Abstract
In this study we compared cell surface staining for human peripheral blood lymphocyte (PBL) CD antigens by flow cytometry, with staining obtained following permeabilization of PBL using the Cytoperm method (Serotec). Six CD antigens (CD20, CD21, CD22, CD32, CD35 and major histocompatibility complex class II antigen) normally found on the surface of B cells, were also found to be expressed within T cells. We also showed, by immunoelectron microscopy, that these inappropriately expressed (‘occult’) CD antigens are located within cytoplasmic vesicles or within the rough endoplasmic reticulum. Following in vitro activation of T cells a distinct increase in expression of all of these cytoplasmic antigens was observed but staining at the cell surface was, by comparison, weak. We therefore propose that up-regulation of various B-cell CD antigens occurs within the cytoplasm of T cells following activation and that these antigens may be synthesized and released into the fluid-phase as soluble immunoregulatory molecules.
INTRODUCTION
Some human peripheral blood lymphocyte (PBL) antigens normally associated with the cell surface may also be found within the cell cytoplasm. This may occur during the early stages of cell development with the surface form gradually replacing the cytoplasmic form. One example of this is the B-cell antigen CD22 which is expressed in a cytoplasmic form (cCD22) between pro- and pre-B-cell stages in the bone marrow. Mature circulating B cells express this important adhesion molecule (BL-CAM) only at the cell surface but expression is lost following B-cell activation.1,2 Similarly, CD3 is found within the cytoplasm of pro-thymocytes (cCD3) but mature T cells express only the membrane form.3 In T-cell acute lymphoblastic leukaemia (T-ALL) both cytoplasmic and membrane forms of CD3 are found.3 Certain CD antigens which function as receptor molecules, e.g. complement and immunoglobulin Fc-receptors, may also be found in the cytoplasm following ligand-mediated internalization.4,5 Some of these receptors may re-cycle between the cell surface and the cytoplasm independently of ligand, with synthesis being required to replace receptors which are constantly being lost from the cell surface.6 It is also well known that the α- and β-chains of major histocompatibility complex (MHC) class II antigen are assembled within the B-cell cytoplasm and following antigen (peptide) binding the entire MHC class II–peptide complex is exported to the cell surface where presentation to the appropriate class of helper T cell (Th) occurs.7
In all of these examples the cytoplasmic and cell surface molecules are associated with the same cell type. Curiously, certain CD antigens were found to be inappropriately expressed within the cytoplasm of completely distinct cell types. For example, CD5, CD14 and CD21 (CR2/EBV–R) which are normally associated with T cells, monocytes and B cells, respectively, were found within large granular lymphocytes (LGL) and CD21 has also been identified within human T cells.8,9 The functional significance of these cytoplasmic ‘occult’ antigens is not known.
In a recent study, weak staining for the B-cell antigen CD32 (FcγRII) was found within the vast majority of normal resting PBL.10 The high percentage of cells (up to 90%) expressing cCD32 suggests that at least some T cells must contain this B-cell antigen within the cytoplasm. Following cell activation, in a two-way 7-day mixed lymphocyte reaction (MLR), expression of CD32 was found at the cell surface but only on days 3–4. By day 7 of the MLR, cell surface expression was lost.11 Cell-free supernatants obtained from day 7 MLR-activated cells contained a soluble IgG binding factor (possibly soluble CD32). Furthermore, mRNA for CD32 (FcγRIIb1 and b2 isoforms) was also demonstrated by in situ hybridization within the cytoplasm of activated PBL, gradually increasing in staining intensity throughout the MLR.11
These observations are therefore consistent with the hypothesis that up-regulation of these inappropriately expressed CD antigens occurs following T-cell activation and that these molecules may be actively synthesized and released into the fluid-phase as soluble immunoregulatory molecules.11
We have extended these studies by comparing cell surface and cytoplasmic antigen expression in parallel using monoclonal antibodies directed against a wide variety of well-known human CD antigens. Cells were permeabilized using a commercial kit (Cytoperm; Serotec, Oxford, UK) and normal unfractionated human PBL, purified T cells (Mini-MACS system; Miltenyi-Biotec, Bisley, Surrey, UK) and in vitro-activated T cells were compared using conventional flow-cytometry and by a novel immunoelectron microscopy (IEM) method.
MATERIALS AND METHODS
Isolation of peripheral blood mononuclear cells (PBMC)
The PBMC were isolated from heparinized blood obtained from healthy individuals using the standard lymphoprep (Nycomed, UK, Ltd. Birmingham, UK) method and resuspended in Iscove’s medium (Gibco BRL, Paisley, UK) supplemented with 10% fetal calf serum (ISC+S) and adjusted to a final concentration of 5×107 cells per ml.
Purification of human T cells using Mini-MACS
Freshly isolated PBMCs were resuspended in ISC+S medium at a final concentration of 107 in 80 μl medium together with 20 μl MACS CD3 Micro Beads (Miltenyi-Biotec, Ltd) and incubated at 4° for 15 min. Cells were washed at 200 g for 5 min in ISC+S and resuspended in 500 μl medium prior to addition to a Mini-MACS column which had been prewashed in phosphate-buffered saline (PBS), pH 7·2. Following extensive washing using ≈4 ml of ISC+S, the magnet was removed and bound T cells were flushed out using the plunger supplied with the column. For further purity these eluted cells were passed over a second fresh column as described above. Cells were adjusted to a final concentration of 5×107 per ml in ISC+S prior to phenotyping by flow-cytometry as described below.
Permeabilization of human PBMC
Cells were fixed and permeabilized using a commercial kit (Cytoperm) obtained from Serotec Ltd. PBMCs were resuspended in Reagent A (50 μl per million cells) and incubated at room temperature for 15 min. Cells were washed once in PBS prior to simultaneous addition of primary monoclonal antibody and permeabilization Reagent B (10 μl per million cells). Following incubation at 4° for 30 min, cells were washed in PBS prior to resuspension in 200-μl membrane-filtered PBS for acquisition on the flow-cytometer.
Flow cytometry
In general, PBMCs were adjusted to 5×107 per ml in PBS and 10-μl cell suspension was incubated with 10 μl of primary antibody (pretitrated to determine the optimum concentration) in Falcon 2052 flow-cytometry tubes.
