Abstract
To investigate the effect of dietary lipids with different fatty acid compositions upon the in vivo cytokine response to bacterial lipopolysaccharide (LPS), mice were fed for 5 weeks on a low-fat diet or on one of four high-fat diets that contained 20%, by weight, of coconut oil (CO), olive oil (OO), safflower oil (SO) or fish oil (FO). The mice were injected intraperitoneally with a non-lethal dose of Escherichia coli LPS (100 μg/20 g body weight) and killed 90 or 180 min later. Plasma tumour necrosis factor-α (TNF-α), interleukin (IL)-1α, IL-6 and IL-10 concentrations were measured by enzyme-linked immunosorbent assay (ELISA). Plasma TNF-α and IL-10 concentrations were higher 90 min postinjection than after 180 min, whereas plasma IL-1β and IL-6 concentrations were higher 180 min postinjection than after 90 min. Peak plasma TNF-α, IL-1β and IL-6 concentrations were lower in the CO- and FO-fed mice than in those fed the SO diet. Peak plasma IL-10 concentrations were higher in CO-fed mice than in those fed some of the other diets. These observations suggest that, relative to the n-6 polyunsaturated fatty acid-rich SO diet, CO and FO diminish production of proinflammatory cytokines in vivo. This indicates that these fatty acids might be useful therapies in acute and chronic inflammatory diseases. The enhanced production of IL-10 following CO feeding appears to be an additional antiinflammatory effect of this oil, which could give added benefit in various clinical conditions.
INTRODUCTION
Severe bacterial infections or sepsis can result in profound physiological changes, including hypotension, fever, tissue breakdown, widespread organ dysfunction and, ultimately, death.1,2 In the case of Gram-negative bacteria this toxicity is caused by endotoxin, a lipopolysaccharide (LPS) component of the bacterial cell wall.3,4
Circulating concentrations of the proinflammatory mediators tumour necrosis factor (TNF) and interleukin (IL)-1 are elevated in patients with sepsis and after LPS administration in humans and laboratory animals.5 These cytokines mimic the effects of LPS,6–8 and animals can be protected from bacterial- and LPS-induced shock by neutralization of these cytokines.9–11 Furthermore, mice deficient in the 55×103 MW TNF-α receptor are resistant to endotoxic shock.12 Thus, the proinflammatory cytokines play a key role in the progression of sepsis, suggesting that inhibition of their production might prevent the pathology of endotoxic shock.
LPS injection in experimental animals also leads to an increase in plasma IL-10 concentrations.13,14 IL-10 suppresses LPS-induced production of TNF-α, IL-1α, IL-1β and IL-6 by human monocytes15,16 and murine peritoneal macrophages17,18in vitro. Neutralization of IL-10 by specific antibodies in LPS-injected mice leads to markedly elevated levels of circulating TNF-α and IL-6 and enhances LPS toxicity.13,14 Administration of IL-10 reduces circulating TNF-α concentrations post-LPS administration19–21 and prevents the onset of septic shock.19,20 These observations suggest that IL-10 protects against the lethal effects of LPS by suppressing the production of the damaging proinflammatory cytokines.
Fish oil (FO) feeding significantly enhances the survival of guinea-pigs following LPS challenge22,23 and decreases the accompanying lactic acidosis,24 fever25 and anorexia.26,27 Likewise, FO feeding to humans results in a slightly reduced febrile response to LPS.28 These effects of FO are often attributed to the changes in eicosanoid metabolism induced by the eicosapentaenoic acid (20:5n-3) found in FO: this fatty acid decreases the production of the proinflammatory eicosanoids, such as prostaglandin E2 (PGE2), generated from arachidonic acid (20:4n-6) and gives rise to an alternative family of eicosanoids that are less biologically active than those produced from arachidonic acid.29
More recently it has become appreciated that FO can alter production of proinflammatory cytokines (see reference 30 for a review). For example, production of TNF-α and IL-1 by Kupffer cells was decreased after feeding rats a FO-rich diet compared with feeding safflower oil (SO)- or corn oil-rich diets, which are rich in the n-6 polyunsaturated fatty acid linoleic acid (18:2n-6).31 Likewise, FO feeding decreased ex vivo production of TNF-α, IL-1 and IL-6 by murine thioglycollate-elicited peritoneal macrophages32,33 and of TNF-α and IL-6 by rat peripheral blood mononuclear cells.34 In humans, FO supplementation has been shown to decrease ex vivo production of TNF,35–38 IL-135–39 and IL-636,37,39 by peripheral blood mononuclear cells. Such effects might account for the prolongation of survival and the decreased pathology in response to LPS which accompanies FO feeding. However, to our knowledge, only one study has examined the effect of FO feeding on cytokine production in vivo40 and that study examined TNF only.
