Abstract
Monoclonal antibodies (mAb) specific for the clonotype of an autoreactive T cell may be useful reagents in the modulation of autoimmune disease. We have previously reported the generation of a set of mAb specific for the clonotypic structure of a human T-cell clone recognizing an epitope of human cartilage gp-39. This glycoprotein was recently identified as a candidate autoantigen in rheumatoid arthritis. Here, we demonstrate for the first time that small amounts of immobilized anticlonotype mAb can induce anergy in the autoreactive clone. Following the anergic stimulus, T cells failed to proliferate upon restimulation as a result of a lack of interleukin-2 (IL-2) gene transcription. In addition, a diminished interferon-γ (IFN-γ) production was found. Our data indicate that anergy was not a result of T-cell receptor (TCR) downmodulation or the absence of free TCR. The anergic state was induced independent of costimulation or the presence of IL-2 and no protein synthesis was required for the induction of anergy. Anticlonotype mAb-induced anergy was prevented by cyclosporin A, suggesting that active signalling via the calcium/calcineurin pathway was required for the induction of anergy. In coculture experiments, anergic T cells were found to suppress the response of reactive cells from the same clone. This bystander suppression led to 90% inhibition of peptide-induced proliferation. Together, these findings suggest that mAb to the clonotypic structure of autoreactive T cells may be suitable reagents for the functional inactivation of these T cells in autoimmune diseases.
INTRODUCTION
Current therapies for autoimmune diseases are often non-specific and suffer from major side effects. The induction of antigen-specific peripheral tolerance is, therefore, considered an attractive approach for treatment of autoimmune diseases. One of the mechanisms underlying peripheral tolerance is T-cell anergy or unresponsiveness,1 which is defined as a state in which the T cell is alive but fails to respond to its antigen presented by functional antigen-presenting cells (APC). Failure of such cells to proliferate is caused by defective interleukin-2 (IL-2) production as a result of an IL-2 transcriptional block.2
Several models of T-cell anergy have been reported (reviewed by Schwartz,2 Johnson and Jenkins3 and Kersh and Allen4). Anergy has been described as a result of T-cell receptor (TCR) occupancy in the absence of costimulation. Anergy has also been obtained by partial triggering of the TCR, mediated by altered peptide ligands (APL) presented on a functional APC. In another model, anergy has been obtained by stimulation of human T-cell clones in the presence of high concentrations of peptide.
Monoclonal antibodies (mAb) directed to the clonotypic structure of relevant T cells may be used to manipulate autoantigen-specific T-cell responses and, hence, treat autoimmune diseases in a specific manner. These T cells may be identified on the basis of their antigen specificity or by demonstration of dominant TCR rearrangements at the site of the lesions.5,6
Clonotype-specific mAb may exert an immunosuppressive effect by depletion of autoreactive T cells or by blocking of antigen-induced proliferation. Another possible mechanism of action for these mAb may be modulation of T cells by the induction of anergy, as has already been shown in a non-specific manner for anti-CD3 mAb.7–9 An antibody approach may have several advantages. Antibodies have a high specificity and high affinity. Furthermore, the functional properties of an antibody can be modified by recombinant DNA techniques. The construction of single chain mAb or specific human Fc-regions coupled to the antigen-binding part of clonotype-specific mAb can provide good pharmacokinetics9–11 and at the same time minimize the risk of unintentional T-cell stimulation.12,13
We have previously reported the identification of human cartilage gp-39 (HC gp-39) as a candidate autoantigen in rheumatoid arthritis.14 Peptides encompassing amino acids 263–275 of this protein are recognized by 30–40% of rheumatoid arthritis patients. We have isolated a T helper 1 (Th1) clone recognizing epitope HC gp-39263–274 in the context of DRB1*0401 and generated a set of mAb directed against the clonotype of this autoreactive human T-cell clone. The mAb were found to block antigen-induced proliferation of the autoreactive T-cell clone. In addition, the mAb were able to trigger the T cell by its receptor.15 In the present study, we show that these mAb can modulate the response of the T-cell clone by the induction of anergy in vitro. The mechanism underlying this type of anergy was investigated and related to other models of T-cell anergy. We further investigated the potency of anergic cells to suppress the response of non-anergic cells.
MATERIALS AND METHODS
Reagents
The medium used in all culture experiments was Dulbecco's modified Eagle's medium (DMEM)/Ham's F12 modified (Gibco BRL, Paisley, UK).
T-cell clone H.243 was derived from a rheumatoid arthritis patient as described by Verheijden et al.14 This Th1-like T-cell clone recognizes epitope RSFTLASSETGV from HC gp-39 in the context of HLA-DRB1*0401. Upon antigen-specific stimulation the clone was found to produce IL-2 and large amounts of interferon-γ (IFN-γ) as opposed to small amounts of IL-4. The TCR of this clone is characterized as Vα8 and Vβ9. Cells were routinely cultured in DMEM/Ham's F12, 10% normal human serum (NHS), 20 U/ml rIL-2, 5 U/ml rIL-4-and periodically restimulated with antigen and histocompatible APC, or with phytohaemagglutinin (PHA) and feeder cells.
Ten mouse anticlonotype mAb to human T-cell clone H.243 were generated as described by Steenbakkers et al.15 mAb ZP 7A directed to zona pellucida was used as a control antibody (provided by Dr M. van Duin, Organon, Oss, The Netherlands).
Peptide HC gp-39261–275 (FGRSFTLASSETGVG) and HC gp-39262–277 (GRSFTLASSETGVGAP) were synthesized by solid phase peptide synthesis using an automated Milligen 9050 synthesizer and purified by reverse phase high pressure liquid chromatography (HPLC).
Primers were synthesized using a DNA synthesizer (Applied Biosystems 394) according to the manufacturer's instructions. IL-2 5′ primer AACTCCTGTCTTGCATTGCA was synthesized according to the sequence reported by Sorg et al.16 and IL-2 3′ primer TGTTTCAGATCCCTTTAGTTC was deduced from the EMBL data bank (fragment length 350 bp). Glyceraldehyde-3-phosphate dehydrogenase (GAP DH) 5′ primer CCCTTCATTGACCTCAACTACATGG and GAPDH 3′ primer GGTCCACCACCCTGTTGCTGTA GCC were deduced from the sequence in the EMBL data bank (fragment length 874 bp).