The monoclonal antibodies used in this study were as follows. Fluorescein isothiocyanate (FITC) -conjugated anti-CD2 (MT910), anti-CD3 (UCHT1), anti-CD4 (MT310), anti-CD5 (DK23), anti-CD20 (BLy1), anti-CD21 (1F8), anti-CD22 (4KB128), anti-CD45 (T29/33) and anti-MHC Class II (CR3/43); phyroerythrin (RPE)-conjugated anti-CD5 (DK23), anti-CD8 (DK25), anti-CD14 (TUC4), anti-CD16 (DJI30c), anti-CD19 (HD37), anti-CD25 (ACT 1) and anti-CD56 (MOC 1); and unconjugated anti-CD79α (HM57 and JCB117) were supplied by Dako Ltd (Denmark House, Cambridge, UK). Serotec Ltd supplied FITC-conjugated anti-CD32 (AT10) and anti-CD35 (E11) and Sigma Chemical Co, (Poole, Dorset, UK) supplied monomeric mouse IgG (sub-class IgG1 and IgG2a). In all experiments, background threshold levels were set using these mouse immunoglobulins and where indirect staining was used FITC-conjugated secondary antibody alone was also used.
PBMCs were incubated with the primary antibody (or monomeric mouse immunoglobulin of the appropriate sub-class) for 30 min at 4°, washed with PBS and centrifuged at 200 g for 5 min. In a few experiments where monoclonal antibodies were not available in a directly conjugated form, a secondary, rabbit F(ab′)2 FITC-conjugated anti-mouse immunoglobulin antibody (Dako Ltd) was added. Following the incubation for 30 min at 4°, cells were washed at 200 g for 5 min in PBS and the supernatants were discarded. The final pellet was resuspended in 200-μl membrane-filtered PBS. Five microlitres of 5 m propidium iodide was added to each tube immediately prior to acquisition on a Becton Dickinson FACScan flow – cytometer. For cell surface values a propidium iodide exclusion gate was pre-set (at 101) to ensure that only viable cells were acquired. In contrast, when cells were permeabilized using the cytoperm method all cells bound propidium iodide, and for this reason an exclusion gate was not used.
In vitro activation of T cells
PBMCs were adjusted to 1×106 per ml in Iscove’s medium containing l-glutamine and were supplemented with 10% heat-inactivated (56°) fetal calf serum. In addition, penicillin (100 units/ml), streptomycin (100 μg/ml) and gentamycin (100 units/ml) were added (Sigma Chemical Co). The final culture medium was membrane filtered using a millipore (0·2-μm) filter prior to use. The T-cell mitogen, phytohaemagglutinin (PHA; Sigma Chemical Co.) was added at a final concentration of 2·5 μg per million cells and 200 μl aliquots were dispensed into the wells of a 96-well round-bottomed sterile tissue culture plate. Plates were sealed with a gas-permeable self-adhesive plastic film (Greiner-Laborotechnik Ltd, Stonehouse, Gloucs, UK) and incubated at 37° in 5% CO2 for 72 hr. The optimum concentration of PHA used in this study was determined by pretitration and was calculated to produce significant T-cell activation with minimum loss of cell viability. Control cells cultured for 3 days in the absence of mitogen were also included.
Detection of cytoplasmic antigens by immunoelectron microscopy
A pellet of 2×107 PBMC was fixed in Reagent A (50 μl per 106 cells) for 15 min at room temperature, washed once in PBS at 200 g for 5 min and resuspended in permeabilization medium Reagent B (10 μl per 106 cells) together with the appropriate mouse monoclonal antibody. Following incubation at 4° for 2 hr, cells were washed twice in PBS and the pellet was resuspended in 100 μl gold (10 nm) anti-mouse immunoglobulin (British BioCell International Ltd, Cardiff, UK) at 4° overnight. Cells were washed twice in PBS and re-fixed for 24 hr in fresh 2% glutaraldehyde. Following a brief rinse in Sorensen’s phosphate buffer, the pellet was allowed to solidify in molten gelatine. The tissue was then embedded in araldite, ultrasectioned and double-stained with uranyl acetate and lead citrate. Sections were then viewed in a Philips CM10 transmission electron microscope.
RESULTS
Comparison of cell surface and cytoplasmic staining by flow-cytometry
Treatment of freshly isolated normal human PBMCs using the cytoperm method (Serotec) produced no obvious change in PBL cell morphology as assessed by dot–plot analysis of forward versus side light scatter. PBL were gated as region 1 (R1) for the purposes of analysis. Successful permeabilization was confirmed by the uptake of propidium iodide. In order to provide control cell surface values, a propidium iodide gate was set to ensure that only viable PBL were acquired.
In the control group, no significant binding of monomeric mouse IgG1 was observed by indirect immunofluorescence, either before or after permeabilization. Similarly, the activation marker CD25 (IL2-R) and the monocyte antigen CD14 also provided useful negative controls. The common leucocyte antigen CD45, which is expressed at high levels on all human PBL, was found to be unchanged by the permeabilization protocol (Fig.1). Similarly, monoclonal anti-intermediate filament antibody (anti-vimentin) did not bind to control PBL but bound to 100% of cells following permeabilization.10
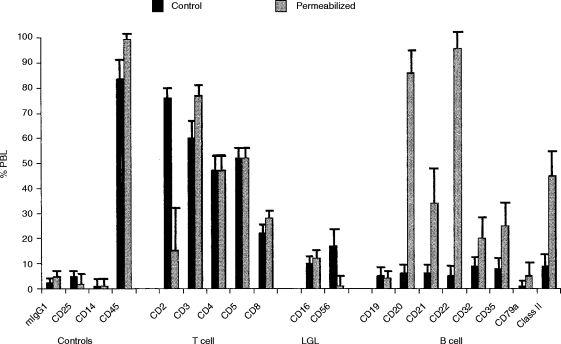
Figure 1.
Comparison of cell surface and cytoplasmic staining by flow-cytometry.The percentage of normal human PBL binding T-cell-, LGL- and B-cell-specific monoclonal antibodies to viable (Control) and to permeabilized PBL is shown as a vertical bar chart indicating the mean+standard error of the mean (SEM) for five normal donors. Negative controls included anti-CD25 (IL-2R), anti-CD14 (monocyte) and anti-CD45.
With antibodies specific for T-cell and large granular lymphocyte (LGL) antigens, as shown in Fig. 1, most were not affected by the permeabilization protocol, i.e. there was no difference between control and permeabilized cell preparations. It was however, noted that expression of CD2 and CD56 (NKH-1) appeared to be adversely affected by the permeabilization protocol. The only T-cell/LGL antigen which showed an increase in staining was CD3. This increase was significant (60·4±5·6% increasing to 77·2±3·4%; P =0·042 paired t-test) and also showed an increase in mean fluorescence intensity (65·2±6·7 increasing to 119±15·5; P =0·012).
