Abstract
Dendritic cells (DC) are professional antigen-presenting cells that can be used as immune adjuvant for anti-tumoural therapies. This approach requires the generation of large quantities of DC that are fully characterized on the immunophenotypical and functional levels. In a murine model, we analysed the in vitro effects of granulocyte–macrophage colony-stimulating factor (GM-CSF) alone or combined with interleukin-4 (IL-4) or Flt3 ligand (Flt3-L) on the number, immunophenotype and functions of bone marrow-derived DC. In GM-CSF cultures, we have identified two populations based on their level of expression of major histocompatibility complex (MHC) class II molecules: MHC-IIhi cells, exhibiting the typical morphology and immunophenotype of myeloid DC (CD11c+ 33D1+ DEC-205+ F4/80+), and MHC-IIlo cells, heterogeneous for DC markers (30% CD11c+; 50% 33D1+; DEC-205−; F4/80+). The addition of Flt3-L to GM-CSF induced a twofold increase in MHC-IIhi DC number; besides, the MHC-IIlo cells lost all DC markers. In contrast, after addition of IL-4 to GM-CSF, the two populations displayed a very similar phenotype (CD11c+ 33D1− DEC-205+ F4/80−), differing only in their expression levels of MHC class II and costimulatory molecules, and showed similar stimulatory activity in mixed leucocyte reaction. We next analysed the migration of these cultured cells after fluorescent labelling. Twenty-four hours after injection into the footpads of mice, fluorescent cells were detected in the draining popliteal lymph nodes, with an enhanced migration when cells were cultured with GM-CSF+Flt3-L. Finally, we showed that MHC-IIhi were more efficient than MHC-IIlo cells in an anti-tumoral vaccination protocol. Altogether, our data highlight the importance of characterizing in vitro-generated DC before use in immunotherapy.
INTRODUCTION
Dendritic cells (DC) are the most potent antigen-presenting cells (APC) of the immune system.1,2 They are capable of initiating primary immune responses3,4 and are the major stimulators of the T-cell-mediated allogeneic responses in mixed leucocyte reaction (MLR).5,6 Thus, DC can be useful to reinforce an otherwise inefficient immune response, such as that found in cancer patients. Studies carried out in murine models have shown that DC pulsed with tumour-associated antigens (TAA) in various forms induced protective and therapeutic anti-tumour immunity.7–9 Based on these results and on additional in vitro data obtained with human DC,10,11 clinical trials have now been initiated.12,13
However, the clinical use of DC requires the in vitro generation of large quantities of cells. This is accomplished through the use of various combinations of cytokines,14–19 which could alter the functional properties of DC. Indeed, it is known that the capacity of DC to capture, process, migrate and present antigens, which varies according to their differentiation/maturation stage,1,20–22 is dependent on in vitro culture conditions.14,16,23,24
To address these issues, we have used murine dendritic cells which can be easily generated from bone marrow-derived precursors under the effect of granulocyte–macrophage colony-stimulating factor (GM-CSF).18 In this work, we have analysed and compared the effects of GM-CSF alone or in combination with two cytokines which play an important role in DC differentiation: interleukin-4 (IL-4), which induces their functional maturation,7,19 and Flt3 ligand (Flt3-L), which dramatically increases their number after in vivo administration to mice.25 In particular, the murine model allows evaluation of the effects of cytokines on DC functions such as homing capacity and efficiency in vaccination protocols. These data underline the heterogeneity of in vitro-generated DC and the importance of their characterization before use for immunotherapy.
MATERIALS AND METHODS
Animals
DBA/2 and C57BL/6 mice were purchased from IFFA-Credo (L'Arbresle, France). Male and female mice were 7–14 weeks of age at the time of the experiments.
Tumour cell line
L1210/hCD4 cells derived from the L1210 murine lymphocytic leukaemia cell line [American Type Culture Collection (ATCC, Rockville, MD) no. CCL219] have been genetically modified to express on the cell membrane the human CD4 molecule (hCD4) as an artificial TAA (B. M. Colombo, R. Lacave, C. Pioche-Durieu, C. Masurier, F. M. Lemoine, M. Guigon & D. Klatzmann, manuscript in preparation). Cells were maintained in RPMI-1640 (Gibco-BRL, Paisley, UK) supplemented with 10% decomplemented fetal calf serum (FCS; Dutscher, Brumath, France), 1% l-glutamine and 1% antibiotics.
Generation of DC from bone marrow
Bone marrow (BM) cells were obtained from DBA/2 mice as previously described26 and cultured at 7×105 cells/ml in RPMI-1640 supplemented with 5% FCS, 1% l-glutamine, 20 μg/ml gentamicin, 50 μm 2β-mercaptoethanol, and 2000 U/ml recombinant murine (rm) GM-CSF (Genzyme, Cambridge, MA) with or without 1000 U/ml of rmIL-4 (PeproTech, London, UK) or with or without 250 ng/ml of human Flt3-L (Immunex, Seattle, WA) in 24-well plates at 37° in 5% CO2. On days 2, 4 and 6, 90% of the medium was replaced by appropriate cytokine-containing medium. On day 6, cells in suspension were harvested, and loosely adherent aggregates of growing DC were dislodged by vigorous pipetting and were then counted. In some experiments, cells were cultured for another 3 days with appropriate cytokine-containing medium at a concentration of 5×105–7×105 cells/ml.
Flow cytometric analysis
Cells (5×105–1×106) were suspended in phosphate-buffered saline (PBS)/3% FCS/0·02% sodium azide, centrifuged at 350 g for 5 min, resuspended in 100 μl of buffer, following by single or double labelling with saturating concentrations of monoclonal antibody (mAb).