Induction of anergy using clonotype-specific mAb
Twenty-four-well culture plates were coated (overnight, room temperature) with 1 ml sheep antimouse immunoglobulin at 40 μg/ml in phosphate-buffered saline (PBS). The wells were washed once and then incubated with 1 ml mAb at a concentration of 2 μg/ml in PBS for 2 hr. Excess of free mAb was removed by washing and 2×106 resting T cells were added in 2 ml DMEM/Ham's F12, 10% NHS. Where indicated, cyclosporin A (Sandoz, Basel, Switzerland), rIL-2, cycloheximide (Sigma, St Louis, MO) or HLA-DRB1*0401 matched APC (3000 rad irradiated) were added at the time of culture initiation. After overnight incubation, the plates were chilled on ice and the T cells were resuspended by pipetting. The cells were washed once with complete culture medium and used for a T-cell proliferation assay, cytokine analysis and fluorescence-activated cell sorting (FACS) analysis, as described below.
T-cell proliferation assay
The antigen-specific proliferative response of T cells was assessed in flat-bottomed microwell cultures containing 2×104 T cells, 1×105 HLA-DRB1*0401 matched, irradiated (3000 rad) peripheral blood mononuclear cells (PBMC) and variable antigen concentrations in 200 μl DMEM/Ham's F12, 10% NHS. After 2 days incubation at 37°, the cells were pulsed with 0·5 μCi [3H]thymidine and incubated for another 16–18 hr. Finally, the cells were harvested onto glass fibre filters and [3H]thymidine incorporation was measured by gas scintillation on a Matrix 96 (Packard, Meriden, CT). Each variable was tested in triplicate. Control cultures were stimulated with either 2·5 μg/ml PHA or blank medium. The proliferative response to IL-2 was assessed by incubating T cells with 20 U/ml rIL-2 in the absence of APC.
Cytokine analysis
For cytokine analysis, wells of 24-well culture plates were inoculated with 5×105 T cells, 2·5×106 DRB1*0401-matched PBMC (3000 rad irradiated) and variable antigen concentrations in 750 μl DMEM/Ham's F12, 10% NHS. Control cultures were performed with either 2·5 μg/ml PHA or blank medium.
After 4 hr incubation at 37°, the cells were harvested for IL-2 mRNA analysis by reverse transcription–polymerase chain reaction (RT–PCR). Briefly, cells were washed once with PBS and RNA was extracted using the RNAzol™ B method (Campro Scientific, Veenendaal, The Netherlands). Then, cDNA was prepared with Superscript II reverse transcriptase (Gibco BRL). Finally, PCR amplification was performed using specific primer sets for IL-2 and for a housekeeping enzyme, GAPDH. cDNAs were amplified for 30 cycles, each one performed at 94° for 30 s, 55° for 30 s and 72° for 1 min. The PCR products were loaded on an agarose gel containing 1% agarose and 0·5 μg/ml ethidium bromide and electrophoresed at 120 V for 1 hr. Fragments of DNA were visualized under UV light.
From duplicate cultures, supernatants were harvested after 24 hr of culture and tested for IFN-γ in a specific capture enzyme-linked immunosorbent assay (ELISA). Anti-IFN-γ (M700 A; Endogen, Cambridge, MA) was used as the capture antibody and anti-IFN-γ (M701; Endogen) conjugated to horseradish peroxidase (HRP) was used for detection. Recombinant human IFN-γ (Campro Scientific) was used as a standard.
RESULTS
Clonotype-specific mAb induce unresponsiveness of human T-cell clone H.243 to subsequent stimulation with antigen and APC
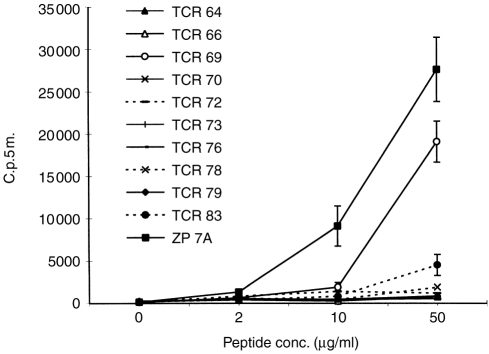
Previously we had found that immobilized anticlonotype mAb to H.243 can functionally trigger the TCR of this clone.15 Here, we have investigated whether the same antibodies could induce anergy. For this purpose, 10 different anticlonotype mAb were immobilized on 24-well plates and incubated overnight with H.243 T cells to give the anergic stimulus. Then, T cells were removed from the plates and it was tested whether they could respond to increasing concentrations of HC gp-39262–277 presented by irradiated, DRB1*0401-matched APC. Figure 1 shows that this response was completely abrogated by 8 out of 10 mAb whereas H.243 cells incubated with a control mAb (ZP 7 A) still responded well to peptide presented by APC. The response of T cells incubated with TCR 69 and TCR 83 was significantly reduced, but not completely abrogated, at higher peptide concentrations.
Figure 1.
Clonotype-specific mAb induce anergy in human Th1-clone H.243. Immobilized anticlonotype mAb or control mAb ZP 7A were incubated overnight with H.243 T cells. After removal of the cells from the anergic stimulus, the cells were challenged with a full stimulus of increasing concentrations of peptide in the presence of DRB1*0401-matched APC. Proliferation was assessed by [3H]thymidine incorporation. Each value represents the mean counts per 5 min (c.p.5m.) of triplicate cultures ±SD.