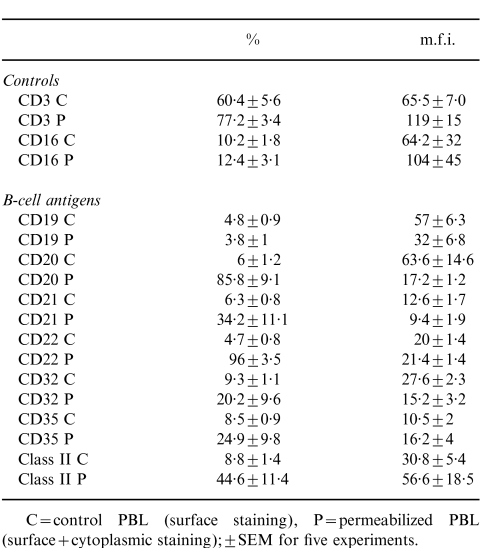
Cell surface expression of eight B-cell-specific CD antigens was found to vary between 5 and 10% of PBL. Following permeabilization, weak staining was observed within a high proportion of PBL with some values reaching 100% (Fig.1). This was observed with six out of eight B-cell CD antigens tested. The two B-cell antigens which did not appear to be affected by the permeabilization protocol were CD19 and CD79α (Fig.1). In general, the mean fluorescence intensity (m.f.i.) of ‘cytoplasmic’ staining for these B-cell antigens was equivalent to, or weaker than, cell surface staining. The only exception to this was MHC class II antigen, where the m.f.i almost doubled following permeabilization (see Table 1).
Comparison of B-cell CD antigens before and after permeabilization using unfractionated human PBL
C = control PBL (surface staining), P = permeabilized PBL (surface + cytoplasmic staining): ± SEM for five experiments.
Demonstration of cytoplasmic CD antigens using ‘pure’ T cells
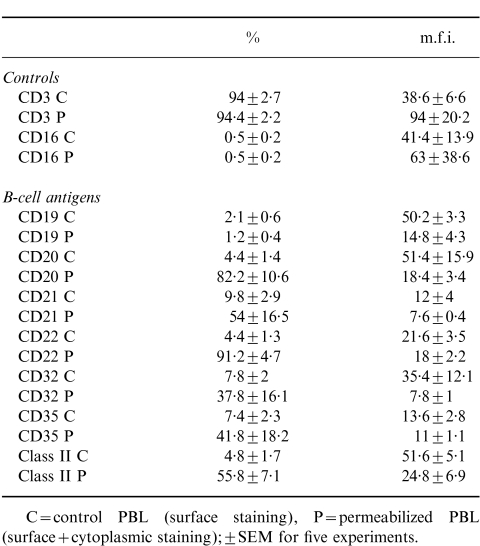
On average, cells eluted from Mini-MACS columns were found to be enriched for T cells (CD3, 94±2·7%; CD16, 0·5±0·2%; CD19, 2·1±0·6%) although in some experiments 99% T-cell purity was achieved.
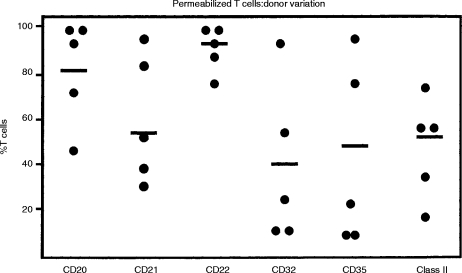
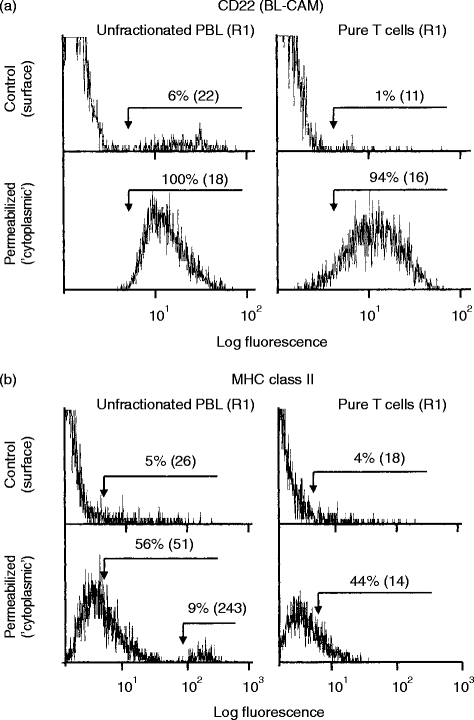
As shown in Table 2, flow-cytometry studies of cell surface versus cytoplasmic staining using ‘pure’ T cells were essentially similar to those observed with control unfractionated PBL. It was also evident that T cells showed considerable donor variation, with the most consistent ‘cytoplasmic’ antigen being CD22 (Fig. 2).
Table 2.
Comparison of B-cell CD antigens before and after permeabilization using ‘pure’ T cells
C = control PBL (surface staining), P = permeabilized PBL (surface + cytoplasmic staining): ± SEM for five experiments.
Figure 2.
Binding of B-cell-specific monoclonal antibodies to permeabilized normal human peripheral blood T cells (Mini-MACS). Individual results obtained from five normal donors are shown with the mean indicated by the horizontal bar.
Some typical examples of normal PBL (and T-enriched PBL) staining for B-cell antigens before and after permeabilization are shown as histograms in Fig. 3(a,b). The weak staining for CD22 observed following permeabilization was essentially similar with unfractionated PBL or with ‘pure’ T cells. This effect was also apparent with CD20, CD21, CD32 and CD35 but the small population (9%) of strong (m.f.i.=243) staining for MHC class II, which was evident following permeabilization of unfractionated PBL, was not observed with ‘pure’ T cells (Fig. 3b). Dual-staining with RPE-conjugated anti-CD19 confirmed that this small population of strong MHC class II-expressing PBL was in fact B cells (data not shown). This is in marked contrast to the relatively weak cell surface staining of B cells for MHC class II antigen (5%, m.f.i.=26).
Figure 3.