Depending on the analysis performed, either fluorescein isothiocyanate (FITC)- or biotin-conjugated 14.4.4S mAb (PharMingen, San Diego, CA) was used against major histocompatibility complex (MHC) class II molecules. The latter was revealed with streptavidin Tri-color (Caltag Laboratories, San Francisco, CA). CD11c expression was analysed with the unlabelled N418 mAb (hamster IgG, ATCC HB224) revealed by a phycoerythrin (PE)-conjugated F(ab′)2 goat anti-hamster immunoglobulin G (IgG) (Caltag Laboratories).
The following antibodies were selected: a panel of FITC-conjugated mAb against B220 (RA3-6B2), mCD3 (500-A2), mCD4 (CT-CD4), mCD8α (CT-CD8a), Mac-1 (M1-70.15), F4/80 (F4/80), from Caltag Laboratories; CD44 (IM7, PharMingen), a panel of biotin-conjugated mAb against CD54 (KAT-1, Caltag Laboratories) and a panel of uncoupled rat mAb against DEC-205 (NLDC 145, Serotec, Oxford UK), spleen DC (33D1, ATCC TIB 227), CD40 (3/23, Serotec), B7-1 (1G10, PharMingen) and B7-2 (GL-1, PharMingen). The biotin-conjugated mAb were revealed with streptavidin phycoerythrin (PharMingen) and the uncoupled rat mAb with an FITC-conjugated F(ab′)2 goat anti-rat IgG (Caltag Laboratories). In order to minimize non-specific binding, cells were preincubated with 2.4G2 (FCγII/IIIR, ATCC HB 197) mAb.
The following isotypic immunoglobulin controls were used: FITC-conjugated rat IgG2b and hamster IgG (Cedarlane, Hornby, Ontario, Canada), biotin-conjugated rat IgG2a, unconjugated hamster IgG, rat IgG2a, and rat IgG2b from Caltag Laboratories.
Cells were fixed in 1% paraformaldehyde, and analyses were performed on a FACScan (Becton Dickinson, Mountain View, CA).
In some experiments, MHC class II-stained DC were sorted on day 6 of the culture on a FACStar Plus (Becton Dickinson).
Allogeneic mixed leucocyte reaction (MLR)
Responder cells from inguinal, brachial and axillary lymph nodes (LN) from C57BL/6 mice and increasing numbers of 25 grey-irradiated BM-derived stimulator cells were cocultured in 0·2 ml of RPMI-1640 supplemented with 10% FCS, 2 mm glutamine, 5×10−5 m 2β-mercaptoethanol, and 20 μg/ml gentamicin in U-bottom 96-well microplates. Four days later, cells were pulsed with 1 μCi/well of [3H]TdR for an additional 12 hr before harvesting and scintillation counting.
Migration studies
Cells (5×106–10×106) were incubated with 2 μm fluorescent dye (PKH2) (Zynaxis, Malvern, PA) at room temperature for 5 min, then RPMI-1640/10% FCS was added to stop the reaction. After three washes, 5×105 cells/25 μl were injected subcutaneously (s.c.) into the footpad of anaesthetized (Avertin 2·5% tribromoethanol) DBA/2 mice. Homolateral popliteal and inguinal LN were removed 6, 14, 24, 48 hr and 5 days after injection.
LN cells were obtained after collagenase digestion as previously described27 and suspended in staining buffer (PBS/3% FCS/0·02% sodium azide) for flow cytometric analyses.
Vaccination of mice with MHC-IIhi and MHC-IIlo cells pulsed with a model TAA
MHC-IIhi and MHC-IIlo cells were sorted on day 6 of a BM culture in the presence of GM-CSF. Unsorted and sorted cells were re-seeded separately in new wells, and pulsed with 10 μg/ml of the antigen (hCD4) in the form of soluble protein.28 On day 7, cells in suspension were harvested and pooled with loosely adherent aggregates of growing DC, and used for vaccination. For this purpose, cells were irradiated (25 grey) and injected intravenously (i.v.) into the retro-orbital sinus of groups of five DBA/2 mice (105 cells/100 μl PBS/mouse). Controls were mice identically injected with unpulsed cells. Eighteen days later, mice were challenged by i.v. injection of 105 L1210/hCD4 tumour cells/100 μl PBS/mouse. Animal survival was followed up to 70 days.
Statistical analysis
Results are presented as the mean±SD of data from individual cultures. The paired Student's t-test was used for statistical analysis of the observed differences, except for the comparison between day 6 and day 9, in which the unpaired Student's t-test was used. Statistical significance was defined as P < 0·05.
RESULTS
Analysis of DC generated in GM-CSF cultures: evidence for two populations
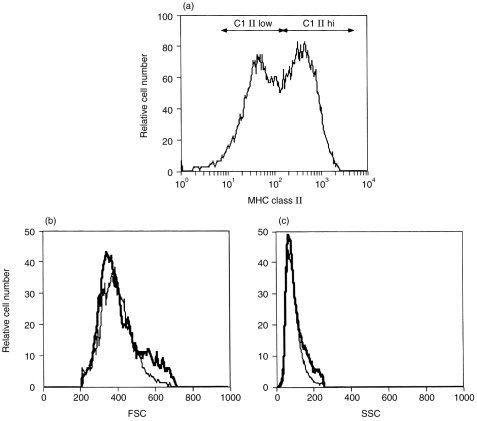
After 6 days of culture of BM cells with GM-CSF, two non-adherent populations with similar size and structural features were distinguished according to their level of expression of MHC class II molecules (Fig. 1): 51±12% of cells had high MHC class II expression (MHC-IIhi), whereas 27±13% of cells displayed weaker expression (MHC-IIlo) (n = 9). The two populations also differed morphologically: the MHC-IIhi cells had numerous and long cellular processes typical of DC, while in MHC-IIlo cells the processes were short or absent. The remaining MHC class II-negative cell population contained essentially granulocytes (not shown).