Therefore, the current study set out to compare the effect of feeding mice fish and other oils upon plasma cytokine concentrations in response to LPS. In doing so, this is the first study to examine the effect of dietary lipids on the in vivo production of IL-1β, IL-6 and IL-10.
MATERIALS AND METHODS
Animals and diets
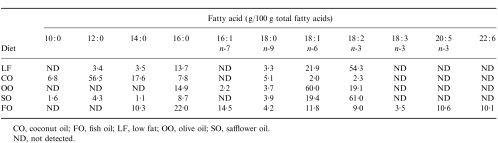
Male C57B16 mice (weighing ≈15 g) were purchased from Charles River (Margate, Kent, UK). They were housed individually in plastic cages in controlled environmental conditions (21·5±0·5°; 45±2% humidity; 12 hr light/12 hr dark cycle). After acclimatization for 1 week, the mice were randomly allocated to receive one of five diets. They were maintained on this diet for 5 weeks until killed. The diets used were a low-fat (LF) diet and four high-fat diets (all prepared by ICN Biomedicals, High Wycombe, Bucks, UK); the LF diet most closely resembled normal mouse chow and contained 2·5% by weight of corn oil. The high-fat diets contained 20% by weight of the lipid under study (coconut oil (CO), olive oil (OO), SO or FO), plus 1% by weight corn oil to prevent essential fatty acid deficiency. All diets contained 20% protein, 20% starch, 29·6% sucrose and 0·12% vitamin E (250 U/g). The fatty acid composition of the diets is shown in Table 1. Apart from the FO diet, which was stored frozen, all diets were stored at 20°. Diets were provided fresh to the animals every 2 days. Mice fed on standard laboratory chow were used for preliminary experiments. All procedures involving experimental animals were approved under the Animals (Scientific Procedures) Act 1986, by the Home Office.
Table 1.
Fatty acid composition of the diets used
CO, coconut oil; FO, fish oil; LF, low fat; OO, olive oil; SO, safflower oil
ND, not detected
LPS administration and blood collection
Mice were injected intraperitoneally (i.p.) with 1 ml of sterile phosphate-buffered saline (PBS) containing Escherichia coli serotype 0111:B4 LPS, purchased from Sigma Chemical Co. (Poole, Dorset, UK; see the Results for the amounts used); control mice received sterile PBS alone. The mice were killed with an overdose of CO2 (see the Results for timing of killing). Blood was collected by cardiac puncture into heparin (≈20 μl heparin/ml blood) and plasma was prepared by centrifugation (4000 g for 5 min). Plasma was aliquoted under sterile conditions and was stored frozen until analysis.
Cytokine measurements
Plasma TNF-α, IL-1α, IL-6 and IL-10 concentrations were measured using Cytoscreen enzyme-linked immunosorbent assay (ELISA) kits purchased from BioSource International (Camarillo, CA). All measurements were made according to the instructions given by the manufacturers.
Statistics
Data are presented as mean±SEM for the indicated number of separate animals. Statistically significant effects of diet were determined by one-way analysis of variance followed by Duncan’s multiple range test. Statistical analyses were performed using SPSS for Windows (SPSS Inc., Chicago, IL).
RESULTS
Timing and dose dependence of the cytokine response to LPS
Prior to investigating the effects of different types of dietary lipids on the cytokine response to i.p. LPS, it was important to define the time course of this response and its dependence upon LPS concentration.
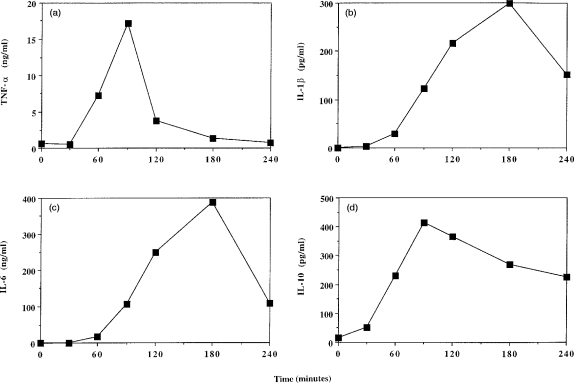
To investigate the time course of the response, chow-fed mice (n=3 per time point) were injected with 100 μg LPS/20 g body weight and killed at 30, 60, 90, 120 and 240 min postinjection; blood was collected from non-injected mice to provide data for time 0. The concentration of TNF-α in the plasma had increased by 60 min and peaked 90 min postinjection (Fig. 1a). Thereafter the TNF-α concentration decreased, returning to 0-time values by 180 min (Fig.1a). IL-1β and IL-6 concentrations in plasma were also elevated by 60 min postinjection and these peaked at 180 min postinjection before decreasing sharply (Fig. 1b, 1c). Plasma IL-10 concentrations were elevated by 30 min postinjection, peaked at 90 min postinjection and decreased slightly thereafter, but still remained elevated relative to baseline levels (Fig. 1d).
