] [PubMed] [Google Scholar]
- 9.Noble A, Staynov DZ, Diaz-Sanchez D, Lee TH, Kemeny DM. Elimination of IgE regulatory rat CD8+ T-cells in vivo increases the co-ordinate expression of Th2 cytokines IL-4, IL-5 and IL-10. Immunology. 1990;80:326. [PMC free article] [PubMed] [Google Scholar]
- 10.Holmes BJ, MacAry PA, Kemeny DM. Depletion of CD8+ T cells following primary immunization with ovalbumin results in a high and persistent IgE response. Int Arch Allergy Immunol. 1997;113:160. doi: 10.1159/000237534. [DOI] [PubMed] [Google Scholar]
- 11.Renz H, Lack G, Saloga J, et al. Inhibition of IgE production and normalisation of airways responsiveness by sensitised CD8 T cells in a mouse model of allergen-induced sensitisation. J Immunol. 1994;152:351. [PubMed] [Google Scholar]
- 12.Olivenstein R, Renzi PM, Yang JP, et al. Depletion of OX-8 lymphocytes from the blood and airways using monoclonal antibodies enhances the late airway response in rats. J Clin Invest. 1993;92:1477. doi: 10.1172/JCI116725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Laberge S, Wu L, Olivenstein R, Xu L-J, Renzi PM, Martin JG. Depletion of CD8+ T cells enhances pulmonary inflammation but not airway responsiveness after antigen challenge in rats. J Allergy Clin Immunol. 1996;98:617. doi: 10.1016/s0091-6749(96)70096-9. [DOI] [PubMed] [Google Scholar]
- 14.Abadie A, Prouvost-Danon A. Specific and total IgE responses to allergenic stimuli in Brown–Norway, Lewis and Sprague–Dawley rats. Immunology. 1980;39:561. [PMC free article] [PubMed] [Google Scholar]
- 15.Like AA, Biron CA, Weringer EJ, Byman K, Sroczynski E, Guberski DL. Prevention of diabetes in BioBreeding/Worcester rats with monoclonal antibodies that recognize T lymphocytes or natural killer cells. J Exp Med. 1986;164:1145. doi: 10.1084/jem.164.4.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Elwood W, Lotvall JO, Barnes PJ, Chung KF. Characterisation of allergen-induced inflammation and bronchial hyperresponsiveness in sensitised Brown–Norway rats. J Allergy Clin Immunol. 1991;88:951. doi: 10.1016/0091-6749(91)90253-k. [DOI] [PubMed] [Google Scholar]
- 17.Chomczynski P, Sacchi N. Single step method of RNA isolation by acid guanidium thiocyanate-phenol chloroform extraction. Anal Biochem. 1987;162:156. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 18.McKnight AJ, Mason DW, Barclay AN. Sequence of rat interleukin 2 and anomalous binding of a mouse interleukin 2 cDNA probe to rat MHC class II-associated invariant chain mRNA. Immunogenetics. 1989;30:145. doi: 10.1007/BF02421547. [DOI] [PubMed] [Google Scholar]
- 19.McKnight AJ, Barclay AN, Mason DW. Molecular cloning of rat interleukin 4 cDNA and analysis of cytokine repertoire of subsets of CD4+ T cells. Eur J Immunol. 1991;21:1187. doi: 10.1002/eji.1830210514. [DOI] [PubMed] [Google Scholar]
- 20.Uberla K, Li WQ, Qin ZH, et al. The rat interleukin-5 gene: characterization and expression by retroviral gene transfer and polymerase chain reaction. Cytokine. 1991;3:72. doi: 10.1016/1043-4666(91)90012-3. [DOI] [PubMed] [Google Scholar]
- 21.Goodman RE, Oblak J, Bell RG. Synthesis and characterization of rat interleukin-10 (IL-10) cDNA clones from the RNA of cultured OX8- OX22- thoracic duct T-cells. Biochem Biophys Res Commun. 1992;189:1. doi: 10.1016/0006-291x(92)91516-s. [DOI] [PubMed] [Google Scholar]
- 22.Dijkema R, van der Meide PH, Pouwels PH, Caspers M, Dubbeld M, Schellekens H. Cloning and expression of the chromosomal immune interferon gene of the rat. EMBO J. 1985;4:761. doi: 10.1002/j.1460-2075.1985.tb03694.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fort P, Marty L, Piechaczyk M, et al. Various rat adult tissues express only one major mRNA species from the glyceraldehyde-3-phosphate dehydrogenase multigenic family. Nucl Acids Res. 1985;13:1431. doi: 10.1093/nar/13.5.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sambrook J, Fritsch EF, Maniatis T. Molecular Cloning: a Laboratory Manual, Book 2. 2. New York: Cold Spring Harbour Laboratory Press; 1989. [Google Scholar]
