Abstract
Although p21ras, raf-1 and MEK have been shown to regulate directly the transcriptional activity of NFAT (nuclear factor of activated T cells) and/or the interleukin-2 (IL-2) promoter, direct evidence that the extracellular signal-regulated protein kinase (ERK) is involved in regulating IL-2 production is still lacking. Here, we demonstrate that transfection of Jurkat cells with a dominant negative mutant of ERK1 (Erk1-K71R) resulted in the suppression of mitogen-stimulated production/secretion of IL-2. This was accompanied by a parallel inhibition of mitogen-stimulated ERK activity. These data provide direct evidence, for the first time, that ERK plays a vital role in regulating the production/secretion of IL-2.
INTRODUCTION
Engagement of the T-cell receptor leads to the activation of a number of intracellular signalling molecules. These include members of the extracellular signal-regulated protein kinase (ERK) cascade which consists of p21ras and/or protein kinase C, raf-1, mitogen-activated protein (MAP) kinase/ERK kinase (MEK)-1 and -2 and ERK.1 Although it is currently not clear how GTP-loaded p21ras stimulates the kinase activity of raf-1, activation of ERK is achieved via the phosphorylation of MEK1 and MEK2 by raf-1 and the subsequent dual phosphorylation of ERK1 and ERK2 by MEK1 and MEK2.2 Cantrell and others have demonstrated that transcriptional activation of NFAT (nuclear factor of activated T cells), a transcription factor that regulates interleukin-2 (IL-2) gene transcription, or the IL-2 promoter required coactivation of the Ca2+/calcineurin- and p21ras-dependent pathways.3–5 The effect of activated p21ras could be mimicked by phorbol 12-myristate 13-acetate (PMA),3–5 a known activator of the ERK cascade. However, there are conflicting reports regarding the ability of members of the ERK cascade to substitute for p21ras in synergizing with the Ca2+ pathway to stimulate the transcriptional activation of NFAT. While studies from Cantrell's laboratory have demonstrated that activated MEK was unable to synergize with ionomycin to stimulate NFAT,5 Whitehurst and Geppert6 reported that activated MEK or raf-1 could synergize with ionomycin to stimulate NFAT-driven reporter gene expression but was unable to stimulate IL-2 promoter-driven reporter gene expression. Studies in Jurkat or EL4 cells have also demonstrated that transfection of these cells with a dominant negative mutant construct of p21ras, raf-1, or MEK, or overexpression of MKP-1, a dual specificity phosphatase which dephosphorylates activated MAP kinases, block NFAT- or IL-2 promoter-driven reporter gene expression.1,6 However, direct evidence that ERK per se participates in the regulation of NFAT or IL-2 promoter activity, or IL-2 production is still lacking since MKP-1 also uses dual-phosphorylated c-jun N-terminal kinase (JNK) and p38 MAP kinases as substrates.7 This problem is compounded by the demonstration that the activity of JNK and p38 is also stimulated in activated T lymphocytes7–9 and studies in U937 cells have demonstrated that conditional expression of MKP-1 preferentially attenuated signalling via p38 and JNK.10
The aim of the present study was therefore to investigate whether ERK played a direct role in regulating IL-2 production. Using Jurkat T leukaemic cells that had been transiently transfected with a dominant negative mutant of ERK, we provide direct evidence that ERK participates in the regulation of mitogen-stimulated IL-2 production.
MATERIALS AND METHODS
DNA constructs
The pSV β-galactosidase was obtained from Promega (Annandale, NSW, Australia). The construction of dominant ERK (pCMV-Erk K71R) has been described previously.11–14 All plasmids were purified using the Qiagen maxi plasmid kit (Qiagen GmbH, Hilden, Germany). The anti-ACTIVE™ ERK antibody was obtained from Promega and anti-ERK2 antibody was obtained from Santa Cruz (CA).