Figure 1.
FACS analysis of bone marrow cells cultured in GM-CSF. Bone marrow cells cultured for 6 days in the presence of GM-CSF were stained with mAb for MHC class II molecules for flow cytometry analysis. (a) Histogram showing two populations expressing MHC class II molecules at high (MHC-IIhi) and low levels (MHC-IIlo). (b) and (c) Histograms representing the size (FSC) and the structural features (SSC) of gated MHC-IIhi (thick line) and MHC-IIlo (thin line) cells. Few MHC class II-negative cells were observed in this experiment.
Detailed immunophenotypical analysis of MHC-class lI-expressing cells was first performed on unsorted cells (n = 3) and then confirmed on sorted cells (Table 1 and Fig. 4). All these cells were negative for B and T lymphocyte (B220, CD3, CD4, CD8α), and granulocyte (GR1) markers, but expressed the myeloid markers F4/80 and Mac-1. Dendritic markers were differently expressed on MHC-IIhi and MHC-IIlo cells: after 6 days of culture, CD11c was expressed by all MHC-IIhi cells and by 30% of MHC-IIlo cells; 50% of MHC-IIhi cells only expressed 33D1 and CD40 markers. Both populations expressed high levels of the CD44 and CD54 adhesion molecules and heterogeneous levels of B7-1 and B7-2 costimulatory molecules, which however, were very low for the MHC-IIlo population.
Table 1.
Immunophenotypical analysis of MHC-IIhi and MHC-IIlo cells cultured in GM-CSF for 6 and 9 days
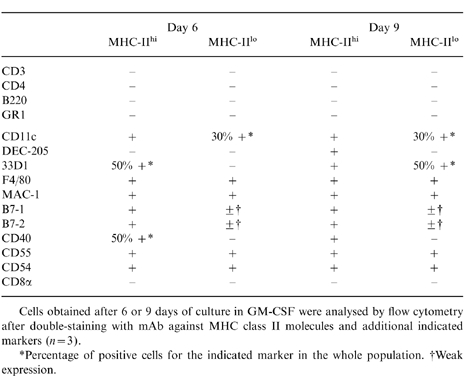
Cells obtained after 6 or 9 days of culture in GM-CSF were analysed by flow cytometry after double-staining with mAb against MHC class II molecules and additional indicated markers (n = 3).
*Percentage of positive cells for the indicated marker in the whole population.
†Weak expression.
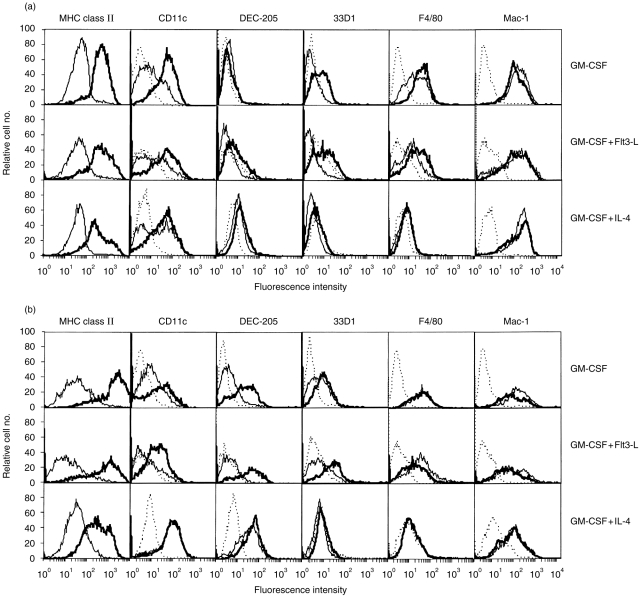
Figure 4.
Cell surface immunophenotype of MHC class II-expressing cells obtained after 6 (a) and 9 days (b) of culture in GM-CSF alone (upper row) or in combination with Flt3-L (middle row) or IL-4(lower row). At day 6 of culture, cells were sorted by flow cytometry according to their high or low expression of MHC class II molecules. Immediately and after 3 days of culture (day 9), cells were double-stained for MHC class II molecules and for additional indicated markers. Histograms: dotted line, negative controls labelled with the same amount of isotype-matched control mAb; thick line, MHC-IIhi cells; thin line, MHC-IIlo cells. Representative data from three independent experiments performed on unsorted cells are shown.
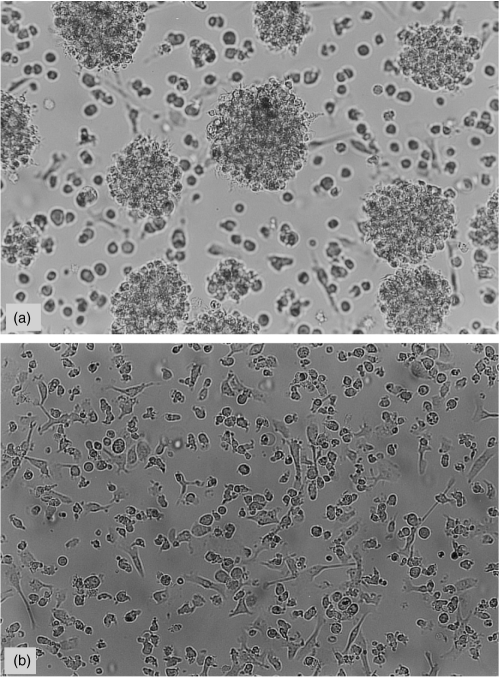
At day 9, the majority of both populations remained non-adherent, displaying the same morphological features as on day 6, but with spontaneous formation of aggregates in the MHC-IIhi population (Fig. 2a,b). Immunophenotypical changes occurred in the MHC-IIhi cell population with expression of 33D1 and CD40 by all cells and appearance of DEC-205 expression. In the MHC-IIlo population, the only change was 33D1 expression by 50% of the cells.