Figure 1.
Time course of plasma concentrations of (a) tumour necrosis factor-α (TNF-α), (b) interleukin (IL)-1β, (c) IL-6, or (d) IL-10 following intraperitoneal (i.p.) lipopolysaccharide (LPS) injection. Chow-fed mice were injected with 100μg LPS/20 g body weight. Data represent the mean value from three animals.
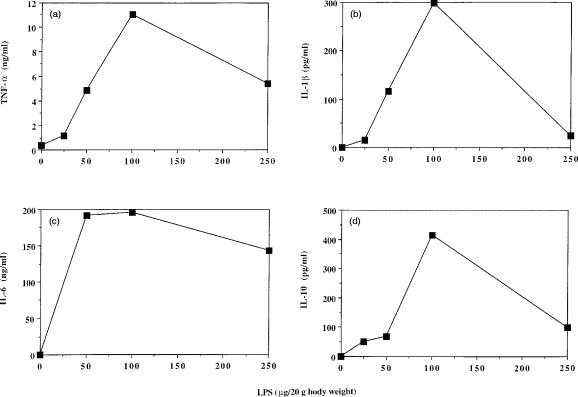
To investigate the response to different concentrations of LPS, chow-fed mice (n=3 per LPS dose) were injected with 25, 50, 100 or 250 μg LPS/20 g body weight and were killed at 90 or 180 min postinjection; mice injected with PBS alone were used as controls. The plasma concentrations of all four cytokines under study were highest following injection of LPS at a dose of 100 μg/20 g body weight (Fig. 2).
Figure 2.
Dependence of plasma concentrations of (a) tumour necrosis factor-α (TNF-α), (b) interleukin (IL)-1β, (c) IL-6, or (d) IL-10 upon dose of lipopolysaccharide (LPS) injected. Chow-fed mice were injected with 0, 25, 50, 100 or 250 μg LPS/20g body weight. Data represent the mean value from three animals killed at 90 min (a, d) or 180 min (b, c) postinjection.
Effects of dietary lipids on plasma cytokine concentrations
None of the cytokines investigated were present in the plasma of PBS-injected mice (n =6 per diet), irrespective of the diet fed (data not shown). Diet had a significant effect on post-LPS concentrations of TNF-α (P =0·019), IL-1β (P =0·045), IL-6 (P < 0·0001) and IL-10 (P =0·041).
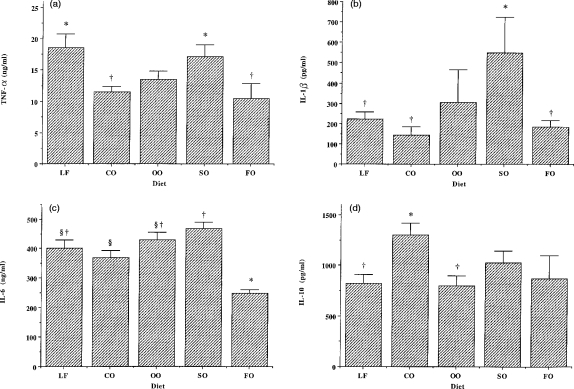
Ninety minutes after i.p. LPS (100 μg/20 g body weight), TNF-α concentrations were significantly lower in the plasma of CO- or FO-fed mice than in the plasma of those fed the LF or SO diets (Fig. 3a). There were no differences in plasma IL-1β concentrations 90 min after LPS injection among mice fed the different diets (data not shown). In contrast, after 180 min, plasma IL-1β concentrations were significantly higher in mice fed the SO diet than in those fed the LF, CO or FO diets (Fig. 3b). FO-fed mice had significantly lower plasma IL-6 concentrations 180 min after LPS injection than any other group (Fig. 3c). Plasma IL-6 concentrations were also significantly lower in CO-fed mice than in SO-fed mice (Fig. 3c). Plasma IL-10 concentrations were significantly higher in CO-fed mice 90 min post-LPS injection than in those fed the LF or OO diets (Fig. 3d).
Figure 3.
Effect of dietary lipids on post-lipopolysaccharide (LPS) plasma cytokine concentrations. Plasma was collected 90 (tumour necrosis factor-α (TNF-α), interleukin (IL)-10) or 180 (IL-1β, IL-6) min after intraperitoneal (i.p.) injection of LPS (100 μg/20 g body weight). Data are expressed as mean±SEM for six mice fed on each diet. Bars not sharing a common symbol are significantly different from one another.