Transfection of Jurkat cells
The Jurkat leukaemic T cells were maintained in a humidified atmosphere of 5% CO2 in 95% air (37°) in RPMI-1640 (Cell Image, Adelaide, Australia), supplemented with penicillin, streptomycin and 10% fetal calf serum (FCS). Cells were washed twice in serum-free RPMI-1640 before use and were transiently transfected using Lipofectamine (Gibco, Melbourne, Australia) under serum-free conditions. Prior to transfection, plasmid DNA (10 μg in 300 μl medium) was mixed with liposome (5 μl in 500 μl medium) and the mixture was left to stand at room temperature for 2·5–3 hr. The DNA–liposome complex was added to cells (4 × 106–1 × 107 in 200 μl medium) and cells were incubated at 37° for 8–12 hr. Control cells received pSV β-galactosidase–liposome complex and a portion of these cells was used to determine transfection efficiency. Then, 3–9 ml fresh RPMI-1640 (20% FCS) were added and cells were further incubated for 36–40 hr. The expression of β-galactosidase did not affect either cytokine production or ERK activity (data not shown). We have consistently found that 60–70% of the pSV μ-galactosidase-transfected cells express the enzyme.
Assay for IL-2
Forty-eight hours after transfection, cells (4 × 106 in 4 ml) were incubated with PMA (100 nm)/phytohaemagglutinin (PHA, 1 μg/ml) for 20 hr and aliquots of the supernatant were taken for cytokine estimation by enzyme-linked immunosorbent assay (ELISA). To examine the role of MEK1, non-transfected cells were preincubated with PD98059 (50 μm) for 45 min prior to the addition of PHA/PMA. Control cells received dimethyl sulphoxide (DMSO).
Partial purification of ERK
ERK was partially purified and assayed according to the methods described by Anderson et al. with some modifications.15–17 Briefly, transfected cells (1 × 107) were incubated with PHA/PMA (37°) for 5 min and incubations were terminated by centrifugation (3000 g for 3 min at 4°). The cell pellets were resuspended in 0·9 ml of 25 mm Tris–HCl (pH 7·5), 2 mm EGTA, 25 mm NaCl, 1 mm Na3VO4, 38 mm P-nitrophenylphosphate, 10 μg/ml pepstatin A, 10 μg/ml aprotinin, 10 μg/ml leupeptin, 2 mm phenylmethylsulphonyl fluoride and 1 mm dithiothreitol, sonicated (3 × 10 seconds, output of 2 units, Ultrasonics Sonicator, Farmingdale, NY) and centrifuged (100 000 g for 15 min). Cytosolic fractions were adsorbed onto phenylsepharose CL4B and after washing the beads with ethylene glycol (2 × 10% followed by 2 × 35%, v/v), ERK was eluted with 200 μl 60% ethylene glycol. Previous studies have demonstrated that ERK is eluted by ethylene glycol at concentrations between 35 and 60% (v/v).15 Kinase activity in the samples was determined as described below. In some experiments, Laemmli buffer was added to the samples which were then stored at – 20° until Western blotted
ERK assay
Partially purified ERK (15 μl) was added to 35 μl of assay mixture (25 mm Tris–HCl, pH 7·4, 50 mm β-glycerophosphate, 0·33 mg/ml myelin basic protein, 1·5 mm EGTA, 0·1 mm sodium orthovanadate, 10 mm MgCl2, 10 μg/ml protein kinase A peptide inhibitor, 40 μm ATP and 0·1 μCi γ[32*sref32*P]ATP) (Betasec, Adelaide, Australia) and the mixture was incubated for 20 min at 30°. Assays were terminated by spotting aliquots of the reaction mixture onto P-81 filter paper followed by extensive washing with 75 mm orthophosphoric acid. Radioactivity was determined by liquid scintillation spectrometry. Since omission of the protein kinase A inhibitor from the assay mix did not affect the incorporation of 32Pi into myelin basic protein (ref. 15, and data not shown) and the assay mixture did not contain a phospholipid, neither protein kinase A nor C could contribute to the enzymatic activity measured. Moreover, Western blotting of the phenylsepharose CL4B-purified extracts demonstrated the presence of immunoreactive p42 and p44 ERK isoforms (data not shown). Our other studies have excluded the possibility that p38 could contribute to the kinase activity in these fractions.18
Western blotting
Denatured proteins were separated on 10% polyacrylamide gels and transferred to nitrocellulose (100 V, 1·5 hr). Immediately after transfer, blots were stained with Ponceau S (0·1% in 5% acetic acid) to confirm equal loading of all lanes of the gels. Affinity-purified polyclonal anti-ACTIVE™ ERK antibody (diluted 1:20 000) and anti-ERK2 antibody (diluted 1:1000 or 1:200) were used to detect dual-phosphorylated and non-phosphorylated ERK isoforms, respectively. The ERK immune complexes were detected by enhanced chemiluminescence as described earlier.16
Statistical analysis
Results were analysed by Student's t-test and were considered significant if P < 0·05.