Unresponsiveness is not due to poor cell viability or lack of free TCR
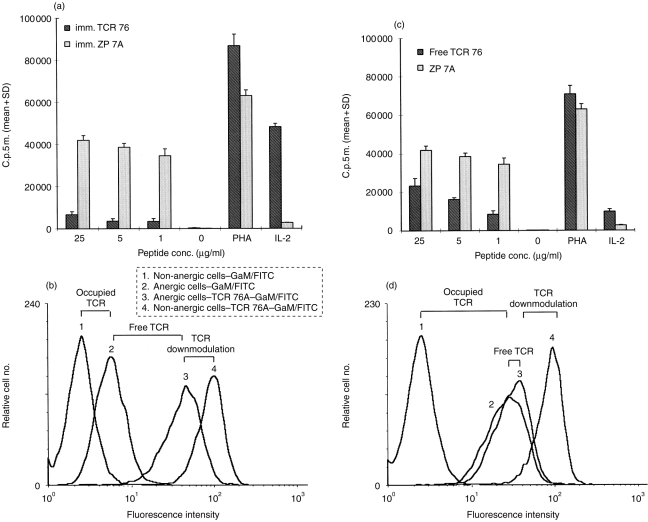
In the next experiment, the properties of the unresponsive T cells obtained from incubation with anticlonotype mAb were characterized in more detail. Anergy was induced by immobilized TCR 76 (anergic cells) and compared with an immobilized control mAb, ZP 7A (non-anergic cells). Following the induction phase, T cells were stimulated with increasing concentrations of HC gp-39261–275 or PHA, and irradiated DRB1*0401-matched APC. In addition, the T cells were stimulated with IL-2 in the absence of APC. HC gp-39261–275 was used in this experiment instead of HC gp-39262–277 because this peptide is superior in stimulating T-cell clone H.243. Nearly complete reduction (90%) of the proliferative response to peptide was observed after incubation with anticlonotype mAb, whereas the cells responded normally to PHA indicating cell viability (Fig. 2a). In addition, anergic cells were found to respond to IL-2 as opposed to non-anergic cells. This is in agreement with an increased level of CD25 expression on anergic cells (data not shown).
Figure 2.
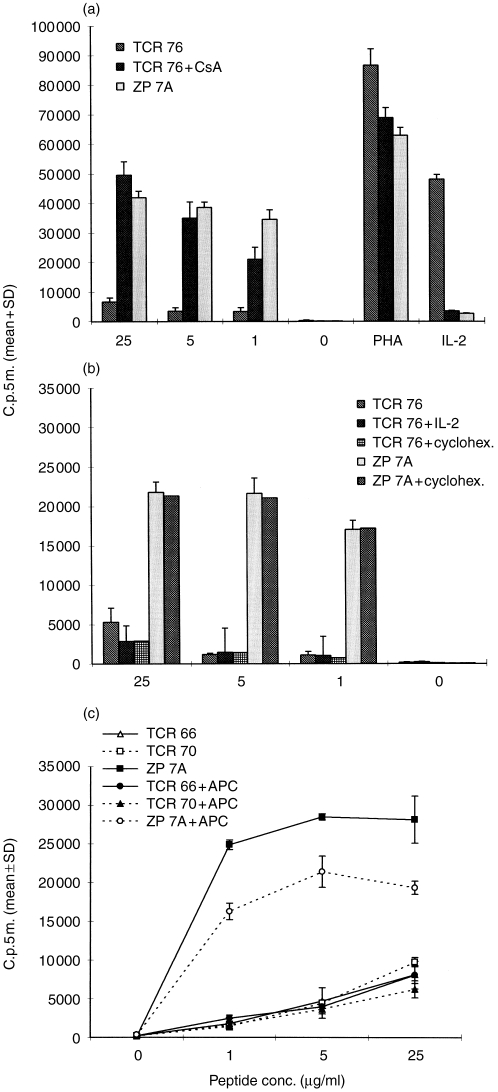
Induction of anergy by anticlonotype mAb TCR 76. (a) Unresponsiveness is not due to cell death or apoptosis. Immobilized TCR 76 (anergic cells) or control mAb ZP 7A (non-anergic cells) were incubated overnight with H.243 T cells. After removal of the cells from the anergic stimulus, the cells were challenged with DRB1*0401-matched APC, and PHA or increasing concentrations of peptide. In addition, T cells were incubated with IL-2 in the absence of APC. Proliferation was assessed by [3H]thymidine incorporation. Each value represents the mean counts per 5 min of triplicate cultures+standard deviation. (b) Unresponsiveness is not caused by TCR blockade or downmodulation. Non-anergic H.243 T cells resulting from incubation with immobilized ZP 7A (curves 1 and 4) or anergic T cells resulting from incubation with immobilized TCR 76 (curves 2 and 3) were stained directly with GAM–FITC (curves 1 and 2) or indirectly with TCR 76 and GAM–FITC (curves 3 and 4). Curve 1 reflects background fluorescence level. Curve 2reflects the number of TCR molecules occupied with anticlonotype mAb after removal from the anticlonotype mAb coated plates. Curve 3 reflects the total number of TCR molecules on anergic cells and curve 4 reflects the total number of TCR molecules on non-anergic cells. (c) and (d) represent similar experiments performed with free TCR 76 instead of immobilized TCR 76.
By FACS analysis, we investigated the availability of free TCR molecules after the anergic stimulus. For this purpose, four different stainings were performed on the cells from the experiment described above. Non-anergic cells were either directly stained with GAM–fluoroscein isothiocyanate (FITC) or indirectly with TCR 76 and goat anti-mouse (GAM)–FITC (Fig. 2b, curves 1 and 4, respectively). Anergic cells were also directly stained with GAM–FITC or indirectly with TCR 76 and GAM–FITC (Fig. 2b, curves 2 and 3, respectively). The difference in fluorescence between curves 1 and 2 reveals that a small amount of anticlonotype mAb was still bound to the T cells after removal from the anergic stimulus. Apparently, some mAb was detached from the plastic surface together with the T cells. Although TCR 76 itself is mitogenic at higher concentrations (Fig. 1c), the small amount of mAb left on the cells did not lead to proliferation (Fig. 2a, no antigen added). Further, from the difference between curves 4 and 3, it can be deduced that TCR expression is slightly downmodulated on the T cells after the overnight anergy-induction phase. However, the difference in fluorescence intensity between curves 3 and 2 reveals that the majority of TCR molecules are still freely available on anergic cells, suggesting that TCR blocking or downmodulation are not responsible for the anergic state. Note that the x-axis scale is logarithmic.
A similar experiment performed with free mAb instead of immobilized mAb showed that only a few TCR molecules are freely available in this experimental setting (Fig. 2d). Note that, here a similar level of TCR downmodulation was observed, but a higher amount of antibody was left on the cells. Despite this observation, the cells responded in a dose-dependent fashion to antigen presented by APC (Fig. 2c). This supports the suggestion that neither TCR blocking by mAb left on the cells after the anergic stimulus nor TCR downmodulation as observed in Fig. 2(b) are responsible for the unresponsiveness of H.243 incubated with immobilized TCR 76.