As a result of the effects of diet on the plasma concentrations of TNF-α and IL-10, the ratio between these cytokines 90 min post-LPS was significantly affected (P=0·042). The TNF-α:IL-10 ratio was significantly lower in mice fed the CO diet (9·3±1·1) compared with those fed the LF diet (24·4±4·1). In mice fed the other diets, this ratio was 18·5±3·1 (OO), 18·0±3·0 (SO) and 18·5±5·5 (FO).
DISCUSSION
This study investigated the effect of different dietary lipids upon the cytokine response to bacterial LPS in vivo. The lipids studied were selected because the fatty acids they contain represent the principal classes of fatty acids found in the human diet and/or because it has been proposed that the fatty acids they contain possess pro- or anti-inflammatory activities.41,42 Furthermore, the types of oils used in this study are the basis of the lipid emulsions found in licensed or experimental parenteral formulae designed for use in patients in trauma situations. As such patients are at risk of sepsis, it seems important to determine the effects of the lipids that are administered upon components of the acute inflammatory response.
The time course of the plasma cytokine responses seen in this study agree with those reported previously in mice,13,14 as are the concentrations observed.14,21 Thus, the cytokine response to LPS observed in the current study appears to be very similar to that reported by others.
This study found that dietary lipids affect the cytokine response to bacterial LPS in vivo. The key effects were: lower TNF-α, IL-1β and IL-6 concentrations following CO or FO feeding than after feeding the SO diet; and higher IL-10 concentrations following CO feeding than after feeding some other oils. These observations suggest that, relative to the n-6 polyunsaturated fatty acid-rich SO diet, CO and FO diminish production of proinflammatory cytokines in vivo. The lower plasma concentrations of TNF-α, IL-1β and IL-6 in LPS-injected mice fed FO might account for the significantly enhanced survival of guinea-pigs given high doses of LPS22,23 and for the decreased pathophysiological responses to LPS.24–28 Taken together, these observations indicate that inclusion of FO, or CO, or the fatty acids they contain, in regimens for intravenous use in patients at risk of developing acute inflammatory responses, might be beneficial. Furthermore, inclusion of these fatty acids in the diet of patients with chronic inflammatory diseases might also be beneficial. The enhanced production of IL-10 following CO feeding appears to be an additional anti-inflammatory effect of this oil, which could convey added benefit in various clinical conditions.
The observations made in the current study may be compared with those of previous studies. FO feeding has been reported to decrease ex vivo production of proinflammatory cytokines by rat Kupffer cells,31 by mouse thioglycollate-elicited macrophages,32,33 by rat blood mononuclear cells34 and by human blood mononuclear cells.35–39 In contrast, some other studies show no effect of FO feeding or even enhanced production (see reference 30 for appropriate references). The outcome from ex vivo measurements might be, in part, affected by the experimental protocols used (e.g. the type of macrophage used and its state of activation at harvesting, the agent used to elicit macrophages where used, the type of serum present in ex vivo cultures and its concentration, the duration of ex vivo culture, etc.). The current study used plasma cytokine concentrations as a measure of the response of the intact physiological system in order to avoid the problems associated with ex vivo culture of purified cells. The lower plasma cytokine concentrations observed after FO feeding agree with some studies of ex vivo production of TNF,31–38 IL-131–33,35–39 and IL-632–34,36,37,39 by LPS-stimulated rodent and human mononuclear cells. Studies that have used CO have not reported any significant differences in ex vivo cytokine production by macrophages compared with those from animals fed other diets such as OO, SO or corn oil.33,40 This indicates a different series of events occurring in vivo compared with those occurring in cell culture. One component that will be absent in cell culture is the interaction between different cell types. No published studies have investigated the effect of dietary lipids on IL-10 production by macrophages or monocytes.
The only previous study reporting the effect of dietary lipids upon the cytokine response to LPS in vivo found enhanced peak plasma TNF concentrations in mice fed 20% FO compared with those fed LF, or 20% corn oil or CO diets.40 The reason for the discrepancy between this observation and those of the current study is unclear.