RESULTS
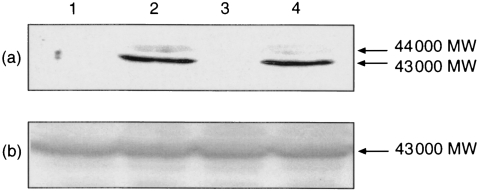
Stimulation of Jurkat cells with PHA (1 μg/ml)/PMA (100 nm) for 5 min resulted in increased ERK activity (Figs 1 and 3). Western blotting of the samples with the anti-ACTIVE™ ERK antibody which detects dual-phosphorylated, active ERK revealed that PHA/PMA caused the appearance of a strong band of immunoreactive material that migrated with an MW of approximately 43 000 (ERK2) (Fig. 2a, lane 2) and a faint band that migrated with an apparent molecular weight of 44 000 (ERK1). Probing parallel blots with an anti-ERK2 antibody (diluted 1:1000) revealed equal loading of ERK2 in all the lanes and at lower antibody dilutions (1:200), the antibody revealed ≈50% more ERK1 in ErkK71R-transfected cells than in control cells (data not shown).
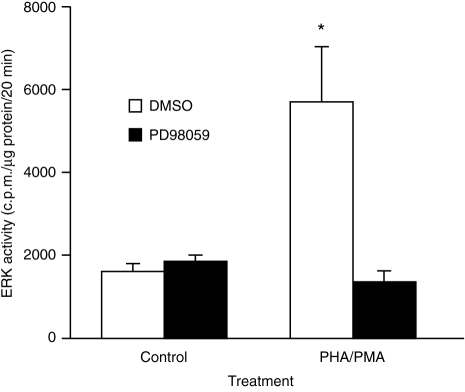
Figure 1.
Inhibition of PHA/PMA-stimulated ERK activity by PD98059. Cells were preincubated with DMSO (0·1% v/v) or PD98059 (50 μm) for 45 min prior to the addition of PHA/PMA. After 5 min, ERK activity was determined as described in the Materials and Methods. Results are means+SEM of three experiments. Significance of difference between unstimulated and mitogen-stimulated cells: *P < 0·05.
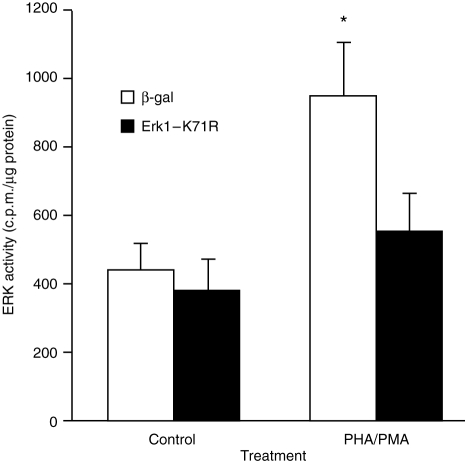
Figure 3.
Inhibition of PHA/PMA-stimulated ERK activity by dominant negative ERK1. Cells were transfected with pSV β-galactosidase or pCMV ErkK71R as described in the Materials and Methods. Forty-eight hours after transfection, the ability of PHA/PMA to stimulate ERK activity was determined. Results are the means+SEM of six to eight individual experiments. Significance of difference between unstimulated and mitogen-stimulated kinase activity: *P < 0·05.