Figure 2.
Morphological features of MHC-IIhi(a) and MHC-IIlo (b) cell populations after 9 days of culture. MHC-IIhi cells have long and numerous cellular processes and spontaneously form aggregates in culture; in MHC-IIlo cells the processes are short or absent. Magnification ×200.
Therefore, BM cells cultured for 9 days with GM-CSF generated a MHC-IIhi myeloid DC population (34±12%) expressing CD11c, F4/80, DEC-205 and 33D1 markers and a heterogeneous population of MHC-IIlo cells (43±10%), containing both DC and cells rather displaying a monocyte–macrophage immunophenotype.
Addition of Flt3-L to GM-CSF cultures resulted in higher MHC-IIhi cell numbers after 9 days
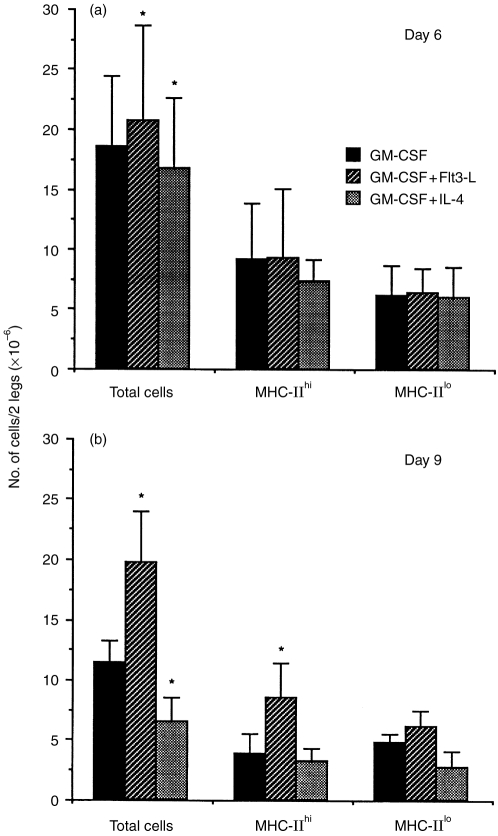
The addition of Flt3-L to GM-CSF cultures slightly increased the total cell numbers after 6 and 9 days (1·2-fold; n = 9; P < 0·02 and 1·7-fold; n = 5; P < 0·01, respectively) as compared to GM-CSF alone. At day 9, the MHC-IIhi cell number was twofold higher than in the GM-CSF culture (n = 5; P < 0·01), whereas that of MHC-IIlo was not significantly different (Fig. 3).
Figure 3.
Cell numbers in culture with GM-CSF, GM-CSF+Flt3-L, GM-CSF+IL-4 after 6 (a) and 9 (b) days. The percentages of MHC-IIhi and MHC-IIlo cells were determined by flow cytometry analysis after double-staining for MHC class II molecules and CD11c markers. These percentages allowed calculation of the number of cells obtained per well for each population. The number of cells of each population is expressed/two legs (tibias and femurs). Mean±SD of six and five independent experiments at day 6 and day 9, respectively. *Significantly different compared to GM-CSF alone.
In contrast, the addition of IL-4 to GM-CSF cultures slightly decreased the total cell number after 6 and 9 days of culture (0·8-fold; n = 6; P = 0·03 and 0·6-fold; n = 5; P < 0·02, respectively) as compared to GM-CSF alone. However, MHC-IIhi or MHC-IIlo cell numbers were not significantly different from GM-CSF culture.
The comparison of cell numbers between days 6 and 9 (unpaired t-test) showed that total cell as well as MHC-IIhi and MHC-IIlo cell numbers were maintained in GM-CSF+Flt3-L cultures, whereas in GM-CSF cultures, the MHC-IIhi cell number was slightly decreased (P < 0·05). On the other hand, in GM-CSF+IL-4 cultures, the total cell number was decreased from day 6 to day 9 (P < 0·04), mostly due to a decrease in MHC-IIhi cells (P < 0·02).
Addition of Flt3-L or IL-4 to GM-CSF cultures induced immunophenotypical changes in both MHC-IIhi and MHC-IIlo populations
The addition of Flt3-L to GM-CSF cultures led to minor immunophenotypical changes in the MHC-IIhi population, which at day 6 did not express 33D1 (Fig. 4a). In contrast, marked changes were observed in the MHC-IIlo population at day 9, mainly involving a decrease in MHC class II molecules and disappearance of CD11c expression ( Fig. 4b). Thus, the MHC-IIlo population no longer expressed characteristic immunophenotypical DC markers at day 9.
The addition of IL-4 induced changes in both populations as compared to cells generated in GM-CSF. After 6 days of culture, they showed weak expression of DEC-205 and no expression of 33D1 or F4/80 markers (Fig. 4a). After 9 days of culture, both populations strongly expressed DEC-205 (Fig. 4b), whereas CD40 was expressed by all MHC-IIhi and by 50% of MHC-II1o cells (not shown). Therefore, after 9 days of culture with GM-CSF+IL-4, both populations showed a very similar immunophenotype except for the expression of MHC class II, B7-1 and B7-2 molecules which remained lower for the MHC-IIlo cells. Thus, these populations, and especially MHC-IIlo cells, appeared immunophenotypically different from cells generated in the presence of GM-CSF.
It should be pointed out that the addition of either cytokine to GM-CSF cultures did not modify the morphology of MHC-IIhi and MHC-IIlo populations.