Similar results were obtained with two other anticlonotype mAb, TCR 66 and TCR 70 (results not shown).
Unresponsive H.243 cells show a reduced level of IL-2 gene transcription and diminished IFN-γ production
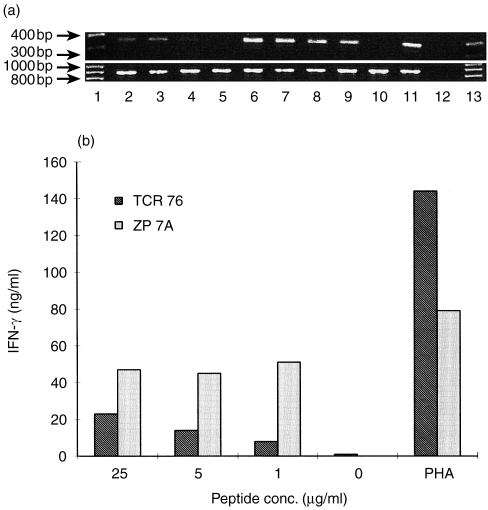
It has previously been shown that T-cell anergy may result from IL-2 gene transcription blockade.2 Thus, we investigated whether the same holds true for unresponsiveness induced by anticlonotype mAb TCR 76. Instead of measuring proliferation, cells were harvested 4 hr after restimulation for the detection of IL-2 mRNA by RT–PCR. PCR for housekeeping enzyme GAPDH was performed on the same cDNA samples as an internal control for RNA isolation and cDNA synthesis. It was found that using equal amounts of RNA, IL-2 gene transcription in anergic cells was significantly reduced after stimulation with 25 or 5 μg/ml peptide or nearly absent after stimulation with 1 μg/ml peptide, compared to IL-2 transcription of non-anergic cells (Fig. 3a).
Figure 3.
Analysis of cytokine production of anergic H.243 T cells. (a) IL-2 transcription is blocked in anergic cells. T-cell clone H.243 was incubated with anergy-inducing anticlonotype mAb, TCR 76, or control mAb, ZP 7A. Subsequently, the cells were stimulated with increasing concentrations of peptide and DRB1*0401-matched APC for 4 hr, and RNA was isolated from the cells. RT–PCRs for IL-2 (upper gel) and a housekeeping enzyme GAPDH (lower gel) were performed on RNA isolated from anergic T cells (lanes 2–6) and non-anergic T cells (lanes 7–11). Lane 1 and 13: 100 bp DNA ladder marker, lanes 2 and 7: 25 μg/ml peptide, lanes 3 and 8: 5 μg/ml peptide, lanes 4 and 9: 1 μg/ml peptide, lanes 5 and 10: medium control, lanes 6 and 11: PHA control, lane 12: PCR control without template. (b) IFN-γ production is diminished in anergic cells. H.243was treated either with anergy-inducing anticlonotype mAb, TCR 76, or a control mAb, ZP 7A. After treatment with mAb, the cells were stimulated with different concentrations of peptide (or PHA) and APC for 24 hr. Then, IFN-γ was determined in the supernatants using a specific sandwich ELISA.
We also investigated the effect of T-cell unresponsiveness on the level of IFN-γ-production. Cultures were set up similar as for the study of IL-2 gene transcription, but instead of harvesting the cells after 4 hr, supernatants were harvested after 24 hr. The amount of IFN-γ in the supernatants was determined in a sandwich ELISA using IFN-γ-specific antibodies. It was found that anergic cells produced a significantly lower amount of IFN-γ compared to non-anergic cells (Fig. 3b).
Unresponsiveness is long lasting
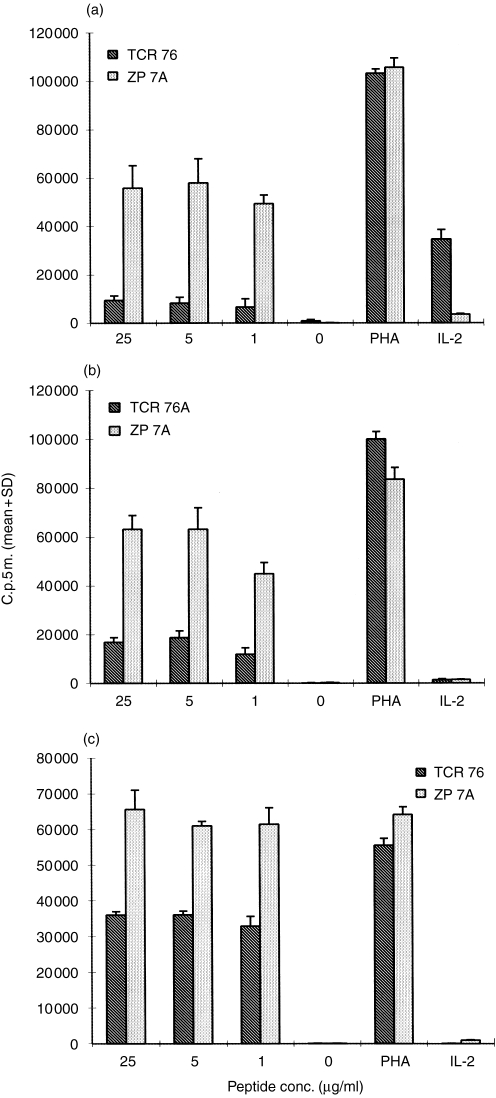
Anergy induced in murine T cells has been reported to have a long-lasting effect in vitro.7,17,18 Conversely, anergy studies with human T cells were not performed with long resting periods between anergy induction and the antigenic stimulus.19–22 This may be related to the fact that human T cells do not survive in the absence of IL-2. We here investigated the duration of anergy induced by clonotype-specific mAb. Anergic cells were allowed to rest for a maximum of 7 days either without IL-2 or in the presence of 9 U/ml IL-2. Cells cultured in the absence of IL-2 did not survive this resting period. In the presence of IL-2, 30–40% of the cells died within 2 days. After 7 days, only 5–20% of the T cells were left. The remaining cells, however, still showed a significantly reduced reactivity upon challenge with peptide presented by APC (Fig. 4b,c). Thus it appears that anergy induced by anticlonotype mAb in vitro can last for at least 7 days.
Figure 4.