Figure 2.
Lack of effect of dominant negative ERK1 on PHA/PMA-stimulated ERK phosphorylation. Cells were transfected with pSV μ-galactosidase (lanes 1 and 2) or pCMV ErkK71R (lanes 3 and 4). Forty-eight hours after transfection, cells were challenged with DMSO (0·1%, v/v, lanes 1 and 3) or with PHA/PMA (lanes 2 and 4). After SDS–PAGE, samples were Western blotted using the anti-ACTIVE™ ERK antibody (a) and immunoreactive material was detected by enhanced chemiluminescence. The presence of equal amounts of ERK2 in all lanes was confirmed in a duplicate blot that was probed with an anti-ERK2antibody (b).
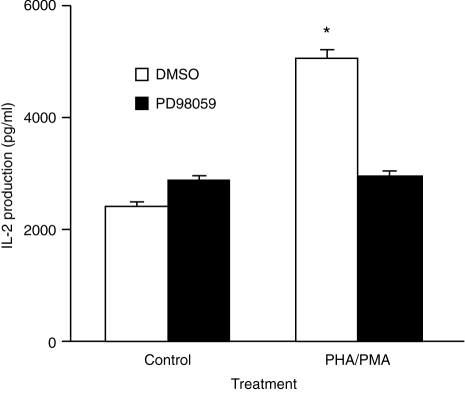
To investigate whether the ERK cascade regulates the production of IL-2, we first examined whether blocking the activation of MEK affected the ability of PHA/PMA to stimulate IL-2 production. This was achieved by using the selective MEK1 antagonist, PD98059, which had previously been demonstrated by Alessi et al.19 to inhibit MEK1 activity by >80% at 50 μm of inhibitor. Preincubation of Jurkat cells with PD98059 (50 μm) for 45 min blocked the stimulation of ERK activity by PHA/PMA (Fig. 1). PD98059 almost completely prevented PHA/PMA from stimulating the production of IL-2 (Fig. 4).
Figure 4.
Inhibition of PHA/PMA-stimulated IL-2 production by PD98059. Cells were pretreated with PD98059 (50 μm) and stimulated as described in the legend to Fig. 1. The supernatants were harvested and the amount of IL-2 that was secreted was assayed by ELISA. Results are the means+SEM of seven experiments. Significance of difference between unstimulated and mitogen-stimulated cells: *P < 0·001.
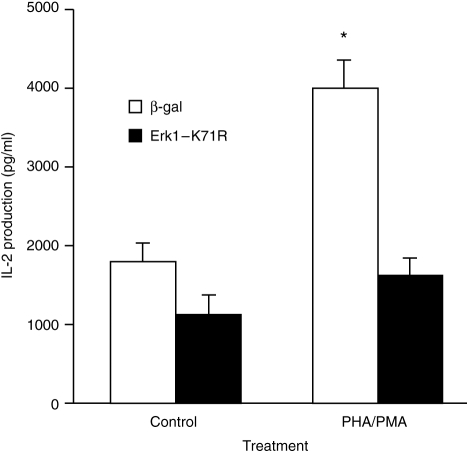
The above data are consistent with the ERK cascade being involved in regulating IL-2 production. To provide direct evidence that ERK regulates IL-2 production, cells were transiently transfected with a dominant negative mutant of ERK (Erk1-K71R), a kinase-dead ERK.20 Previous studies have demonstrated that transfection of cells with Erk1-K71R blocked both ERK1- and ERK2-mediated responses and cotransfection of cells with dominant negative ERK1 and ERK2 constructs did not result in a greater degree of inhibition than that observed when cells were transfected with only the ERK1 mutant.14 Transient transfection of Jurkat cells with Erk1-K71R suppressed the ability of PHA/PMA to stimulate the production of IL-2 (Fig. 5). The ERK mutant also suppressed the ability of PHA/PMA to stimulate ERK activity (Fig. 3). In contrast to kinase activity, PHA/PMA-stimulated ERK2 dual-phosphorylation was not affected by Erk1-K71R although Erk1-K71R reduced the level of ERK1 dual phosphorylation (Fig. 2a, lane 2 versus lane 4).