Influence of the cytokines on the function of MHC-IIhi and MHC-IIlo populations in allogeneic MLR
The stimulating activity in MLR is a characteristic property of DC.18,19 MLR were first performed using, as stimulators, total BM-derived cells generated with different combinations of cytokines. Cells cultured in GM-CSF with either Flt3-L or IL-4 for 6 days were always more potent stimulators of MLR than cells cultured in GM-CSF alone (n = 3). Cells cultured in GM-CSF or in GM-CSF+Flt3-L for 9 days had similar stimulatory activities, but were always less efficient than cells cultured with GM-CSF+IL-4 (n = 3) (not shown).
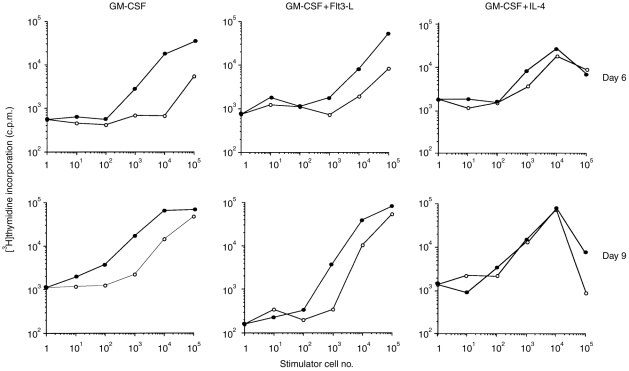
In order to compare the stimulatory activities of MHC-IIhi and MHC-IIlo cell populations, similar experiments were performed with sorted cells (Fig. 5). The MHC-IIhi cell population generated in GM-CSF with or without Flt3-L was always a more potent stimulator in MLR than the MHC-IIlo cell population at day 6 of culture and to a lesser extent at day 9. When cells were cultured with GM-CSF+IL-4, the difference in the stimulatory effect of MHC-IIhi and MHC-IIlo populations was smaller at day 6 and no longer observed at day 9.
Figure 5.
MLR with sorted MHC-IIhi(•) and MHC-IIlo (○) cells after a 6 (upper panel) or 9-day (lower panel) culture in GM-CSF alone (left panel) or in combination with Flt3-L (middle panel) or IL-4 (right panel). Cells from DBA/2 mice were sorted at day 6 and subcultured until day 9 as described in Fig. 2. Graded numbers of cells were irradiated and cocultured with 105 responding LN cells from C57BL/6 mice. Proliferative responses were measured (c.p.m.) on day 4, which corresponds to the peak of response under our experimental conditions.
Cells obtained after culture with different combinations of cytokines migrate differently to draining lymph nodes
Another specific property of DC is their capacity to home to the T-cell areas of peripheral lymphoid tissues.29–31
We first determined the homing kinetics of total cells generated in 6-day cultures with the three combinations of cytokines. Cells stained with the fluorescent dye PKH2 were s.c. injected into the mouse footpad. Six, 14, 24, 48 hr and 5 days later, the draining popliteal LN were harvested and analysed by flow cytometry. Regardless of the culture conditions, the number of fluorescent cells recovered in the popliteal LN peaked at 24 hr (not shown).
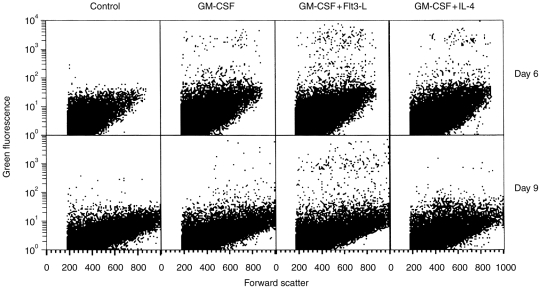
Then, we compared the homing capacity of cells cultured for 6 and 9 days with the different combinations of cytokines by analysing the draining LN 24 hr after injection. Cells obtained after 6 days of culture were able to migrate to the draining popliteal LN regardless of the cytokine combination used (Fig. 6). Nevertheless, a significantly higher number of PKH2-stained cells was found in the LN after injection of cells cultured in GM-CSF+Flt3-L (577±316 stained cells/106 LN cells) as compared to GM-CSF alone (280±86 stained cells/106 LN cells) (P = 0·02). The number of fluorescent cells was also increased, albeit not significantly, after injection of GM-CSF+IL-4 cultured cells (587±455 stained cells/106 LN cells; P = 0·08, n = 3).
Figure 6.
FACS analysis of popliteal LN cells 24 hr after injection of cells cultured in GM-CSF, GM-CSF+Flt3-L, or GM-CSF+IL-4 for 6(upper panel) or 9 days (lower panel). Cells were stained with PKH2 and injected s.c into the footpad of syngeneic mice. Twenty-four hours later, the popliteal draining LN were harvested and digested by collagenase treatment. LN cells (1·5×105) were analysed by flow cytometry for their size (forward scatter) and PKH2 green fluorescence. Controls were popliteal LN cells from non-injected mice. Representative data from one of three independent experiments performed with three or four mice per group.
After injection of cells cultured for 9 days in GM-CSF+Flt3-L, the number of fluorescent cells in the LN remained unchanged (529±315 stained cells/106 LN cells), whereas it was strongly reduced after injection of cells cultured with GM-CSF or GM-CSF+IL-4 (i.e. 56±17 and 32±5 stained cells/106 LN cells, respectively).