Comparison of cell surface and cytoplasmic staining by flow-cytometry.The effects of permeabilization on expression of selected CD antigens are shown as histogram overlays. Control ‘viable’ cells provide baseline values for cell surface staining. Permeabilized cells (cytoperm method) provide values for cell surface plus cytoplasmic staining. Results for unfractionated PBL and ‘pure’ T cells obtained from the same donor are shown. (a) CD22 (BL-CAM): cell surface staining for CD22 was confined to B cells. Following permeabilization, virtually all unfractionated PBL and purified T cells expressed CD22. (b) MHC class II: cell surface staining for MHC class II was observed on all B cells and on a small proportion of T cells. Following permeabilization a high proportion of unfractionated PBL and T cells and showed weak staining for MHC class II with a second strong peak of staining (9%, m.f.i.=243) observed with unfractionated PBL but not with T cells.
Demonstration of cytoplasmic CD antigens within activated human T cells
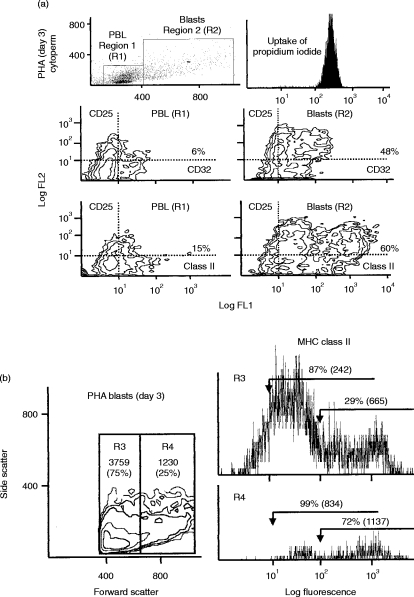
Normal human PBMCs were incubated with a low concentration (2·5 μg per million cells) of the T-cell mitogen (PHA) for 72 hr. Large blast cells were gated as region 2 (R2) while normal sized PBL in these preparations were gated as region 1 (R1) (see Fig. 4). Control cells incubated for 3 days without mitogen showed no increase in cells in R2. Normal sized PBL in R1 also showed no change in cell surface phenotype throughout the time–course of this experiment.
Figure 4.
Dual staining for CD25 (IL-2R) and CD antigens using permeabilized activated T cells. (a) PHA-activated T cells (day 3) were gated as region 2 (R2) using dot-plots of forward versus side light scatter and distinguished from normal sized PBL gated as region 1 (R1). Permeabilized cells showed uniform uptake of propidium iodide.Two examples of double labelling are shown as contour plots: CD25 versus CD32 (FcγRII) and CD25 versus MHC class II antigen. Normal-sized PBL in R1 showed some weak staining for CD32 and MHC class II with a small proportion showing double staining with CD25 whereas cells in R2 showed very strong staining for CD25 with co-expression of CD32 and MHC class II. This pattern of double labelling was also found for CD20, CD21, CD22 and CD35 (data not shown). It was also evident from these contour plots that a sub-population of R2 cells expressed extremely high levels of MHC class II antigen (and to a lesser extent CD20, CD21, CD22, CD32 and CD35). (b) Activated T cells (R2) were arbitrarily subdivided on the basis of size into small (R3) and large (R4) cells and analysed separately. ‘Cytoplasmic’ MHC class II expression was found to be much stronger in R4. Similar results (not shown) were obtained with the other B cell antigens (CD20, CD21, CD22, CD32 and CD35).
Following permeabilization of PHA-activated T cells, dual-staining was conducted using the activation marker anti-CD25 (IL-2R)–RPE together with the appropriate FITC-conjugated anti-B-cell CD antigen.
In all of these experiments it was evident that the large blast cells in R2 expressed higher levels of the cytoplasmic B-cell antigens than did the normal-sized PBL gated in R1. This was found with CD20, CD21, CD22, CD32, CD35 and MHC class II but not with CD19 or CD79α.
Double-labelling between CD25 and CD32 and CD25 and MHC class II are shown as contour plots for R1 and R2 in Fig. 4(a). Essentially similar results were obtained for CD20, CD21, CD22 and CD35. From contour–plot analysis it was evident that a sub-population of CD25+ blast cells showed much stronger expression of B-cell CD antigens. It was also noted that low levels of CD25 (IL-2R) were present on the surface and within the normal sized PBL (R1) in these preparations (see Fig. 4a), suggesting that many of the cells in this region are in the early stages of activation prior to transformation into large blast cells and cell division. When blast cells were sub-divided arbitrarily into small (region 3 – representing 75% of cells) and large (region 4) cells (Fig. 4b) it was found that much stronger cytoplasmic staining for B-cell CD antigens was observed within the large blast cells gated in R4. The results for MHC class II antigen are shown in Fig. 4(b) but similar results were found for CD20, CD21, CD22, CD32 and CD35 although the m.f.i. for these antigens was somewhat weaker.
In some experiments, cell surface staining and cytoplasmic staining were compared in parallel. In general, staining for B-cell CD antigens was relatively weak at the surface of activated T-cell blasts: CD20 (26%, m.f.i.=70), CD21 (20%, m.f.i.=9), CD22 (14%, m.f.i.=27), CD32 (38%, m.f.i.=10), CD35 (26%, m.f.i.=6), MHC class II (36%, m.f.i.=77). Much stronger staining was observed following permeabilization, i.e. ‘cytoplasmic’ staining: CD20 (94%, m.f.i.=46), CD21 (92%, m.f.i.=10), CD22 (99%, m.f.i.=42), CD32 (87%, m.f.i.=16), CD35 (88%, m.f.i.=8), MHC class II (75%, m.f.i.=357).
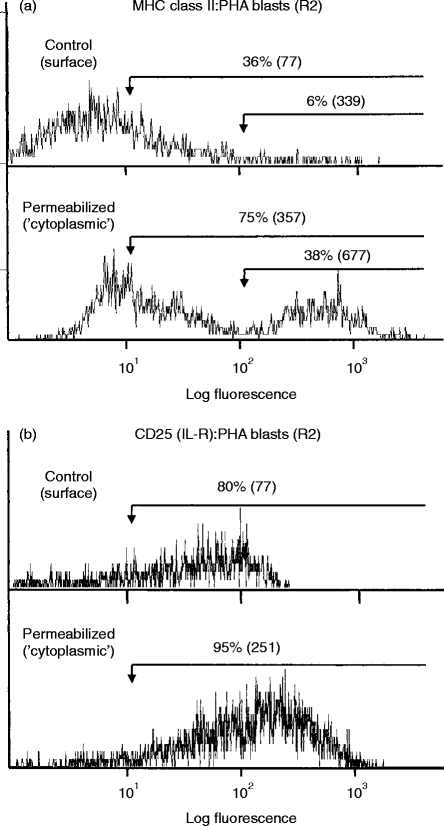
Some examples of this comparison are shown as histograms. Figure 5(a) shows MHC class II antigen being expressed mainly within the cytoplasm of activated T cells while Fig. 5(b) shows that CD25 (IL-2R) is expressed on the cell surface (control=80%, m.f.i.=77) and probably also within the cytoplasm (permeabilized=95%, m.f.i.=251) of T-cell blasts.