Figure 5.
Inhibition of PHA/PMA-stimulated IL-2 production by dominant negative ERK1 Cells were transfected as described in legend to Fig 3 and 48(h) after transfection, cells were stimulated with PHA/PMA. Twenty hours after stimulation, aliquots of the culture medium were removed and the amount of IL-2 which was produced over this period determined by ELISA. Results are means+SEM of 6–8 experiments. Significance of difference between unstimulated and mitogen-stimulated cells: *P < 0·05.
DISCUSSION
Studies by Cantrell and others have demonstrated that the transcriptional activation of NFAT and the IL-2 promoter is regulated by Ca2+/calcineurin-and p21ras-, raf-1- and MEK-dependent pathways.1,3–6 Thus, interfering with the function of p21ras, raf-1, or MEK alters the transcriptional activation of NFAT or the IL-2 promoter and this is associated with changes in ERK activity. Although indicative of an involvement of ERK, these observations do not provide direct evidence that ERK is involved in regulating IL-2 production since interfering with p21ras, raf-1, or MEK signalling will also affect the function of non-ERK effectors of p21ras, raf-1, or MEK. While ERK is currently the only known effector of MEK, multiple effectors of ras and raf have either been characterized or proposed.21,22 Thus, the flow of signal via these effectors will also be affected. Similarly, the data obtained with cells that overexpress MKP16 are consistent but do not prove that ERK regulates IL-2 production since MKP1 also dephosphorylates activated JNK and p38, in addition to activated ERK.7 Studies in U937 cells have, in fact, demonstrated that overexpressed MKP-1 has a preference for JNK and p38 over ERK.10 Hence, direct evidence demonstrating that ERK regulates IL-2 production/secretion is still lacking.
Using PHA/PMA as stimuli, we now provide direct evidence that ERK regulates the production of IL-2. Thus, transfection of Jurkat cells with a dominant negative construct of ERK1 greatly reduced the production of IL-2 in PHA/PMA-stimulated cells. In our other studies, we have also demonstrated that the dominant negative ERK1 mutant blocked lymphotoxin production (manuscript submitted).
Using HeLa cells, we have recently demonstrated that expression of Erk1-K71R reduced the ability of PMA to stimulate p90rsk (unpublished data), a kinase which is phosphorylated and activated by ERK.23 Using an Elk1 reporter construct, Westwick et al. demonstrated that transfection of NIH3T3 cells with Erk1-K71R blocked both ERK1- and ERK2-mediated increase in the transcriptional activity of Elk1 and that cotransfection of cells with Erk1-K71R and Erk2-K52R did not result in greater inhibition than that observed with Erk1-K71R alone.14 These studies therefore indicate that transfection of Jurkat cells with Erk1-K71R was sufficient to block ERK1-and ERK2-mediated signalling events.
Our studies with the anti-ACTIVE™ ERK antibody demonstrated a stronger staining of phosphorylated ERK2 than ERK1. This suggests a preferential activation of ERK2 over ERK1. Although recombinant MEK1/MEK2 do not show preferences between ERK1 and ERK2 as substrates in in vitro assays,24 a previous study in B cells has reported preferential activation of ERK2 over ERK1 following engagement of the B-cell antigen receptor.25
Expression of Erk1-K71R did not affect the dual phosphorylation ERK2, indicating that the antagonistic action of Erk1-K71R was exerted at a site(s) post-ERK2 phosphorylation. In contrast, dual phosphorylation of ERK1 was reduced in cells that expressed Erk1-K71R. This was despite a higher level of immunoreactive ERK1 being detected in Erk1-K71R-transfected cells than in control cells. The data suggest that Erk1-K71R could interfere with the ability of MEK to interact with wild-type/mutant ERK1 but not with ERK2. It needs to be stressed that under the conditions of forced expression of kinase-dead ERK,20 changes in the level of ERK dual phosphorylation could not be interpreted as changes in signalling via ERK since Erk1-K71R, although not affecting the dual phosphorylation of ERK2, prevented wild-type ERK from phosphorylating myelin basic protein in vitro and blocked the biological functions of ERK1 and ERK2 in intact cells.