- 25.Kemeny DM, Noble A, Holmes BJ, Diaz-Sanchez D. Immune regulation: a new role for the CD8+ T cells. Immunol Today. 1994;15:107. doi: 10.1016/0167-5699(94)90152-X. [DOI] [PubMed] [Google Scholar]
- 26.Hirji N, Lin T-J, Befus AD. A novel CD8 molecule expressed by alveolar and peritoneal macrophages stimulates nitric oxide production. J Immunol. 1997;158:1833. [PubMed] [Google Scholar]
- 27.Thepen T, Van Rooijen N, Kraal G. Alveolar macrophage elimination in vivo is associated with an increase in pulmonary immune response in mice. J Exp Med. 1989;170:499. doi: 10.1084/jem.170.2.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thepen T, McMenamin C, Girn B, Kraal G, Holt PG. Regulation of IgE production in pre-sensitized animals: in vivo elimination of alveolar macrophages preferentially increases IgE responses to inhaled allergen. Clin Exp Allergy. 1992;22:1107. doi: 10.1111/j.1365-2222.1992.tb00137.x. [DOI] [PubMed] [Google Scholar]
- 29.Hirji N, Lin T-J, Bissonnette E, Belosevic M, Befus AD. Mechanisms of macrophage stimulation through CD8: macrophage CD8α and CD8β induce nitric oxide production and associated killing of the parasite Leishmania major. J Immunol. 1998;160:6004. [PubMed] [Google Scholar]
- 30.Underwood SL, Kemeny DM, Lee TH, Raeburn D, Karlsson J-A. IgE production, antigen-induced inflammation and airway hyperreactivity in the Brown–Norway rat: the effects of ricin. Immunology. 1995;85:256. [PMC free article] [PubMed] [Google Scholar]
- 31.Noble A, Staynov DZ, Kemeny DM. Generation of rat Th2-like cells in vitro is interleukin-4-dependent and inhibited by interferon-γ. Immunology. 1993;79:562. [PMC free article] [PubMed] [Google Scholar]
- 32.Iwamoto I, Nakajima H, Endo H, Yoshida S. Interferon γ regulates allergen-induced eosinophil recruitment into the mouse airways by inhibiting the infiltration of CD4+ T cells. J Exp Med. 1993;177:573. doi: 10.1084/jem.177.2.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Salgame P, Abrams JS, Clayberger C, et al. Differing lymphokine profiles of functional subsets of human CD4 and CD8 T cell clones. Science. 1991;254:279. doi: 10.1126/science.254.5029.279. [DOI] [PubMed] [Google Scholar]
- 34.McMenamin C, Holt PG. The natural immune response to inhaled soluble protein antigen involves major histocompatibility complex (MHC) class I-restricted CD8+ T cell-mediated but MHC class II-restricted CD4+ T cell-dependent immune deviation resulting in selective suppression of immunoglobulin E production. J Exp Med. 1993;178:889. doi: 10.1084/jem.178.3.889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Morgan DA, Ruscetti FW, Gallo R. Selective in vitro growth of T lymphocytes from normal human bone marrows. Science. 1976;193:1007. doi: 10.1126/science.181845. [DOI] [PubMed] [Google Scholar]
- 36.Smith KA. Interleukin-2: inception, impact, and implications. Science. 1988;240:1169. doi: 10.1126/science.3131876. [DOI] [PubMed] [Google Scholar]
- 37.Sedgwick JB, Frick WE, Sondel PM, Hank JA, Borden E, Busse WW. The appearance of hypodense eosinophils during interleukin-2 treatment. J Allergy Clin Immunol. 1990;85:557. doi: 10.1016/0091-6749(90)90093-j. [DOI] [PubMed] [Google Scholar]
- 38.Renzi PM, Sapienza DU.T, Waserman R, Olivenstein R, Martin JG. Effects of interleukin-2 on the acute and late phase response to ovalbumin in the rat. Am Rev Respir Dis. 1992;146:163. doi: 10.1164/ajrccm/146.1.163. [DOI] [PubMed] [Google Scholar]
- 39.Renzi PM, Du T, Sapienza S, Wang NS, Martin JG. Acute effects of interleukin-2 on lung mechanics and airway responsiveness in rats. Am Rev Respir Dis. 1991;143:380. doi: 10.1164/ajrccm/143.2.380. [DOI] [PubMed] [Google Scholar]