MHC-IIhi cells are more efficient than MHC-IIlo cells in an anti-tumoral vaccination protocol
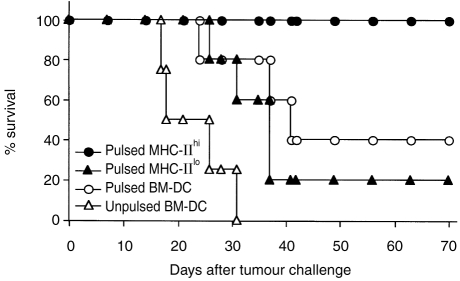
BM-derived DC pulsed with tumour antigens have been used for immunotherapy of murine tumours.32 In the following experiment, a vaccination protocol was used to analyse the respective protective effects of MHC-IIhi and MHC-IIlo cells generated in GM-CSF culture and pulsed with the soluble antigen hCD4 in the form of protein. As shown in Fig. 7, only hCD4-pulsed MHC-IIhi cells resulted in 100% tumour protection 70 days after the challenge with L1210/hCD4 tumour cells. In contrast, the percentage of animal survival was only 20% in the group of mice vaccinated with hCD4-pulsed MHC-IIlo cells. When vaccination was performed with unsorted hCD4-pulsed BM-DC, the percentage of mouse survival 70 days after L1210/hCD4 tumour challenge was 40%, but it may vary in relation to the amount of MHC-IIhi and MHC-IIlo cell populations present in the culture (data not shown). All control mice vaccinated with unpulsed BM-DC died before 31 days after tumour challenge.
Figure 7.
Effect of vaccination with MHC-IIhi and MHC-IIlo cell populations generated in GM-CSF culture pulsed with hCD4 soluble antigen. Survival of mice vaccinated with sorted hCD4-pulsed MHC-IIhi and MHC-IIlo cell populations, unsorted, pulsed or unpulsed BM-derived cells, after challenge with L1210/hCD4 tumour cells (five mice/group).
Altogether, these data indicate that vaccination with either unpulsed BM-DC or with pulsed MHC-IIlo sorted cells did not protect, or only weakly protected, mice against tumour challenge. In contrast, vaccinations with pulsed-unsorted BM-DC and with pulsed MHC-IIhi sorted cells efficiently protected mice against tumour challenge.
DISCUSSION
Bone marrow was chosen for this study, because it is a potential source of DC in both mice and humans. Our data confirm the heterogeneity of DC generated from murine bone marrow as already noted by others33 and provide further information on the different cell populations generated in GM-CSF cultures. Indeed, one cell population of typical DC coexists with cells characterized by a lower expression of MHC class II and costimulatory molecules and immunophenotypical heterogeneity.
The nature of the MHC-IIlo cell population, which represents about 40% of total cells, is not yet known. Several points should be emphasized. First, the level of MHC class II expression remained low in these cells throughout the duration of the culture, even after cell sorting and after addition of Flt3-L or IL-4 to GM-CSF. Immunohistochemical analysis of MHC-II distribution showed that its expression was both membrane and cytoplasmic whereas MHC-IIhi cells showed a preferential membrane expression (data not shown), indicating differences in maturation level.34 Second, MHC-IIlo cells were heterogeneous and their immunophenotype appeared to be dependent on the cytokines present in the culture. In cultures with GM-CSF, some MHC-IIlo cells were CD11c−, probably committed to the monocyte/macrophage lineage. In cultures with GM-CSF and IL-4, they displayed DC markers similar to MHC-IIhi cells although still expressing low levels of MHC class II and costimulatory molecules and thus, their relationship with other DC is still unclear. In cultures with GM-CSF+Flt3-L, MHC-IIlo cells did not display characteristic markers of DC, but appeared more committed to the macrophage–monocyte lineage.
We did not find differences in phagocytosis of latex beads which was low for both MHC-IIlo and MHC-IIhi cells as compared to macrophages (data not shown). However, MHC-IIlo cells were functionally different from MHC-IIhi cells. Indeed, they were generally less potent stimulators in MLR than MHC-IIhi cells, with the exception of cells generated in GM-CSF+IL-4. Furthermore, tumour-pulsed MHC IIlo cells were inefficient in an anti-leukaemic vaccination protocol. This was shown using the non-immunogenic L1210 B-lymphocytic leukaemia expressing on the cell surface a candidate exogenous TAA, the hCD4. This antigen has been previously used as TAA in an anti-tumour vaccination approach in mice based on DNA immunization.35 The results showed that only pulsed MHC IIhi cells were efficient in the in vivo anti-leukaemic protection, indicating that the protective effect of pulsed cells may be correlated with the immunophenotype of the injected population.
Immunotherapy also required the generation of large numbers of DC. In mice, DC numbers can be dramatically increased in lymphoid and non-lymphoid organs by in vivo administration of Flt3-L.25,36 Under our in vitro conditions, Flt3-L combined with GM-CSF for 9 days resulted in a twofold increase in MHC-IIhi DC number, but at the same time, since there were no MHC-IIlo cells displaying the DC phenotype, only half of the total population was typical myeloid-related DC. This slight increase in the DC number is comparable to the in vivo effect obtained on BM-derived DC.36
Interestingly, cells generated in GM-CSF and Flt3-L showed an increased capacity to migrate to draining LN. Further studies with purified populations are necessary to determine the population involved in this phenomenon.
Another important parameter is the functional maturation of DC, which can be induced in vitro by IL-4.7,8,19 Indeed, after 9 days of culture with GM-CSF+IL-4, both MHC-IIhi and MHC-IIlo cells displayed a similar DC phenotype but did not express 33D1 and F4/80 markers, and were thus phenotypically different from cells generated in GM-CSF. In line with their maturation state, cells obtained in GM-CSF+IL-4 cultures were more potent stimulators in MLR than cells generated with GM-CSF with or without Flt3-L.
Altogether, our results demonstrate the heterogeneity of DC generated in vitro from murine bone marrow. This is in agreement with the heterogeneity of DC previously reported in vivo and in vitro.27,37–39 Furthermore, we show that cytokines influence both the immunophenotype and the in vivo functions of in vitro-generated DC. Our data highlight the need to characterize the type of DC to be used in order to induce an efficient in vivo anti-tumoral immune response.