In summary, this study provides direct evidence, for the first time, that ERK is involved in the regulation of IL-2 production/secretion.
Acknowledgments
This work was supported by funds from the Channel 7 Children' Research Foundation, the Women's and Children's Hospital Research Foundation and the National Health and Medical Reseach Council.
REFERENCES
- 1.Pastor MI, Woodrow M, Cantrell D. Regulation and function of p21ras in T lymphocytes. Cancer Surv. 1995;22:75. [PubMed] [Google Scholar]
- 2.Cobb MH, Goldsmith EJ. How MAP kinases are regulated. J Biol Chem. 1995;270:14843. doi: 10.1074/jbc.270.25.14843. [DOI] [PubMed] [Google Scholar]
- 3.Woodrow MA, Rayter S, Downward J, Cantrell D. p21ras and calcineurin synergise to regulate the nuclear factor of activated T cells. J Immunol. 1993;150:3853. [PubMed] [Google Scholar]
- 4.Baldari CT, Heguy A, Telford JL. Calcium-dependent cyclosporin-A sensitive activation of the interleukin-2 promoter by p56lck. J Biol Chem. 1993;268:2693. [PubMed] [Google Scholar]
- 5.Genot E, Cleverly S, Henning S, Cantrell D. Multiple p21ras effector pathways regulate nuclear factor of activated T cells. Embo J. 1996;15:3923. [PMC free article] [PubMed] [Google Scholar]
- 6.Whitehurst CE, Geppert TD. MEK1 and the extracellular signal-regulated kinases are required for the stimulation of IL-2 gene transcription in T cells. J Immunol. 1996;156:1020. [PubMed] [Google Scholar]
- 7.Chu Y, Solski PA, Khosravi-Far R, Der CJ, Kelly K. The mitogen-activated protein kinase phosphatases PAC1, MKP-1 and MKP-2 have unique substrate specificities and reduced activity in vivo towards the ERK2 sevenmaker mutation. J Biol Chem. 1996;271:6497. doi: 10.1074/jbc.271.11.6497. [DOI] [PubMed] [Google Scholar]
- 8.Su B, Jacinto E, Hibi M, Kallunki T, Karin M, Ben-Neriah Y. JNK is involved in signal integration during co-stimulation of T lymphocytes. Cell. 1994;77:727. doi: 10.1016/0092-8674(94)90056-6. [DOI] [PubMed] [Google Scholar]
- 9.Crawley JB, Rawlinson L, Lali FV, Page TH, Saklatvala J, Foxwell BM. T cell proliferation in response to interleukins 2 and 7 requires p38 MAP kinase activaion. J Biol Chem. 1997;272:15023. doi: 10.1074/jbc.272.23.15023. [DOI] [PubMed] [Google Scholar]
- 10.Franklin CC, Kraft AS. Conditional expression of the mitogen-activated protein kinase (MAPK) phosphatase, MKP-1, preferentially inhibits p38 MAPK and stress-activated protein kinase in U937 cells. J Biol Chem. 1997;272:16917. doi: 10.1074/jbc.272.27.16917. [DOI] [PubMed] [Google Scholar]
- 11.Quilliam LA, Kato K, Rabun KM, et al. Identification of residues critical for Ras (17N) growth inhibitory phenotype and for Ras interaction with guanine nucleotide-exchange factors. Mol Cell Biol. 1994;14:1113. doi: 10.1128/mcb.14.2.1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen SY, Huff SY, Lai CC, Der CJ, Bower S. Ras-15A protein shares highly similar dominant-negative biological properties with Ras-17N and forms a stable, guanine-nucleotide resistant complex with CDC25 exchange factor. Oncogene. 1994;9:2691. [PubMed] [Google Scholar]
- 13.Cox AD, Brtva TR, Lowe DG, Der CJ. R-ras induces maglinant, but not morphologic, transformation of NIH 3T3 cells. Oncogene. 1994;9:3281. [PubMed] [Google Scholar]
