Abstract
The development of more effective antituberculosis vaccines would assist in the control of the global problem of infection with Mycobacterium tuberculosis. One recent vaccination strategy is immunization with DNA plasmids encoding individual microbial genes. Using the genes for the M. tuberculosis-secreted proteins, MPT64 (23 000 MW) and Ag85B (30 000 MW) as candidate antigens, we previously prepared DNA vaccines and demonstrated their ability to stimulate T-cell responses and confer protection in a mouse model of aerosol tuberculosis (TB). The protective efficacy of the DNA vaccines was less than that promoted by the current vaccine Mycobacterium bovis bacille Calmette–Guèrin (BCG). To improve the immunogenicity and protective efficacy of these mycobacterial vectors, co-immunization of a plasmid expressing granulocyte–macrophage colony-stimulating factor (GM-CSF) was investigated. Intramuscular immunization with DNA expressing MPT64 or Ag85B and GM-CSF enhanced the antigen-specific cellular immune response, with increased proliferative response and production of interferon-γ (IFN-γ). The titre of antimycobacterial protein immunoglobulin G (IgG) antibodies was unchanged. Mice immunized with DNA vaccines showed reduced pulmonary bacterial load following an aerosol challenge of M. tuberculosis, but codelivery of the plasmid expressing GM-CSF did not increase the protective effect. Therefore, despite modifying the cellular immune response to DNA vaccines, GM-CSF does not improve their protective efficacy at the peak of infection after an aerosol challenge with 100 c.f.u. of M. tuberculosis.
INTRODUCTION
Infection with Mycobacterium tuberculosis continues to be a major cause of morbidity and mortality throughout the world, with over eight million new cases of tuberculosis (TB) each year resulting in three million deaths.1 The current vaccine, Mycobacterium bovis bacille Calmette–Guérin (BCG), has variable protective efficacy, ranging from 0 to 85% in different studies2 and second generation antituberculosis vaccines are urgently required. The development of new vaccines requires an understanding of the protective immune response against M. tuberculosis and the development of delivery vectors with the ability to mimic this protective response. The critical component of protective immunity against TB is a Tcell-mediated response characterized by the secretion of interferon-γ (IFN-γ) and other cytokines.3 Although subunit vaccines were previously considered ineffective against mycobacteria, vaccines based on culture filtrate proteins of M. tuberculosis and adjuvant have induced protective immunity in mice4 and guinea-pigs.5 A new strategy of delivering these protective antigens is via DNA vaccination.
In a number of disease models, DNA immunization induces both antibody and cell-mediated immune responses involving the CD4+ and CD8+ T-cell compartments (reviewed by Tighe et al.6) and protective immunity against a number of pathogens. Recently, we and others have shown that DNA vaccines encoding proteins of mycobacteria stimulates antimycobacterial immune responses and partial protection against tuberculosis7–10. Strategies to improve the immune response generated by DNA vaccines include increasing antigen uptake, the efficiency of antigen presentation or modulating the immune response to encoded antigen by codelivery of plasmids encoding cytokines11 or costimulatory molecules.12
The cytokine, granulocyte–macrophage colony-stimulating factor (GM-CSF) increases the immunostimulatory potential of antigen-presenting cells (APCs),13–17 and co-immunization with a plasmid expressing GM-CSF with DNA vaccines has enhanced the response against viral and tumour antigens.18–26 In this study, we investigated the effect of a plasmid expressing GM-CSF codelivered with DNA vaccines encoding secreted proteins of M. tuberculosis. The cellular immune response, in terms of proliferative response and cytokine production, elicited by DNA-immunized mice was augmented by the codelivery of the GM-CSF gene. Though improving the antimycobacterial immune response by DNA vaccination, co-immunization of the plasmid expressing GM-CSF did not enhance protection against M. tuberculosis challenge.
MATERIALS AND METHODS
Bacteria
For aerosol challenge, M. tuberculosis H37Rv (ATCC 27294) was grown in Proskauer and Beck liquid media, for 14 days at 37°. M. bovis BCG CSL (CSL Bioscience, Melbourne, Australia) was grown in Middlebrook 7H9 broth with supplement for 14 days at 37°. The bacteria were enumerated on supplemented Middlebrook 7H11 Agar, and stored at −70°. Escherichia coli strain MC1061 was grown in Luria–Bertani (LB) broth or Circlegrow broth (Bio101, Vista, CA) supplemented with ampicillin as required.