Acknowledgments
We are indebted to Immunex (Immunext, Seattle, WA) for the generous gift of F1t3-L. This work was supported by the ‘ssociation pour la Recherche sur le Cancer’, by the ‘Agence Nationale de Recherche contre le SIDA’ and by the ‘Foundation de France–Foundation contre la Leucémie’. C.M. was supported by a private fellowship from ARDIVI and ARMO.
Abbreviations
- APC
antigen-presenting cells
- DC
dendritic cells
- Flt3-L
Flt3 ligand
- GM-CSF
granulocyte–macrophage colony-stimulating factor
- IL-4
interleukin-4
- LN
lymph nodes
- L1210/hCD4
L1210 murine lymphocytic leukaemia cell line expressing the human CD4 molecule
- MLR
mixed lymphocyte reactions
- MHC-IIhi
high major histocompatibility complex class II expression
- MHC-IIlo
low MHC class II expression
REFERENCES
- 1.Steinman RM. The dendritic cell system and its role in immunogenicity. Annu Rev Immunol. 1991;9:271. doi: 10.1146/annurev.iy.09.040191.001415. [DOI] [PubMed] [Google Scholar]
- 2.Guéry J-C, Adorini L. Dendritic cells are the most efficient in presenting endogenous naturally processed self-epitopes to class II-restricted T cells. J Immunol. 1995;154:536. [PubMed] [Google Scholar]
- 3.Inaba K, Steinman RM. Protein-specific helper T-lymphocyte formation initiated by dendritic cells. Science. 1985;229:475. doi: 10.1126/science.3160115. [DOI] [PubMed] [Google Scholar]
- 4.Croft M, Duncan DD, Swain SL. Response of naive antigen-specific CD4+ T cells in vitro: characteristics and antigen-presenting cell requirements. J Exp Med. 1992;176:1431. doi: 10.1084/jem.176.5.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Steinman RM, Gutchinov B, Witmer MD, Nussenzweig MC. Dendritic cells are the principal stimulators of the primary mixed leukocyte reaction in mice. J Exp Med. 1983;157:613. doi: 10.1084/jem.157.2.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Metlay JP, Puré E, Steinman RM. Distinct features of dendritic cells and anti-Ig activated B cells as stimulators of the primary mixed leukocyte reaction. J Exp Med. 1989;169:239. doi: 10.1084/jem.169.1.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mayordomo JI, Zorina T, Storkus WJ, et al. Bone marrow-derived dendritic cells pulsed with synthetic tumour peptides elicit protective and therapeutic antitumour immunity. Nat Med. 1995;1:1297. doi: 10.1038/nm1295-1297. [DOI] [PubMed] [Google Scholar]
- 8.Zitvogel L, Mayordomo JI, Tjandrawan T, et al. Therapy of murine tumors with tumor peptide-pulsed dendritic cells: dependance on T cells, B7 costimulation, and T helper cell 1-associated cytokines. J Exp Med. 1996;183:87. doi: 10.1084/jem.183.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ashley DM, Faiola B, Nair S, Hale LP, Bigner DD, Gilboa E. Bone marrow-generated dendritic cells pulsed with tumor extracts or tumor RNA induce antitumor immunity against central nervous system tumors. J Exp Med. 1997;186:1177. doi: 10.1084/jem.186.7.1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bakker ABH, Marland G, de Boer AJ, et al. Generation of antimelanoma cytotoxic T lymphocytes from healthy donors after presentation of melanoma-associated antigen-derived epitopes by dendritic cells in vitro. Cancer Research. 1995;55:5330. [PubMed] [Google Scholar]
- 11.van Elsas A, van der Burg SH, van der Minne C, et al. Peptide-pulsed dendritic cells induce tumoricidal cytotoxic T lymphocytes from healthy donors against stably HLA-A*0201-binding peptides from the melan-A/MART-1 self antigen. Eur J Immunol. 1996;26:1683. doi: 10.1002/eji.1830260803. [DOI] [PubMed] [Google Scholar]
- 12.Hsu FJ, Benike C, Fagnoni F, et al. Vaccination of patients with B-cell lymphoma using autologous antigen-pulsed dendritic cells. Nat Med. 1996;2:52. doi: 10.1038/nm0196-52. [DOI] [PubMed] [Google Scholar]
- 13.Nestle FO, Alijagic S, Gilliet M, et al. Vaccination of melanoma patients with peptide-or tumor lysate-pulsed dendritic cells. Nat Med. 1998;4:328. doi: 10.1038/nm0398-328. [DOI] [PubMed] [Google Scholar]
- 14.Sallusto F, Lanzavecchia A. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor α. J Exp Med. 1994;179:1109. doi: 10.1084/jem.179.4.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reid CD, Stackpoole A, Meager A, Tikerpae J. Interactions of tumor necrosis factor with granulocyte-macrophage colony-stimulating factor and other cytokines in the regulation of dendritic cell growth in vitro from early bipotent CD34+ progenitors in human bone marrow. J Immunol. 1992;149:2681. [PubMed] [Google Scholar]
- 16.Rosenzwajg M, Camus S, Guigon M, Gluckman J-C. The influence of interleukin (IL) -4, IL-13 and Flt3 ligand on human dendritic cell differentiation from cord blood CD34+ progenitor cells. Exp Hematol. 1998;26:63. [PubMed] [Google Scholar]