How the lipids used in the current study act to influence cytokine production is not clear and was not investigated. However, dietary FO has been shown to significantly lower TNF-α mRNA levels in LPS-stimulated murine peritoneal macrophages,32 IL-1β mRNA levels in LPS-stimulated murine spleen lymphocytes,43 and TNF-α, IL-1β and IL-6 mRNA levels in the kidneys of lupus-prone mice.44 These observations suggest that one or more components of FO can alter proinflammatory cytokine gene expression, perhaps by altering the intercellular signalling mechanisms that lead to activation of proinflammatory cytokine genes. This might occur through inhibition of activation of transcription factors, such as nuclear factor κB (NFκB), which regulate activation of the TNF-α, IL-1β and IL-6 genes. NFκB is activated by phosphorylation, often by protein kinase C, and subsequent dissociation of its inhibitory subunit. The n-3 polyunsaturated fatty acids found in FO have been shown to directly inhibit protein kinase C from brain,45 spleen lymphocytes46 and macrophages47 and so these fatty acids might prevent the activation of NFκB by this mechanism. This effect could account for the reduced plasma TNF-α, IL-1β and IL-6 concentrations, seen in the current study, in FO-fed mice following LPS administration.
Eicosanoids derived from the n-6 polyunsaturated fatty acid, arachidonic acid, might activate NFκB via protein kinase C.48 This might account for the fact that plasma TNF-α, IL-1β and IL-6 concentrations were highest in mice fed diets rich in the arachidonic acid precursor linoleic acid (i.e. the LF and SO diets). Furthermore, the concentrations of these cyokines were lowest in the plasma of mice fed diets that were poor in linoleic acid (i.e. the CO and FO diets). FO is known to diminish production of arachidonic acid-derived eicosanoids29,33 and this effect could account for the reduced plasma TNF-α, IL-1β and IL-6 concentrations, seen in the current study, in FO-fed mice following LPS administration.
IL-10 suppresses LPS-induced production of TNF-α, IL-1β and IL-6 in vitro,15–18 neutralization of IL-10 in LPS-injected mice leads to markedly elevated levels of circulating TNF-α and IL-6,13,14 and administration of IL-10 reduces circulating TNF-α concentrations post-LPS administration.19–21 IL-10 concentrations were not elevated in the plasma of FO-fed mice post-LPS, suggesting that the decreased plasma TNF-α, IL-1β and IL-6 concentrations in these animals were not a result of enhanced IL-10 production. In contrast, feeding CO elevated plasma IL-10 concentrations such that the ratio of TNF-α:IL-10 was lowered in mice fed this diet. Thus, CO might reduce proinflammatory cytokine production post-LPS by enhancing production of IL-10. How this might occur is not clear but, given the important protective role of IL-10 in LPS-induced shock, this is an exciting observation.
The observations made in the current study are of relevance to acute and chronic inflammatory conditions. With respect to the former, critically ill patients are often provided with artificial nutritional support, although the nature of the precise components of this remain controversial. The most widely used formulae for parenteral nutrition include lipid emulsions based upon n-6 polyunsaturated fatty acid-rich oils, such as soybean oil or SO. Concern has been expressed that the high levels of n-6 polyunsaturated fatty acids in these emulsions may be responsible for some of the observed deleterious effects on reticuloendothelial system and immune function during total parenteral nutrition.49–51 The findings of the current study support this concern because they suggest that such oils might favour enhanced proinflammatory cytokine production in patients already at risk of acute overproduction of such cytokines and the resulting septic shock and multiorgan failure. This study supports the idea of using alternative lipid sources in such formulae, particularly CO or FO. Lipid emulsions that include FO or the medium-chain saturated fatty acids typical of CO, or mixtures of the two, are available52 and warrant study in experimental and pathological acute inflammation.
Glossary
Abbreviations
- CO
coconut oil
- FO
fish oil
- IL
interleukin
- i.p.