Unresponsiveness is long lasting.Immobilized TCR 76 or control mAb ZP 7A were incubated overnight with H.243 T cells. Subsequently, the cells were challenged with increasing concentrations of peptide and DRB1*0401-matched APC immediately (a), or following a rest period of 2 days (b) or 7 days in the presence of IL-2 (c). Proliferation was assessed by [3H]thymidine incorporation. Each value represents the mean counts per 5 min of triplicate cultures+standard deviation.
Cyclosporin A prevents the induction of anergy by anticlonotype mAb, whereas IL-2 or cycloheximide does not prevent anergy
Different effects of cyclosporin A (CsA) and IL-2 on the induction of anergy have been reported depending on the way anergy was induced.7,17,19,23–25 Further, it has been reported that cycloheximide prevents the induction of anergy.18 We investigated the effect of these compounds on the induction of anergy by anticlonotype mAb. The experiments were performed as described above using TCR 76 as the anergy-inducing mAb and ZP 7A as the control mAb. During the induction phase either 1 μg/ml CsA, or 500 U/ml rIL-2 or 3 μg/ml cycloheximide was added to the cultures. These compounds were washed out before initiation of the proliferation assay.
The calcineurin inhibitor CsA prevented the induction of anergy by TCR 76, suggesting that an active signal via the calcium/calcineurin pathway is required for the induction of anergy (Fig. 5a). The addition of IL-2 during the induction phase had no effect (Fig. 5b), indicating that lack of IL-2 production during the induction phase is not responsible for anergy. Further, cycloheximide did not prevent the induction of anergy, indicating that protein synthesis is not required to obtain anergy.
Figure 5.
Effects of CsA, IL-2, cycloheximide and costimulation on clonotype-specific anergy induction. (a) CsA prevents the induction of anergy. H.243 cells were incubated overnight with immobilized anticlonotype mAb TCR 76 either in the presence or absence of 1 μg/ml CsA, or with control mAb ZP 7A. Subsequently, the cells were restimulated with increasing concentrations of peptide or PHA, and APC. In addition, T cells were incubated with IL-2 in the absence of APC. Proliferation was assessed by [3H]thymidine incorporation. Each value represents the mean counts per 5 min of triplicate cultures+standard deviation. (b) IL-2 and cycloheximide do not prevent the induction of anergy by anticlonotype mAb TCR 76. This experiment was performed under the same conditions as the experiment described in (a) except that 500 U/ml rIL-2 or 3 μg/ml cycloheximide were used instead of CsA. (c) Induction of anergy by anticlonotype mAb is costimulation independent. Immobilized TCR 66, TCR 70 or control mAb ZP 7A were incubated overnight with H.243 T cells either in the presence or absence of DRB1*0401-matched APC. Subsequently, the cells were restimulated with increasing concentrations of peptide and fresh APC from the same donor as used during the induction phase. Proliferation was assessed by [3H]thymidine incorporation. Each value represents the mean counts per 5min of triplicate cultures±SD.
Similar results were obtained with another two clonotype-specific mAb, TCR 66 and TCR 70 (results not shown).
Induction of anergy by anticlonotype mAb is costimulation independent
Triggering the TCR in the absence of costimulation can induce anergy in T cells. However, anergy can also be induced in the presence of costimulation using altered peptide ligands (APL) or high concentrations of soluble peptides.2 Apparently, the role of costimulators may vary with the nature of the anergizing stimulus. To gain more insight into the mechanism of anergy induction by anticlonotype mAb, we investigated the influence of costimulation. In this experiment, 2×105 T cells and 5×105 irradiated, DR-matched APC were added to wells coated with anticlonotype mAb, TCR 66 or TCR 70. Control cultures with mAb in the absence of APC were performed as described above. After overnight incubation, the cells were collected, washed and restimulated in a T-cell proliferation assay with peptide HC gp-39261–275 and fresh irradiated, DR-matched APC from the same donor as the APC used during the induction phase. The results show that the induction of anergy by TCR 66 and TCR 70 was independent of costimulation (Fig. 5c). This observation was confirmed by using anti-CD28 for costimulation instead of APC. Co-immobilization of anti-CD28 and anticlonotype mAb resulted in the same level of unresponsiveness than immobilization of anticlonotype mAb alone (data not shown).
Anergic cells suppress the response of non-anergic cells
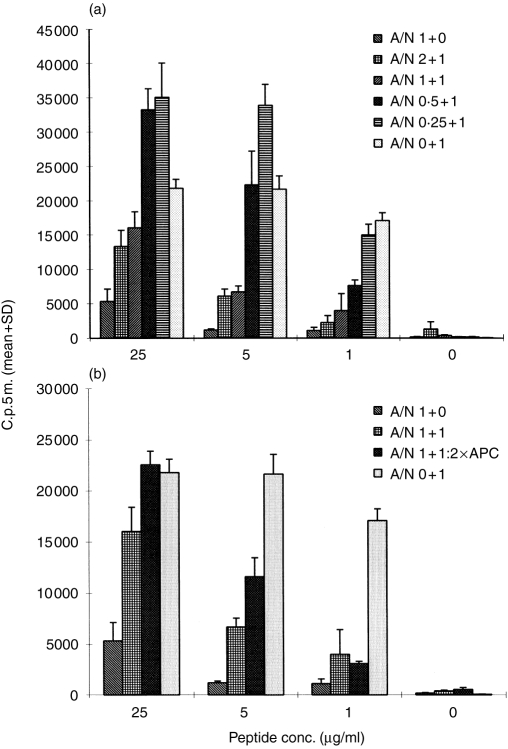
Recently, it was suggested that anergic T cells can have a role in bystander suppression.26–28 Thus, we investigated whether anergic H.243 cells were able to suppress the response of non-anergic H.243 cells. Anergy was induced with TCR 76 and non-anergic cells were obtained from incubation with control mAb ZP 7A. Subsequently, proliferation assays were performed using different T-cell populations, namely, 2×104 anergic T cells per well, 2×104 non-anergic T cells per well, or mixtures of decreasing concentrations of anergic T cells (4×104, 2×104, 1×104 and 0·5×104) and 2×104 non-anergic T cells per well. The response of non-anergic cells was significantly reduced, in a dose-related fashion, by the addition of anergic cells (Fig. 6a). Approximately 90% reduction of proliferation was obtained at an antigen concentration of 1 μg/ml and a ratio anergic cells versus non-anergic cells (A/N ratio) of 2:1. At low A/N ratios and higher antigen concentrations, less reduction (or enhancement) of proliferation was observed. This may be explained by the fact that at low A/N ratios the non-anergic cells are not completely inhibited and may be stimulated to produce an excess of IL-2. Because anergic cells can respond to IL-2 (Fig. 2a) and because we did not irradiate these cells, total proliferation may be less reduced or even higher than proliferation of non-anergic cells alone.