- 14.Westwick JK, Cox AD, Der CJ, et al. Oncogeni Ras activates c-Jun via a separate pathway from the activation of extracellular signal-regulated kinases. Proc Natl Acad Sci USA. 1994;91:6030. doi: 10.1073/pnas.91.13.6030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Anderson NG, Maller JL, Tonks NK, Sturgill TW. Requirement for integration of signals from two distinct phosphorylation pathways for activation of MAP kinase. Nature. 1990;343:651. doi: 10.1038/343651a0. [DOI] [PubMed] [Google Scholar]
- 16.Hii CST, Ferrante A, Edwards YS, et al. Activation of the mitogen-activated protein kinase by arachidonic acid in rat liver epithelial WB cells by a protein kinase C-dependent mechanism. J Biol Chem. 1995;270:4201. doi: 10.1074/jbc.270.9.4201. [DOI] [PubMed] [Google Scholar]
- 17.Hii CST, Oh SY, Schmidt S, Clark KJ, Murray AW. Lysophosphatidic acid inhibits gap-junctional communication and stimulates phosphorylation of connexin 43 in WB cells: possible involvement of the mitogen-activated protein kinase cascade. Biochem J. 1994;303:475. doi: 10.1042/bj3030475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hii CST, Huang ZH, Bilney AJ, et al. Stimulation of p38 phosphorylation and activity by arachidonic acid in HeLa cells, HL60 promyelocytic leukaemic cells and human neutrophils: evidence for cell-type specific activation of MAP kinases. J Biol Chem. 1998;273:192772. doi: 10.1074/jbc.273.30.19277. [DOI] [PubMed] [Google Scholar]
- 19.Alessi DR, Cuenda A, Cohen P, Dudley DT, Saltiel AR. PD098059 is a specific inhibitor of the activation of mitogen-activated protein kinase kinase in vitro and in vivo. J Biol Chem. 1995;270:27489. doi: 10.1074/jbc.270.46.27489. [DOI] [PubMed] [Google Scholar]
- 20.Robbins DJ, Zhen E, Owaki H, et al. Regulation and properties of extracellular signal-regulated protein kinases 1 and 2 in vitro. J Biol Chem. 1993;268:5097. [PubMed] [Google Scholar]
- 21.Vojtek AB, Der CJ. Increasing complexity of the ras signalling pathway. J Biol Chem. 1998;273:199258. doi: 10.1074/jbc.273.32.19925. [DOI] [PubMed] [Google Scholar]
- 22.Chung KC, Gomes I, Wang D, Lau LF, Rosner MR. Raf and fibroblast growth factor phosphorylate Elk-1 and activate the serum response element of the immediate early gene pip92 by mitogen-activated protein kinase-independent as well as -dependent signalling pathways. Mol Cell Biol. 1998;18:2272. doi: 10.1128/mcb.18.4.2272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sale EM, Atkinson PG, Sale GJ. Requirement of MAP kinase for differentiation of fibroblasts to adipocytes, for insulin activation of p90, S6 kinase and for insulin or serum stimulation of DNA synthesis. Embo J. 1995;14:674. doi: 10.1002/j.1460-2075.1995.tb07046.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zheng CF, Guan KL. Properties of MEKs, the kinases that phosphorylate and activate the extracellular signal-regulated kinases. J Biol Chem. 1993;268:239339. [PubMed] [Google Scholar]
- 25.Sutherland CL, Heath AW, Pelech SL, Young PR, Gold MR. Differential activation of the ERK, JNK and p38 mitogen-activated protein kinases by CD40 and the B cell antigen receptor. J Immunol. 1996;157:3381. [PubMed] [Google Scholar]