intraperitoneal
- LF
low fat
- LPS
lipopolysaccharide
- NFκB
nuclear factor κB
- OO
olive oil
- PBS
phosphate-buffered saline
- SO
safflower oil
- TNF-α
tumour necrosis factor-α
REFERENCES
- 1.Bone RC, Fischer C, Clemmer TP, Slotman GJ, Metz CA, Balk RA. Sepsis syndrome: a valid clinical entity. Crit Care Med. 1989;17:389. [PubMed] [Google Scholar]
- 2.Parrillo JE, Parker MM, Natanson C, et al. Septic shock in humans: advances in the understanding of pathogenesis, cardiovascular dysfunction, and therapy. Ann Intern Med. 1990;113:227. doi: 10.7326/0003-4819-113-3-227. [DOI] [PubMed] [Google Scholar]
- 3.Westphal O. Bacterial endotoxins. Int Arch Allergy Appl Immunol. 1975;49:1. [PubMed] [Google Scholar]
- 4.Gilbert RP. Mechanisms of the hemodynamic effects of endotoxin. Physiology. 1960;40:245. doi: 10.1152/physrev.1960.40.2.245. [DOI] [PubMed] [Google Scholar]
- 5.Cannon JG, Tompkins RG, Gelfrand JA, et al. Circulating interleukin-1 and tumor necrosis factor in septic shock and experimental endotoxin fever. J Infect Dis. 1990;161:79. doi: 10.1093/infdis/161.1.79. [DOI] [PubMed] [Google Scholar]
- 6.Tracey KJ, Beutler B, Lowry SF, et al. Shock and tissue injury induced by recombinant human cachetin. Science. 1986;234:470. doi: 10.1126/science.3764421. [DOI] [PubMed] [Google Scholar]
- 7.Mathison JC, Wolfson E, Ulevitch RJ. Participation of tumor necrosis factor in the mediation of Gram negative bacterial lipopolysaccharide-induced injury in rabbits. J Clin Invest. 1988;81:1925. doi: 10.1172/JCI113540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Okusawa S, Gelfand JA, Ikejima T, Connolly RJ, Dinarello CA. Interleukin 1 induces a shock-like state in rabbits. Synergism with tumor necrosis factor and effect of cyclooxygenase inhibition. J Clin Invest. 1988;81:1162. doi: 10.1172/JCI113431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beutler B, Milsark IW, Cerami AC. Passive immunization against cachetin/tumor necrosis factor protects mice from lethal effect of endotoxin. Science. 1985;229:869. doi: 10.1126/science.3895437. [DOI] [PubMed] [Google Scholar]
- 10.Alexander HR, Doherty GM, Buresh CM, Venzon DJ, Norton JA. A recombinant human receptor antagonist to interleukin 1 improves survival after lethal endotoxemia in mice. J Exp Med. 1991;173:1029. doi: 10.1084/jem.173.4.1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tracey KJ, Fong Y, Hesse DG, et al. Anti-cachectin/TNF monoclonal antibodies prevent septic shock during lethal bacteraemia. Nature. 1987;330:662. doi: 10.1038/330662a0. [DOI] [PubMed] [Google Scholar]
- 12.Pfeffer K, Matsuyama T, Kundig TM, et al. Mice deficient for the 55 kd tumor necrosis factor receptor are resistant to endotoxic shock, yet succumb to L. monocytogenes infection. Cell. 1993;73:457. doi: 10.1016/0092-8674(93)90134-c. [DOI] [PubMed] [Google Scholar]
- 13.Marchant A, Bruyns C, Vandenabeele P, et al. Interleukin-10 controls interferon-γ and tumor necrosis factor production during experimental endotoxemia. Eur J Immunol. 1994;24:1167. doi: 10.1002/eji.1830240524. [DOI] [PubMed] [Google Scholar]
- 14.Standiford TJ, Streiter RM, Lukacs NW, Kunkel SL. Neutralization of IL-10 increases lethality in endotoxaemia. J Immunol. 1995;155:2222. [PubMed] [Google Scholar]
- 15.De Waal Malefyt R, Abrams J, Bennett B, Frigdor C, De Vries JE. IL-10 inhibits cytokine synthesis by human monocytes: an autoregulatory role of IL-10 produced by monocytes. J Exp Med. 1991;174:1209. doi: 10.1084/jem.174.5.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cassatella MA, Meda L, Bonora S, Ceska M, Constantin G. Interleukin 10 (IL-10) inhibits the release of proinflammatory cytokines from human polymorphonuclear leucocytes: evidence for an autocrine role of tumor necrosis factor and IL-1β in mediating the production of IL-8 triggered by lipopolysaccharide. J Exp Med. 1993;178:2207. doi: 10.1084/jem.178.6.2207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fiorentino DF, Zlotnik A, Mosmann TR, Howard M, O’Garra A. IL-10 inhibits cytokine production by activated macrophages. J Immunol. 1991;147:3815. [PubMed] [Google Scholar]