Figure 6.
Anergic H.243 T cells suppress the response of non-anergic H.243 T cells. (a) Immobilized TCR 76 or control mAb ZP 7A were incubated overnight with H.243 T cells. Then, both T-cell populations were used in a proliferation assay with increasing concentrations of peptide (or PHA) and APC using 2×104 T cells per well. In addition, variable amounts of anergic cells (incubated with TCR 76) were mixed with 2×104 non-anergic cells (incubated with ZP 7A), and a proliferation assay was performed. A/N: ratio of anergic cells versus non-anergic cells; 1–2×104 cells. Proliferation was assessed by [3H]thymidine incorporation. Each value represents the mean counts per 5 min of triplicate cultures+SD. (b) Equal amounts (2×104 cells) of anergic and non-anergic cells were mixed and proliferation was assessed in a proliferation assay using 1×105 and 2×105 irradiated APC per well.
A twofold increase in APC concentration does not restore the response of non-anergic cells at a nearly saturating concentration of antigen (1 μg/ml), nor does doubling the concentration of APC at a fivefold higher antigen concentration (Fig. 6b). Therefore, steric hindrance or competition for antigen are not likely to explain the reduced reactivity of non-anergic cells.
DISCUSSION
In the present study, we have shown that clonotype-specific mAb are able to modulate the response of an autoreactive, DRB1*0401-restricted T-cell clone specific for an epitope on HC gp-39. Anergy was induced by immobilized anticlonotype mAb. After removal of the anergic stimulus, the clone could not be stimulated with antigen presented on DRB1*0401-matched APC. Anergy seems to be a result of defective IL-2 gene transcription upon restimulation. Besides a defective IL-2 gene transcription the anergic cells also showed a diminished IFN-γ production.
Cell death or apoptosis did not account for T-cell unresponsiveness as anergic cells retained the ability to respond to PHA and exogenous IL-2. Compared to non-anergic cells, anergic cells displayed a slightly reduced number of freely accessible TCR molecules at the cell surface as a result of both TCR downmodulation and the presence of a residual amount of clonotype-specific mAb left on the cells after the anergic stimulus. However, it is unlikely that this small reduction of TCR is responsible for the observed anergy since only a very small number of TCR are required for T-cell activation as shown by Viola and Lanzavecchia29 and by our own data (using free mAb instead of immobilized mAb). Further, we conclude that continuous presence of a small amount of anticlonotype mAb on the cells was not responsible for the anergic effect, because incubation with free anticlonotype mAb resulted in a larger amount of antibody on the T cells and still did not result in the same level of unresponsiveness.
Previously, we have demonstrated that immobilized anticlonotype mAb are able to trigger the TCR of the specific T-cell clone leading to proliferation. However, the same stimulus does not lead to proliferation if the T cells are removed from the antibody-coated plates after overnight incubation (no background proliferation in the proliferation assay without antigen). From the latter observation, it can also be concluded that the residual amount of mAb left on the cells after the anergic stimulus does not lead to proliferation, which is in agreement with our findings that low amounts of anticlonotype mAb (<100 ng/ml; data not shown) are not mitogenic.
It appeared that very small amounts of anticlonotype mAb are required for anergy induction. Coating wells with as little as 8 ng/ml antibody was sufficient to induce anergy (results not shown). The high affinity of mAb for the TCR (nm range) may account for this quantitative effect in vitro. In vivo, the prolonged bioavailability of antibodies may be an additional benefit. Furthermore, the biological effect of antibodies can be manipulated by changing their molecular design.12,13 This makes antibodies attractive candidates for the induction of anergy in autoimmune disease. In vivo, crosslinking of the TCR by mAb (which seems to be required for efficient induction of anergy; Fig. 2) may be obtained by Fc-receptor-bearing cells.15,30,31
The anticlonotype induced anergy described in this study is distinct from T-cell anergy induced by TCR occupancy in the absence of costimulation because, in the latter model, anergy induction is prevented by costimulation or by the addition of IL-2.2,22 Moreover, anergy induction requires protein synthesis,18 which is in contradiction to our findings. One may argue that anticlonotype mAb behave like APL. Thus, partial triggering the TCR by anticlonotype mAb might lead to the induction of anergy. Indeed anticlonotype mAb induced anergy resembles APL-induced anergy in murine T-cell clones in that anergy is prevented by CsA24 and is not prevented by the presence of costimulation.2 However, it was recently reported17 that APL-induced anergy could be rescued by addition of IL-2 during the induction phase, which is opposed to our findings with clonotype-specific mAb. The same holds true for anergy induced by high concentrations of peptide. CsA and costimulation added during the induction of this form of anergy showed similar effects, as we found for the induction of anergy by anticlonotype mAb.2,32 However, Essery et al.19 reported that high concentrations of IL-2 (300–1000 U/ml) prevented the induction of anergy, whereas in our experiments anergy was not prevented by as much as 500 U/ml IL-2.
Further, several investigators have used anti-CD3 for the induction of anergy. Anti-CD3-induced anergy in naive CD4+ mouse T cells8 and in human resting T cells33 differs from the anticlonotype mAb-induced anergy reported here by the observation that anergy in these experiments could not be prevented by CsA33 (F. Andris, personal communication). Anti-CD3 induced anergy in murine Th1 clones7 was prevented in the presence of accessory cells, thereby suggesting that anti-CD3-induced anergy is distinct from clonotype-induced anergy. In this respect, it is worth noting that triggering the TCR/CD3 complex by anti-CD3 may be different from triggering the TCR/CD3 complex by anti-TCR, as was recently reported by Kishimoto et al.34 Thus, we believe that the anticlonotype mAb-induced anergy model presented in this study is different from previously reported models. Nevertheless, we fully appreciate that it is hard to compare the great variety of anergy data in the literature, because of the differences in experimental conditions (naive T cells, T-cell clones and different species).