Figure 5.
Comparison of cell surface versus cytoplasmic staining with activated T cells. Cell surface staining of viable PHA-activated human peripheral blood T cells (R2 cells) was compared with ‘cytoplasmic’ staining observed following permeabilization of cells. Results are shown as the percentage of positive cells with the mean fluorescence intensity shown in brackets. For comparison, gates were set at the positions indicated by the horizontal arrows. (a) Weak staining for MHC class II antigen was found at the cell surface with much stronger ‘cytoplasmic’ staining evident following permeabilization. (b) Moderate staining for CD25(IL-2R) was observed at the cell surface with stronger ‘cytoplasmic’ staining evident following permeabilization.
Demonstration of cell surface and cytoplasmic antigens by immunoelectron microscopy
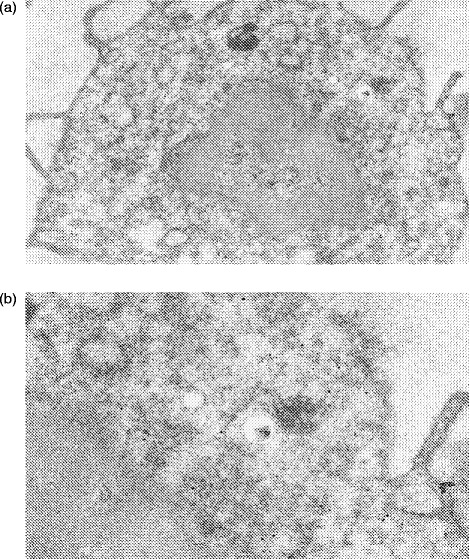
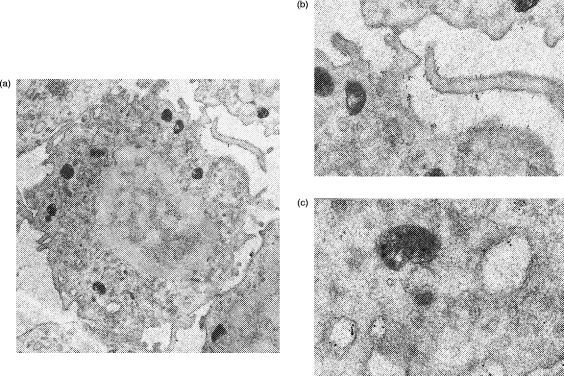
As shown in Fig. 6, it was possible to detect cytoplasmic antigens, e.g. intermediate filaments, within normal human PBL using pre-embedding immunoelectron microscopy as described in the Materials and Methods. Pre-fixed and permeabilized PBL were incubated with mouse monoclonal anti-vimentin, which in turn was visualized by 10-nm gold particles conjugated with anti-mouse immunoglobulin. Binding of gold particles was found only within the cytoplasm of the cells, with none being detected at the cell surface. The nucleus of the cell did not stain nor was any staining observed on or within any platelets found as contaminants in these preparations. Similarly, no background binding of gold particles was observed in control preparations where monomeric mouse immunoglobulin G1 (IgG1) was used in place of the primary mouse monoclonal antibody. A small proportion of PBL (presumably B cells) were found to show good cell surface labelling for B-cell CD antigens with cytoplasmic staining within these cells largely confined to the rough endoplasmic reticulum (RER) (Fig. 7). As expected, most PBL (non-B cells) did not show any cell surface labelling for B-cell CD antigens (Fig. 8) but cytoplasmic staining was observed. Typically, these cells showed gold particles either lying free within the cytosol or associated with vesicles (Fig. 8).
Figure 6.
Immunoelectron microscopy using permeabilized normal human peripheral blood lymphocytes. Binding of primary mouse monoclonal antibody (anti-intermediate filament vimentin) was visualized by 10-nm gold particles conjugated with anti-mouse immunoglobulin. (a) Low-power of normal lymphocyte (×20 300) showing whole cell. (b) High-power (×44 000) detail showing beads scattered throughout the cytoplasm with no binding to the cell nucleus or at the cell surface evident.
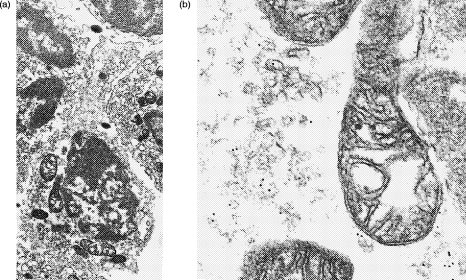
Figure 7.
Immunoelectron microscopy of permeabilized normal human peripheral blood lymphocytes. Binding of primary mouse monoclonal antibody (mouse monoclonal anti-CD32 – clone AT10) was visualized by 10-nm gold particles conjugated with anti-mouse immunoglobulin. (a) Low-power of normal lymphocyte (×16 750) showing whole cell. (b) High-power (×37 155) showing beads at the cell surface. (c) High-power (×70 815) showing beads associated with dilated cisternae of rough endoplasmic reticulum.
Figure 8.
Immunoelectron microscopy of permeabilized normal human peripheral blood lymphocytes. Binding of primary mouse monoclonal antibody (anti-CD22 BL-CAM: clone 4KB128) was visualized by 10-nm gold particles conjugated with anti-mouse immunoglobulin. (a) Low-power of normal lymphocyte (×12 625) showing whole cell. (b) High-power (× 55 230) showing beads associated with vesicles.
DISCUSSION
Many studies have now shown that cytoplasmic antigens can be readily detected by flow-cytometry following cell permeabilization with no obvious adverse effect on cell surface antigenicity.12–16 Using a commercial permeabilization kit (Cytoperm, Serotec) we found that in general there was no increase in expression of the majority of human PBL CD antigens, particularly with monoclonal antibodies specific for T cells or LGLs. The only exception to this was CD3, which showed a small but statistically significant increase in percentage and in m.f.i. following permeabilization. Using ‘pure’ T cells, a significant increase (P =0·02 by paired t-test) in m.f.i. for CD3 was noted (see Table 2): an observation consistent with low-level cytoplasmic expression within normal resting T cells. Although cCD3 has previously been found within human thymocytes and malignant T cells3 it has, as far as we are aware, never been described within resting peripheral blood T cells. The possibility that CD3 may be present within some non-T cells requires further study using pure B-cell and LGL populations.