- 18.Rolph P, Nakoinz I, Sampson-Johannes A, et al. IL-10, T lymphocyte inhibitor of human blood cell production of IL-1 and tumor necrosis factor. J Immunol. 1992;148:808. [PubMed] [Google Scholar]
- 19.Gerard C, Bruyns C, Marchant A, et al. Interleukin 10 reduces the release of tumor necrosis factor and prevents lethality in experimental endotoxemia. J Exp Med. 1993;177:547. doi: 10.1084/jem.177.2.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Howard M, Muchamuel T, Andrade S, Menon S. Interleukin 10 protects mice from lethal endotoxemia. J Exp Med. 1993;177:1205. doi: 10.1084/jem.177.4.1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smith SR, Teeminelli C, Kenworthy-Bott L, Calzetta A, Donkin J. The cooperative effects of TNF-α and IFN-γ are determining factors in the ability of IL-10 to protect mice from lethal endotoxemia. J Leukocyte Biol. 1994;55:711. doi: 10.1002/jlb.55.6.711. [DOI] [PubMed] [Google Scholar]
- 22.Mascioli EA, Leader L, Flores E, Trimbo S, Bistrian B, Blackburn G. Enhanced survival to endotoxin in guinea pigs fed i.v. fish oil emulsion. Lipids. 1988;23:623. doi: 10.1007/BF02535609. [DOI] [PubMed] [Google Scholar]
- 23.Mascioli EA, Iwasa Y, Trimbo S, Leader L, Bistrian BR, Blackburn GL. Endotoxin challenge after menhaden oil diet: effects on survival of guinea pigs. Am J Clin Nutr. 1989;49:277. doi: 10.1093/ajcn/49.2.277. [DOI] [PubMed] [Google Scholar]
- 24.Pomposelli JJ, Flores E, Hirschberg Y, et al. Short-term TPN containing n-3 fatty acids ameliorates lactic acidosis induced by endotoxin in guinea pigs. Am J Clin Nutr. 1990;52:548. doi: 10.1093/ajcn/52.3.548. [DOI] [PubMed] [Google Scholar]
- 25.Pomposelli J, Mascioli EA, Bistrian BR, Flores SM. Attenuation of the febrile response in guinea pigs by fish oil enriched diets. J Parent Ent Nutr. 1990;13:136. doi: 10.1177/0148607189013002136. [DOI] [PubMed] [Google Scholar]
- 26.Hellerstein MK, Meydani SN, Meydani M, Wu K, Dinarello CA. Interleukin-1-induced anorexia in the rat: influence of prostaglandins. J Clin Invest. 1989;84:228. doi: 10.1172/JCI114145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mulrooney HM, Grimble RF. Influence of butter and of corn, coconut and fish oils on the effects of recombinant human tumour necrosis factor-α in rats. Clin Sci. 1993;84:105. doi: 10.1042/cs0840105. [DOI] [PubMed] [Google Scholar]
- 28.Endres S, Lonnemann G, Van Der Meer JM.W, et al. Effects of dietary omega-3 fatty acids on the acute phase response during endotoxin fever in humans. J Leukocyte Biol. 1987;42:591. [Google Scholar]
- 29.Kinsella JE, Lokesh B, Broughton S, Whelan J. Dietary polyunsaturated fatty acids and eicosanoids: potential effects on the modulation of inflammatory and immune cells: an overview. Nutrition. 1990;6:24. [PubMed] [Google Scholar]
- 30.Calder PC. n-3 Polyunsaturated fatty acids and cytokine production in health and disease. Ann Nutr Metab. 1997;41:203. doi: 10.1159/000177997. [DOI] [PubMed] [Google Scholar]
- 31.Billiar T, Bankey P, Svingen B, et al. Fatty acid uptake and Kupffer cell function: fish oil alters eicosanoid and monokine production to endotoxin stimulation. Surgery. 1988;104:343. [PubMed] [Google Scholar]
- 32.Renier G, Skamene E, De Sanctis J, Radzioch D. Dietary n-3 polyunsaturated fatty acids prevent the development of atherosclerotic lesions in mice: modulation of macrophage secretory activities. Arterioscler Thomb. 1993;13:1515. doi: 10.1161/01.atv.13.10.1515. [DOI] [PubMed] [Google Scholar]
- 33.Yaqoob P, Calder PC. Effects of dietary lipid manipulation upon inflammatory mediator production by murine macrophages. Cell Immunol. 1995;163:120. doi: 10.1006/cimm.1995.1106. [DOI] [PubMed] [Google Scholar]
- 34.Grimm H, Tibell A, Norrlind B, Blecher C, Wilker S, Schwemmle K. Immunoregulation by parenteral lipids: impact of the n-3 to n-6 fatty acid ratio. J Parent Ent Nutr. 1994;18:417. doi: 10.1177/0148607194018005417. [DOI] [PubMed] [Google Scholar]
- 35.Endres S, Ghorbani R, Kelley VE, et al. The effect of dietary supplementation with n-3 polyunsaturated fatty acids on the synthesis of interleukin-1 and tumor necrosis factor by mononuclear cells. N Engl J Med. 1989;320:265. doi: 10.1056/NEJM198902023200501. [DOI] [PubMed] [Google Scholar]