Peripheral tolerance is an important mechanism for generating unresponsiveness against both self and external antigens. It is mediated by T cells using at least three non-mutally exclusive mechanisms: active suppression, apoptosis or clonal deletion, and anergy.1,35,36 It was reported that tolerance can be induced by epitopes of the antigen even if they are different from the autoreactive epitope.37 Recently, it was also found that anergic T cells are able to transfer unresponsiveness to a separate population of antigen-responsive T cells26–28 and it was suggested that this phenomenon called infectious anergy might be responsible for T-cell-mediated suppression in oral tolerance.27 In this report, we have shown that anergic T cells can inhibit the response of non-anergic cells from the same T-cell clone in a dose-dependent fashion. Up to 90% inhibition was found at nearly saturating concentrations of antigen. At higher antigen concentrations, less inhibition was observed but these high concentrations are not likely to reflect a physiological situation. To date, we have not extensively investigated the mechanism underlying this form of bystander suppression. Our preliminary data suggest that competition for APC surface (steric hindrance) or competition for antigen are not responsible for this suppresive effect. Further, it seems unlikely that competition for locally produced IL-2 as proposed by Lombardi et al.26 is responsible for this suppresive effect, as anergic cells also respond to IL-2 and the net result would be proliferation as well. The anergic cells may exert their suppressive effect by secretion of soluble factors or another, as yet undefined, mechanism.
We have previously shown that the anticlonotype mAb used in this study were able to inhibit antigen-driven proliferation of the HC gp-39-specific Th1 clone. In this report, we have shown that small amounts of these mAb induce anergy in this autoreactive T-cell clone, which is, at the same time, the first demonstration of human T-cell anergy by clonotype-specific antibodies. These findings suggest that mAb to the clonotypic structure of autoreactive T cells may be suitable reagents for the functional inactivation of these T cells in autoimmune diseases. However, before starting therapeutic development of such mAb, it will be necessary to establish the presence of a limited set of clonal or oligoclonal expanded T cells with identical clonotypic structures at the inflammatory sites in autoimmune diseases. Anticlonotype mAb may be helpful in performing these studies.
Acknowledgments
We would like to thank Mrs L. den Hoed for cytokline analysis, and Dr G. Verheijden and Dr. A. Miltenburg for critical reading of the manuscript. Dr. C. van Staveren is acknowledged for peptide synthesis.
REFERENCES
- 1.Melamed D, Friedman A. In vivo tolerization of Th1 lymphocytes following a single feeding with ovalbumin: anergy in the absence of suppression. Eur J Immunol. 1994;24:1974. doi: 10.1002/eji.1830240906. [DOI] [PubMed] [Google Scholar]
- 2.Schwartz RH. Models of T cell anergy: is there a common molecular mechanism. J Exp Med. 1996;184:1. doi: 10.1084/jem.184.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Johnson JG, Jenkins MK. The role of anergy in peripheral T cell unresponsiveness. Life Sci. 1994;55:1767. doi: 10.1016/0024-3205(94)90087-6. [DOI] [PubMed] [Google Scholar]
- 4.Kersh GJ, Allen PM. Essential flexibility in the T-cell recognition of antigen. Nature. 1996;380:495. doi: 10.1038/380495a0. [DOI] [PubMed] [Google Scholar]
- 5.Oksenberg JR, Panzara MA, Begovich AB, et al. Selection for T-cell receptor Vβ-Dβ-Jβ gene rearrangements with specificity for a myelin basic protein peptide in brain lesions of multiple sclerosis. Nature. 1993;362:68. doi: 10.1038/362068a0. [DOI] [PubMed] [Google Scholar]
- 6.Allegretta M, Albertini RJ, Howell MD, et al. Homologies between T cell receptor junctional sequences unique to multiple sclerosis and T cells mediating experimental allergic encephalomyelitis. J Clin Invest. 1994;94:105. doi: 10.1172/JCI117295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jenkins MK, Chen C, Jung G, Mueller DL, Schwartz RH. Inhibition of antigen-specific proliferation of type 1 murine T cell clones after stimulation with immobilized anti-CD3 monoclonal antibody. J Immunol. 1990;144:16. [PubMed] [Google Scholar]
- 8.Andris F, Van Mechelen M, De Mattia F, Baus E, Urbain J, Leo O. Induction of T cell unresponsiveness by anti-CD3 antibodies occurs independently of co-stimulatory functions. Eur J Immunol. 1996;26:1187. doi: 10.1002/eji.1830260534. [DOI] [PubMed] [Google Scholar]
- 9.Ben-Amor A, De Leite–Moraes MC, Lepault F, et al. In vitro T cell unresponsiveness following low-dose injection of anti-CD3 MoAb. Clin Exp Immunol. 1996;103:491. doi: 10.1111/j.1365-2249.1996.tb08307.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fischman AJ, Babich JW, Strauss HW. A ticket to ride: peptide radiopharmaceuticals. J Nucl Med. 1993;34:2253. [PubMed] [Google Scholar]
- 11.Molthoff CF, Buist MR, Kenemans P, Pinedo HM, Boven E. Experimental and clinical analysis of the characteristics of a chimeric monoclonal antibody, MOv18, reactive with an ovarian cancer-associated antigen. J Nucl Med. 1992;33:2000. [PubMed] [Google Scholar]
- 12.Jolliffe LK. Humanized antibodies: Enhancing therapeutic utility though antibody engineering. Int Rev Immunol. 1993;10:241. doi: 10.3109/08830189309061699. [DOI] [PubMed] [Google Scholar]
- 13.Knulst AC, Noort WA, Tibbe GJ, Benner R, Savelkoul HF. Prevention of lethal graft-versus-host disease in mice by monoclonal antibodies directed to T cells or their subsets. II. Differential effectiveness of IgG2a and IgG2b isotypes of anti-CD3 and anti-CD4 moAb. Bone Marrow Transplant. 1994;14:535. [PubMed] [Google Scholar]