In marked contrast to the T and LGL antigens we found that following permeabilization there was a striking increase in the percentage of PBL expressing certain B-cell antigens [i.e. CD20, CD21(CR2/EBV-R), CD22 (BL-CAM), CD32 (FcγRII), CD35 (CR1) and MHC class II antigen]. This staining was however, weak in comparison with cell surface staining. These antigens were also observed within peripheral blood T cells purified using the Mini-MACS method. This inappropriate or ‘occult’ expression within T cells was not characteristic of all B-cell CD antigens since cytoplasmic staining for CD19 or CD79α was not found.
B cells were also found to express high levels of cytoplasmic MHC class II antigen whereas resting T cells showed relatively weak and variable cytoplasmic staining. This observation is therefore consistent with the well-documented antigen-presenting properties of B cells.7 Donor variation was also evident with the other ‘occult’ CD antigens, although all five normal donors showed very consistent staining for cCD22.
The function of these CD antigens on the B-cell surface is well known but the function of these ‘occult’ antigens within T cells is not. It is probably not valid to simply extrapolate these functions to T cells and would in any event depend on their expression at the cell surface at some point during the T-cell cycle: possibly following activation. For this reason we looked for cell surface and cytoplasmic expression of the six ‘occult’ cytoplasmic B-cell antigens within PHA-activated human T cells. Strong cytoplasmic staining for all of the six B-cell antigens described above was observed within large T-cell blasts. In contrast, cell surface staining was relatively weak. It was also evident that ≈40% of activated T cells, subsequently shown to be the larger blast cells, expressed exceptionally high levels of B-cell cytoplasmic antigen (see Fig. 4b).
Many of these antigens, which are widely regarded as B-cell specific, have recently been found to be expressed on the surface of a small sub-set of normal peripheral blood T cells, on cortical thymocytes and on certain T-cell lines.17–25 Furthermore, expression of these surface antigens may increase following T-cell activation in vitro and in vivo.26–31
Activated T cells have also been shown to actively synthesize MHC class II antigen,32,33 and soluble MHC class II antigen can be detected in cell supernatants and in human plasma.34,35 Similarly, soluble forms of CD21,36 CD3237 and CD3538,39 have been found in human plasma with elevated levels reported in a variety of pathological conditions including autoimmune disease, viral infections, graft versus host disease, cancer and in acute allograft rejection.40–43 Recent studies have also shown that although resting human T cells do not express appreciable amounts of MHC class II antigen or CD21 at the cell surface they do express levels of mRNA for these antigens within the cytoplasm which are comparable to B-cell levels.44–46 Following T-cell activation, increased mRNA levels can be detected within 15 min, with increased surface expression evident within 30 min.44 It therefore seems likely that T cells express low levels of preformed antigen within the cell cytoplasm required for rapid cell surface expression and/or synthesis. Since this rapid response occurs well before DNA synthesis it has been suggested that the genes for these antigens may be regulated post-transcriptionally within human T cells45 with surface expression being controlled by cytokines such as tumour necrosis factor-α.47
In this study we have shown by immunoelectron microscopy that cells which express cell surface antigens (presumably B cells) also express these antigens within the RER (see Fig. 7) and cells which do not express surface antigen show cytoplasmic antigen localized mainly within vesicles (see Fig. 8). These observations therefore lend further support to the hypothesis that these CD antigens are actively synthesized by both B cells and by activated T lymphocytes.
The function of soluble CD antigens is not yet known but it seems likely that they play an important role in immunoregulation.
For example, soluble FcγRII and complement receptors may bind to immune complexes, thus interfering with the signal pathways that normally occur when they bind to B cells. Similarly, soluble BL-CAM and MHC class II would block the normal interaction between antigen-presenting cells and Th cells, thus providing a negative feedback loop within this particular arm of the immune response. Soluble class II–peptide complexes have been shown to bind to the appropriate specific T-cell receptor for antigen but in the absence of the normal co-stimulatory cell activation signals provided by the antigen-presenting cells the T cells undergo apoptosis and die.48 This mechanism is thought to operate within the thymus where autoreactive clones of T cells are eliminated by negative selection. Similarly, soluble class II–peptide complexes may also play a role in peripheral tolerance by eliminating autoreactive clones of T cells, i.e. induction of ‘clonal anergy’.48
In conclusion, we therefore propose that activated T cells have the capacity to synthesize a variety of CD antigens normally associated with the B-cell surface. These soluble antigens may play an important role in the regulation of ligand–receptor binding. In particular, the surprisingly high levels of class II found within the cytoplasm of activated T cells may reflect biosynthesis of soluble class II molecules with the capacity to present self-peptides.49 These MHC class II–self-peptide complexes may play an important role in the elimination of autoreactive clones in man and failure to produce these regulatory molecules may result in autoimmune disease.