- 17.Romani N, Gruner S, Brang D, et al. Proliferating dendritic cell progenitors in human blood. J Exp Med. 1994;180:83. doi: 10.1084/jem.180.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Inaba K, Inaba M, Romani N, et al. Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte/macrophage colony-stimulating factor. J Exp Med. 1992;176:1693. doi: 10.1084/jem.176.6.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lu L, McCaslin D, Starzl TE, Thomson AW. Bone marrow-derived dendritic cell progenitors (NLDC 145+, MHC class II+, B7dim, B7−) induce alloantigen-specific hyporesponsiveness in murine T lymphocytes. Transplantation. 1995;60:1539. doi: 10.1097/00007890-199560120-00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Romani N, Koide S, Crowley M, et al. Presentation of exogenous protein antigens by dendritic cells to T cell clones. Intact protein is presented best by immature epidermal Langerhans cells. J Exp Med. 1989;169:1169. doi: 10.1084/jem.169.3.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Larsen CP, Steinman RM, Witmer-Pack M, Hankins DF, Morris PJ, Austyn JM. Migration and maturation of Langerhans cells in the skin transplants and explants. J Exp Med. 1990;172:1483. doi: 10.1084/jem.172.5.1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Larsen CP, Ritchie SC, Hendrix R, et al. Regulation of immunostimulatory function and costimulatory molecule (B7 and B7) expression on murine dendritic cells. J Immunol. 1994;152:5208. [PubMed] [Google Scholar]
- 23.Caux C, Dezutter-Dambuyant C, Schmitt D, Banchereau J. GM-CSF and TNF-α cooperate in the generation of dendritic Langerhans cells. Nature. 1992;360:258. doi: 10.1038/360258a0. [DOI] [PubMed] [Google Scholar]
- 24.Zhang Y, Mukaida N, Wang J-B, Harada A, Akiyama M, Matsushima K. Induction of dendritic cell differentiation by granulocyte-macrophage colony stimulating factor, stem cell factor, and tumor necrosis factor αin vitro from lineage phenotypes negative c-kit+ murine hematopoietic progenitor cells. Blood. 1997;90:4842. [PubMed] [Google Scholar]
- 25.Maraskovsky E, Brasel K, Teepe M, Roux ER, Lyman SD, Shortman K, McKenna HJ. Dramatic increase in the numbers of functionally mature dendritic cells in Flt3 ligand-treated mice: multiple dendritic cell subpopulations identified. J Exp Med. 1996;184:1953. doi: 10.1084/jem.184.5.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Salomon B, Lorès P, Pioche C, Racz P, Jami J, Klatzmann D. Conditional ablation of dendritic cells in transgenic mice. J Immunol. 1994;152:537. [PubMed] [Google Scholar]
- 27.Salomon B, Cohen JL, Masurier C, Klatzmann D. Three populations of mouse lymph node dendritic cells with different origins and dynamics. J Immunol. 1998;160:708. [PubMed] [Google Scholar]
- 28.Idziorek T, Klatzmann D. Functional expression of the CD4 protein after cross-linking to red blood cells with a bifunctional reagent. Biochem Biophys Acta. 1991;1062:39. doi: 10.1016/0005-2736(91)90332-3. [DOI] [PubMed] [Google Scholar]
- 29.Austyn JM, Kupiec-Weglinski JW, Hankins DF, Morris PJ. Migration patterns of dendritic cells in the mouse. Homing to T cell-dependent areas of spleen, and binding within marginal zone. J Exp Med. 1988;167:646. doi: 10.1084/jem.167.2.646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Inaba K, Steinman RM, Witmer Pack M, et al. Identification of proliferating dendritic cell precursors in mouse blood. J Exp Med. 1992;175:1157. doi: 10.1084/jem.175.5.1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lu L, Hsieh M, Oriss TB, et al. Generation of DC from mouse spleen cell cultures in response to GM-CSF: immunophenotypic and functional analyses. Immunology. 1995;84:127. [PMC free article] [PubMed] [Google Scholar]
- 32.Troy AJ, Hart DNJ. Dendritic cells and cancer: progress toward a new cellular therapy. J Hematother. 1997;6:523. doi: 10.1089/scd.1.1997.6.523. [DOI] [PubMed] [Google Scholar]
- 33.Garrigan K, Moroni-Rawson P, McMurray C, et al. Functional comparison of spleen dendritic cells and dendritic cells cultured in vitro from bone marrow precursors. Blood. 1996;88:3508. [PubMed] [Google Scholar]
- 34.Pierre P, Turley SJ, Gatti E, et al. Developmental regulation of MHC class II transport in mouse dendritic cells. Nature. 1997;388:787. doi: 10.1038/42039. [DOI] [PubMed] [Google Scholar]
- 35.Wang B, Merva M, Dang K, Ugen KE, Williams WV, Weiner DB. Immunization by direct DNA inoculation induces rejection of tumor cell challenge. Hum Gene Ther. 1995;6:407. doi: 10.1089/hum.1995.6.4-407. [DOI] [PubMed] [Google Scholar]
- 36.Shurin MR, Pandharipande PP, Zorina TD, et al. Flt3 ligand induces the generation of functionally active dendritic cells in mice. Cell Immunol. 1997;179:174. doi: 10.1006/cimm.1997.1152. [DOI] [PubMed] [Google Scholar]
- 37.Kraal G, Breel M, Janse M, Bruin G. Langerhans' cells, veiled cells, and interdigitating cells in the mouse recognized by a monoclonal antibody. J Exp Med. 1986;163:981. doi: 10.1084/jem.163.4.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Crowley M, Inaba K, Witmer-Pack M, Steinman RM. The cell surface of mouse dendritic cells: FACS analyses of dendritic cells from different tissues including thymus. Cell Immunol. 1989;118:108. doi: 10.1016/0008-8749(89)90361-4. [DOI] [PubMed] [Google Scholar]
- 39.Gluckman JC, Canque B, Chapuis F, Rosenzwajg M. In vitro generation of human dendritic cells and cell therapy. Cytokines, Cellular and Molecular Therapy. 1997;3:187. [PubMed] [Google Scholar]