- 14.Verheijden GF, Rijnders AWM, Bos E, et al. Human cartilage glycoprotein-39 is a candidate autoantigen in rheumatoid arthritis. Arhritis Rheum. 1997;40:1115. doi: 10.1002/art.1780400616. [DOI] [PubMed] [Google Scholar]
- 15.Steenbakkers PGA, Boots AMH, Rijnders AWM. Generation and functional characterization of anti-clonotype antibodies to human T-cell receptors. J Immunol Methods. 1997;210:51. doi: 10.1016/s0022-1759(97)00176-2. [DOI] [PubMed] [Google Scholar]
- 16.Sorg R, Enczmann J, Sorg U, Heermeier K, Schneider EM, Wernet P. Rapid and sensitive mRNA phenotyping for interleukins (IL-1 to IL-6) and colony-stimulating factors (G-CSF, M-CSF, and GM-CSF) by reverse transcription and subsequent polymerase chain reaction. Exp Hematol. 1991;19:882. [PubMed] [Google Scholar]
- 17.Madrenas J, Schwartz RH, Germain RN. Interleukin 2 production, not the pattern of early T-cell antigen receptor-dependent tyrosine phosphorylation, controls anergy induction by both agonists and partial agonists. Proc Natl Acad Sci USA. 1996;93:9736. doi: 10.1073/pnas.93.18.9736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Quil H, Schwartz RH. Stimulation of normal inducer T cell clones with antigen presented by purified Ia molecules in planar lipid membranes: specific induction of a long-lived state of proliferative nonresponsiveness. J Immunol. 1987;138:3704. [PubMed] [Google Scholar]
- 19.Essery G, Feldmann M, Lamb JR. Interleukin-2 can prevent and reverse antigen-induced unresponsiveness in cloned human T lymphocytes. Immunology. 1988;64:413. [PMC free article] [PubMed] [Google Scholar]
- 20.Vergelli M, Hemmer B, Utz U, et al. Differential activation of human autoreactive T cell clones by altered peptide ligands derived from myelin basic protein peptide (87) Eur J Immunol. 1996;26:2624. doi: 10.1002/eji.1830261113. [DOI] [PubMed] [Google Scholar]
- 21.Sidhu S, Deacock S, Bal V, Batchelor JR, Lombardi G, Lechler RI. Human T cells cannot act as autonomous antigen-presenting cells, but induce tolerance in antigen-specific and alloreactive responder cells. J Exp Med. 1992;176:875. doi: 10.1084/jem.176.3.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boussiotis VA, Barber DL, Nakarai T, et al. Prevention of T cell anergy by signaling through γc chain of the IL-2 receptor. Science. 1994;266:1039. doi: 10.1126/science.7973657. [DOI] [PubMed] [Google Scholar]
- 23.Van Gool S, De Boer M, Ceuppens JL. The combination of anti-B7 monoclonal antibody and cyclosporin A induces alloantigen-specific anergy during a primary mixed lymphocyte reaction. J Exp Med. 1994;179:715. doi: 10.1084/jem.179.2.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sloan-Lancaster J, Evavold BD, Allen PM. Induction of T-cell anergy by altered T-cell-receptor ligand on live antigen-presenting cells. Nature. 1993;363:156. doi: 10.1038/363156a0. [DOI] [PubMed] [Google Scholar]
- 25.Williams ME, Shea CM, Lichtman AH, Abbas AK. Antigen receptor-mediated anergy in resting T lymphocytes and T cell clones. J Immunol. 1992;149:1921. [PubMed] [Google Scholar]
- 26.Lombardi G, Sidhu S, Batchelor R, Lechler R. Anergic T cells as suppressor cells in vitro. Science. 1994;264:1587. doi: 10.1126/science.8202711. [DOI] [PubMed] [Google Scholar]
- 27.Mannie MD, Rendall SK, Arnold PY, Nardella JP, White GA. Anergy-associated T cell antigen presentation. A mechanism of infectious tolerance in experimental autoimmune encephalomyelitis. J Immunol. 1996;157:1062. [PubMed] [Google Scholar]
- 28.Diepolder HM, Jung M, Wierenga E, et al. Anergic Th1 clones specific for hepatitis B virus (HBV) core peptides are inhibitory to other HBV core-specific CD4+ T cells in vitro. J Virol. 1996;70:7540. doi: 10.1128/jvi.70.11.7540-7548.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Viola A, Lanzavecchia A. T cell activation determined by T cell receptor number and tunable thresholds. Science. 1996;273:104. doi: 10.1126/science.273.5271.104. [DOI] [PubMed] [Google Scholar]
- 30.Lobo PI, Patel HC. Murine monoclonal IgG antibodies: Differences in their IgG isotypes can affect the antibody effector activity when using human cells. Immunol Cell Biol. 1997;75:267. doi: 10.1038/icb.1997.41. [DOI] [PubMed] [Google Scholar]
- 31.Duits AJ, Aarden LA, Ernst LK, Capel PJA, Van de Winkel JGJ. Isotype-specific cross-linking of select human FcγR isoforms triggers release of IL-6. Clin Exp Immunol. 1993;92:225. doi: 10.1111/j.1365-2249.1993.tb03384.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lombardi G, Hargreaves R, Sidhu S, et al. Antigen presentation by T cells inhibits IL-2 production and induces IL-4 release due to altered cognate signals. J Immunol. 1996;156:2769. [PubMed] [Google Scholar]
- 33.Willems F, Andris F, Abramowicz D, et al. Induction of T-cell anergy by OKT3 requires cyclosporine-insensitive activation signals. Transplant Proc. 1995;27:1425. [PubMed] [Google Scholar]
- 34.Kishimoto H, Kubo RT, Yorifuji H, Nakayama T, Asano Y, Tada T. Physical dissociation of the TCR-CD3 complex accompanies receptor ligation. J Exp Med. 1995;182:1997. doi: 10.1084/jem.182.6.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thomson HS, Harper N, Bevan DJ, Staines NA. Suppression of collagen induced arthritis by oral administration of type II collagen: changes in immune and arthritic responses mediated by active peripheral suppression. Autoimmunity. 1993;16:189. doi: 10.3109/08916939308993327. [DOI] [PubMed] [Google Scholar]
- 36.Liblau RS, Tisch R, Shokat K, et al. Intravenous injection of soluble antigen induces thymic and peripheral T-cell apoptosis. Proc Natl Acad Sci USA. 1996;93:3031. doi: 10.1073/pnas.93.7.3031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Friedman A, Al-Sabbagh A, Santos LM, et al. Oral tolerance: a biologically relevant pathway to generate peripheral tolerance against external and self antigens. Chem Immunol. 1994;58:259. [PubMed] [Google Scholar]