- 36.Meydani SN, Endres S, Woods MM, et al. Oral (n-3) fatty acid supplementation suppresses cytokine production and lymphocyte proliferation: comparison between young and older women. J Nutr. 1991;121:547. doi: 10.1093/jn/121.4.547. [DOI] [PubMed] [Google Scholar]
- 37.Meydani SN, Lichtenstein AH, Cornwall S, et al. Immunologic effects of national cholesterol education panel step-2 diets with and without fish-derived n-3 fatty acid enrichment. J Clin Invest. 1993;92:105. doi: 10.1172/JCI116537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Caughey GE, Mantzioris E, Gibson RA, Cleland LG, James MJ. The effect on human tumor necrosis factor α and interleukin 1β production of diets enriched in n-3 fatty acids from vegetable oil or fish oil. Am J Clin Nutr. 1996;63:116. doi: 10.1093/ajcn/63.1.116. [DOI] [PubMed] [Google Scholar]
- 39.Cooper AL, Gibbons L, Horan MA, Little RA, Rothwell NJ. Effect of dietary fish oil supplementation on fever and cytokine production in human volunteers. Clin Nutr. 1993;12:321. doi: 10.1016/0261-5614(93)90027-2. [DOI] [PubMed] [Google Scholar]
- 40.Chang HR, Arsenijevic D, Pechere JC, et al. Dietary supplementation with fish oil enhances in vivo synthesis of tumor necrosis factor. Immunol Lett. 1992;34:13. doi: 10.1016/0165-2478(92)90021-f. [DOI] [PubMed] [Google Scholar]
- 41.Grimble RF. Interaction between nutrients, pro-inflammatory cytokines and inflammation. Clin Sci. 1996;91:121. doi: 10.1042/cs0910121. [DOI] [PubMed] [Google Scholar]
- 42.Calder PC. Immunomodulatory and anti-inflammatory effects of n-3 polyunsaturated fatty acids. Proc Nutr Soc. 1996;55:737. doi: 10.1079/pns19960069. [DOI] [PubMed] [Google Scholar]
- 43.Robinson DR, Urakaze M, Huang R, et al. Dietary marine lipids suppress continuous expression of interleukin-1β gene expression. Lipids. 1996;31:S23. doi: 10.1007/BF02637046. [DOI] [PubMed] [Google Scholar]
- 44.Chandrasekar B, Fernandes G. Decreased pro-inflammatory cytokines and increased antioxidant enzyme gene expression by ω-3 lipids in murine lupus nephritis. Biochem Biophys Res Commun. 1994;200:893. doi: 10.1006/bbrc.1994.1534. [DOI] [PubMed] [Google Scholar]
- 45.Speizer LA, Watson MJ, Brunton LL. Differential effects of omega-3 fish oils on protein kinase activities in vitro. Am J Physiol. 1991;261:E109. doi: 10.1152/ajpendo.1991.261.1.E109. [DOI] [PubMed] [Google Scholar]
- 46.May CL, Southworth AJ, Calder PC. Inhibition of lymphocyte protein kinase C by unsaturated fatty acids. Biochem Biophys Res Commun. 1993;195:823. doi: 10.1006/bbrc.1993.2119. [DOI] [PubMed] [Google Scholar]
- 47.Tappia PS, Man WJ, Grimble RF. Influence of unsaturated fatty acids on the production of tumour necrosis factor and interleukin-6 by rat peritoneal macrophages. Mol Cell Biochem. 1995;143:89. doi: 10.1007/BF01816941. [DOI] [PubMed] [Google Scholar]
- 48.Camandola S, Leonarduzzi G, Musso T, et al. Nuclear factor κB is activated by arachidonic acid but not eicosapentaenoic acid. Biochem Biophys Res Commun. 1996;229:643. doi: 10.1006/bbrc.1996.1857. [DOI] [PubMed] [Google Scholar]
- 49.Fischer GW, Hunter KW, Wilson SR, Mease AD. Diminished bacterial defences with Intralipid. Lancet. 1980;ii:819. doi: 10.1016/s0140-6736(80)90171-3. [DOI] [PubMed] [Google Scholar]
- 50.Freedman Z, Marks KH, Maisels J. Effect of parenteral fat emulsions on pulmonary reticuloendothelial systems in the new born infant. Pediatrics. 1978;61:694. [PubMed] [Google Scholar]
- 51.Freeman J, Goldman DA, Smith NE, Sidebottom DG, Epsten MF, Platt R. Association of intravenous lipid emulsion and coagulase-negative Staphylococcal bacteremia in neonatal intensive care units. N Engl J Med. 1989;323:301. doi: 10.1056/NEJM199008023230504. [DOI] [PubMed] [Google Scholar]
- 52.Chan S, McCowen KC, Bistrian B. Medium-chain triglyceride and n-3 polyunsaturated fatty acid-containing emulsions in intravenous nutrition. Curr Opin Clin Nutr Metab Care. 1998;1:163. doi: 10.1097/00075197-199803000-00004. [DOI] [PubMed] [Google Scholar]