Acknowledgments
M. P. was supported by a BSc senior honours project grant awarded by the Arthritis and Rheumatism Council.
REFERENCES
- 1.Stamenkovic I, Seed B. The B cell antigen CD22 mediates monocyte and erythrocyte adhesion. Nature. 1990;345:74. doi: 10.1038/345074a0. [DOI] [PubMed] [Google Scholar]
- 2.Law CL, Sidorenko SP, Clark EA. Regulation of lymphocyte activation by the cell-surface molecule CD22. Immunol Today. 1994;15:442. doi: 10.1016/0167-5699(94)90275-5. [DOI] [PubMed] [Google Scholar]
- 3.Campana D, Thomson JS, Amlot P, Brown S, Janossy G. The cytoplasmic expression of CD3 antigens in normal and malignant cells of the T lymphoid lineage. J Immunol. 1987;138:648. [PubMed] [Google Scholar]
- 4.Fearon DT, Ahearn JM. Complement receptor type-1 (C3b, C4b receptor–CD35): and complement receptor type 2 (C3d, EBV–R-CD21) Curr Top Microbiol Immunol. 1990;153:83. doi: 10.1007/978-3-642-74977-3_5. [DOI] [PubMed] [Google Scholar]
- 5.Jost CR, de Goede R, Fransen JA, Daha MR, Ginsel LA. On the origin of FcγRIII (CD16)-containing vesicle population in human neutrophil granulocytes. Eur J Cell Biol. 1991;54:313. [PubMed] [Google Scholar]
- 6.Mellman I, Plunter H, Ukkonen P. Internalization and rapid recycling of macrophage Fc receptors tagged with monocovalent anti-receptor antibody: possible role of a pre-lysosomal compartment. J Cell Biol. 1984;8:1170. doi: 10.1083/jcb.98.4.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cresswell P. Invariant chain structure and MHC class II function. Cell. 1996;84:505. doi: 10.1016/s0092-8674(00)81025-9. [DOI] [PubMed] [Google Scholar]
- 8.Schroff RW, Bucana CD, Klein RA, Farrel MM, Morgan AC. Detection of intracytoplasmic antigens by flow cytometry. J Immunol Methods. 1984;70:167. doi: 10.1016/0022-1759(84)90182-0. [DOI] [PubMed] [Google Scholar]
- 9.Morgan AC, Jr, Schroff RW, Klein RA, et al. Occult (non-surface expression) of T, B and monocyte markers in human large granular lymphocytes. Eur J Immunol. 1986;335:117. doi: 10.1016/0161-5890(87)90083-6. [DOI] [PubMed] [Google Scholar]
- 10.Sandilands GP, McLaren AP, Howie D, MacSween RNM. Occult expression of CD32 (FcγRII) in normal human peripheral blood mononuclear cells. Immunology. 1995;86:525. [PMC free article] [PubMed] [Google Scholar]
- 11.Sandilands GP, MacPherson SA, Burnett ER, Russell AJ, Downie I, MacSween RN.M. Differential expression of CD32 isoforms following alloactivation of human T cells. Immunology. 1997;91:204. doi: 10.1046/j.1365-2567.1997.00241.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hallden G, Andersson U, Hed J, Johansson SG. A new membrane permeabilisation method for the detection of intracellular antigens. J Immunol Methods. 1989;124:103. doi: 10.1016/0022-1759(89)90191-9. [DOI] [PubMed] [Google Scholar]
- 13.Jacob MC, Favre M, Bensa JC. Membrane cell permeabilisation with saponin and multiparametric analysis by flow cytometry. Cytometry. 1991;12:550. doi: 10.1002/cyto.990120612. [DOI] [PubMed] [Google Scholar]
- 14.Pollice AA, McCoy JP, Shackney SE, Smith CA, et al. Sequential paraformaldehyde and methanol fixation for simultaneous flow cytometric analysis of DNA, cell surface proteins, and intracellular proteins. Cytometry. 1992;13:432. doi: 10.1002/cyto.990130414. [DOI] [PubMed] [Google Scholar]
- 15.Pizzolo G, Vincenzi C, Nadali G, Veneri D, et al. Detection of membrane and intracellular antigens by flow cytometry following ORTHO PermeaFix fixation. Leukaemia. 1994;8:672. [PubMed] [Google Scholar]
- 16.Francis C, Connelly MC. Rapid single-step method for flow cytometric detection of surface and intracellular antigens using whole blood. Cytometry. 1996;25:58. doi: 10.1002/(SICI)1097-0320(19960901)25:1<58::AID-CYTO7>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 17.Lanier L, Kipps TJ, Phillips JH. Functional properties of a unique sub-set of CD3+ T lymphocytes that express Fc receptors for IgG (CD16/leu 11a) J Exp Med. 1985;162:2089. doi: 10.1084/jem.162.6.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tsoukas CD, Lambris JD. Expression of CR2/EBV receptors on human thymocytes detected by monoclonal antibodies. Eur J Immunol. 1988;18:1299. doi: 10.1002/eji.1830180823. [DOI] [PubMed] [Google Scholar]
- 19.Fischer E, Delibrias C, Kazatchkine MD. Expression of CR2 (the C3dg/EBV receptor, CD21) on normal human peripheral blood T lymphocytes. J Immunol. 1991;146:865. [PubMed] [Google Scholar]
- 20.Delibrias CC, Fischer E, Bismuth G, Kazatchkine MD. Expression, molecular association, and functions of C3 complement receptors CR1 (CD35) and CR2 (CD21) on the human T cell line HPB-ALL. J Immunol. 1992;149:768. [PubMed] [Google Scholar]
- 21.Hultin LE, Hausner MA, Hultin PM, Giorgi JV. CD20 (pan-B cell) antigen is expressed at low level on a sub-population of human T lymphocytes. Cytometry. 1993;14:196. doi: 10.1002/cyto.990140212. [DOI] [PubMed] [Google Scholar]
- 22.Baudeau C, Falkenrodt A, Parissiadis A, Tongio MM. A significant percentage of normal T lymphocytes express HLA-DP in the peripheral blood. Tissue Antigens. 1993;42:111. doi: 10.1111/j.1399-0039.1993.tb02176.x. [DOI] [PubMed] [Google Scholar]
- 23.Delibrias CC, Mouhoub A, Fischer E, Kazatchkine MD. CRI (CD35) and CR2 (CD21) complement C3 receptors are expressed on normal thymocytes and mediate infection of thymocytes with opsonised human immunodeficiency virus. Eur J Immunol. 1994;24:2784. doi: 10.1002/eji.1830241131. [DOI] [PubMed] [Google Scholar]
- 24.Sandor M, Galon J, Takacs L, Tatsumi Y, et al. An alternative Fc gamma-receptor ligand: potential role in T-cell development. Proc Natl Acad Sci USA. 1994;91:12857. doi: 10.1073/pnas.91.26.12857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leclercq G, Plum J. Expression and function of Fc receptors in the thymus. Crit Rev Immunol. 1995;15:215. doi: 10.1615/critrevimmunol.v15.i3-4.20. [DOI] [PubMed] [Google Scholar]
- 26.Yu DT.Y, Winchester RJ, Fu SM, Gibofsky A, Ko HS, Kunkel HG. Peripheral blood Ia-positive T cells. J Exp Med. 1980;151:91. doi: 10.1084/jem.151.1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Matsui Y, Shapiro HM, Sheehy MJ, Christenson L, et al. Differential expression of T cell differentiation antigens and major histocompatability antigens on activated T cells during the cell cycle. Eur J Immunol. 1986;16:248. doi: 10.1002/eji.1830160307. [DOI] [PubMed] [Google Scholar]
- 28.Levy E, Ambrus J, Kahl L, Molina H, et al. T lymphocyte expression of complement receptor 2 (CR2/CD21): a role in adhesive cell–cell interactions and dysregulation in a patient with systemic lupus erythematosus (SLE) Clin Exp Immunol. 1992;90:235. doi: 10.1111/j.1365-2249.1992.tb07935.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Engelhardt W, Matzke J, Schmidt RE. Activation-dependent expression of low affinity IgG receptors Fc gamma II (CD32) and Fc gamma RIII (CD16) in subpopulations of human T lymphocytes. Immunobiology. 1995;192:297. doi: 10.1016/s0171-2985(11)80172-5. [DOI] [PubMed] [Google Scholar]
- 30.Rodgaard A, Thomsen BS, Bendixen G, Bentdzen K. Increased expression of complement receptor type 1 (CR1, CD35) on human peripheral blood T lymphocytes after polyclonal activation in vitro. Immunol Res. 1995;14:69. doi: 10.1007/BF02918498. [DOI] [PubMed] [Google Scholar]
- 31.Yaskanin DD, Waxman FJ. Expression of CR1 receptor on human leukaemia-derived CD4+ T cell lines. Cell Immunol. 1995;163:139. doi: 10.1006/cimm.1995.1108. [DOI] [PubMed] [Google Scholar]
- 32.Weyand CM, Jendro M, Goronzy JJ. Soluble HLA-DR molecules in patients with HLA class II versus class I associated disorders. Autoimmunity. 1991;8:281. doi: 10.3109/08916939109007635. [DOI] [PubMed] [Google Scholar]
- 33.Barnaba V, Watts C, de Boer M, Lane P, Lanzavecchia A. Professional presentation of antigen by activated human T cells. Eur J Immunol. 1994;24:71. doi: 10.1002/eji.1830240112. [DOI] [PubMed] [Google Scholar]
- 34.Westhoff U, Thinnes P, Gotz H, Grosse-Wilde H. Quantitation of soluble HLA Class II molecules by an enzyme-linked immunosorbent assay. Vox Sang. 1991;61:106. doi: 10.1111/j.1423-0410.1991.tb00255.x. [DOI] [PubMed] [Google Scholar]
- 35.Hausmann S, Claus R, Buchwald S, Kohler H, et al. Quantitation of soluble HLA-DR antigens in human serum and other body fluids. Beitr Infusionsther Transfusionmed. 1994;32:281. [PubMed] [Google Scholar]
- 36.Fremeux-Bacchi V, Fischer E, Kazatchkine MD. Membrane and soluble forms of CD21 (the C3dg/EBV receptor) Immunol Lett. 1996;54:210. doi: 10.1016/s0165-2478(96)02673-9. [DOI] [PubMed] [Google Scholar]
- 37.Fridman WH. Regulation of B cell activation and antigen presentation by Fc receptors. Curr Opin Immunol. 1993;5:35. doi: 10.1016/0952-7915(93)90053-u. [DOI] [PubMed] [Google Scholar]
- 38.Pascual M, Duchosal MA, Steiger G, Giostra E, et al. Circulating soluble CR1 (CD35). Serum levels in disease and evidence for its release by human leukocytes. J Immunol. 1993;151:1702. [PubMed] [Google Scholar]
- 39.Danielsson C, Pascual M, French L, Steiger I, Schifferli JA. Soluble complement receptor type 1 (CD35) is released from leukocytes by surface cleavage. Eur J Immunol. 1994;24:2725. doi: 10.1002/eji.1830241123. [DOI] [PubMed] [Google Scholar]
- 40.Hara T, Fukuda H, Massaoka T, Matsumoto M, Seya T. Development of an ELISA assay for soluble CD35 (C3b/C4b receptor): high levels of soluble CD35 in LE-positive patients with haematological malignancies. Immunol Lett. 1994;41:249. doi: 10.1016/0165-2478(94)90141-4. [DOI] [PubMed] [Google Scholar]
- 41.Filaci G, Contini P, Brenci S, et al. Increased serum concentration of soluble HLA-DR antigens in HIV infection and following transplantation. Tissue Antigens. 1995;46:117. doi: 10.1111/j.1399-0039.1995.tb02487.x. [DOI] [PubMed] [Google Scholar]
- 42.Larcher C, Kempkes B, Kremmer E, Prodinger WM, et al. Expression of Epstein–Barr virus nuclear antigen-2 (EBNA 2) induces CD21/CR2 on B and T cell lines and shedding of soluble CD21. Eur J Immunol. 1995;25:1713. doi: 10.1002/eji.1830250634. [DOI] [PubMed] [Google Scholar]
- 43.Astier A, Merle-Beral H, de la Salle Moncuit J, et al. Soluble Fc gamma receptor, Fc gamma RIIa2, is present in two forms in human serum and is increased in patients: with stage C chronic lymphocytic leukaemia. Leuk Lymphoma. 1997;26:317. doi: 10.3109/10428199709051781. [DOI] [PubMed] [Google Scholar]
- 44.Zier K, Gansbacher B, Ikegaki N, Kennet R, Polakova K. Expression of HLA-DR mRNA in T cells following activation is early and can precede DNA synthesis. Autoimmunity. 1989;5:59. doi: 10.3109/08916938909029143. [DOI] [PubMed] [Google Scholar]
- 45.Caplen HS, Salvadori S, Gansbacher B, Zier KS. Post-transcriptional regulation of MHC class II expression in human T cells. Cell Immunol. 1992;139:98. doi: 10.1016/0008-8749(92)90103-v. [DOI] [PubMed] [Google Scholar]
- 46.Braun M, Melchers I, Peter HH, Illges H. Human B and T lymphocytes have similar amounts of CD21 mRNA, but differ in surface expression of the CD21 glycoprotein. Int Immunol. 1998;10:1197. doi: 10.1093/intimm/10.8.1197. [DOI] [PubMed] [Google Scholar]
- 47.Kamoun M, Zerva L, Sloan S, Zmijewski C, et al. Induction of HLA Class II molecules on human T cells: relationship to immunoregulation and the pathogenesis of AIDS. DNA Cell Biol. 1992;11:265. doi: 10.1089/dna.1992.11.265. [DOI] [PubMed] [Google Scholar]
- 48.Guery JC, Adorini L. Selective Immunosuppression of class II-restricted T cells by MHC Class II binding peptides. Crit Rev Immunol. 1993;13:195. [PubMed] [Google Scholar]
- 49.Lasalle JM, Ota K, Hafler DA. Presentation of autoantigen by human T cells. J Immunol. 1991;147:774. [PubMed] [Google Scholar]