Production of DNA vaccines and protein antigens
The gene for the MPT64 protein was amplified from the plasmid, pTJ1, while the gene for Ag85B was amplified from M. tuberculosis genomic DNA, and were cloned into pJW4303 to yield plasmids, DNA-64 and DNA-85B, as described previously.8 Murine GM-CSF cDNA, kindly provided by the Genetics Institute Inc., was excised from plasmid pXMT2-muGM-CSF, using Kpn I restriction endonuclease, and inserted in to the same site of the pCMV polylinker27 to yield pCMV-muGM-CSF (pGM-CSF). DNA for immunization was purified by CsCl centrifugation, and stored in PBS (1 mg/ml) at −20° until required. MPT64 and Ag85B proteins were expressed, purified, and stored as described previously.28
Immunization and animals
C57Bl/6 female mice were supplied as specific pathogen-free (SPF) mice by ARC (Perth, Australia), and were maintained in SPF conditions. Mice were immunized between 8 and 12 weeks of age. Fifty micrograms of each plasmid was injected intramuscularly (imi) into each tibialis anterior muscle. Control mice were immunized with phosphate-buffered saline (PBS) or the parental vector, pJW4303.
Mycobacterial challenge
Four weeks after the last boost of the DNA vaccine, mice were exposed to an aerosol dose of ≈100 c.f.u. of viable M. tuberculosis H37Rv. After 4 weeks, the number of bacteria in one lung lobe was enumerated by homogenising the tissue, and plating 10-fold dilutions in water on supplemented Middlebrook 7H11 agar. The colonies were counted visually after 21 days. In challenge experiments, control vaccinated mice were immunized with a subcutaneous injection of 5×104 c.f.u. of BCG CSL, 100 days before M. tuberculosis infection.
Antibody determination
Serum antigen-specific antibodies were determined by enzyme-linked immunosorbent assay (ELISA). Briefly, mycobacterial protein (10 μg/ml) was coated onto the wells, and fivefold dilutions of mouse sera were used. Detection was achieved with goat antimurine IgG alkaline phosphatase conjugate (Sigma, St. Louis, MO, diluted 1:2000) and the chromogen, n-nitrophenylphosphate (Sigma, 1 mg/ml). To determine the titre of the antigen-specific antibody, the mean absorbance plus 3 standard deviations of normal mouse sera, at 1:100, was adopted as the cut-off absorbance.
Lymphocyte proliferation and cytokine assays
The inguinal, axillary, and para-aortic lymph nodes and the spleen were collected from immunized mice and single cell suspensions prepared in complete RPMI media, supplemented with 2 mm glutamate, 50 μm β-mercaptoethanol and 10% fetal calf serum (FCS). T-cells were enriched by passing leucocytes through nylon wool. Semi-purified T-cells and γ-irradiated syngeneic splenocytes, as APCs (2×105 cells each) were added to 96-well plates and were incubated with varying concentrations of antigen, the mitogen concancavalin A (Sigma), or media alone for 3 days, then pulsed with 1 μCi 3H-thymidine (NEN Life Sciences, Boston, MA) per well for 6 hr and incorporation of 3H-thymidine was determined.
Cytokines present in culture supernatants were determined by capture ELISA, using the monoclonal antibodies, R46A2 and biotinylated XMG 1.2 (Endogen, Woburn, MA) for IFN-γ and 11B11 and biotinylated BVDG-24G2 (kindly donated by Dr P. Hodgkin, Centenary Institute, Australia) for interleukin-4 (IL-4).
RESULTS
Enhanced T-cell activation by co-immunization with the plasmid encoding GM-CSF
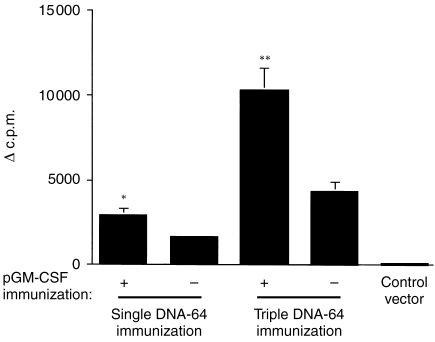
A single immunization with the DNA-64, encoding the mycobacterial secreted protein, MPT64, stimulated an antigen-specific proliferative response of T cells from the spleen (Fig. 1) and lymph nodes (data not shown). Co-immunization with the GM-CSF plasmid significantly increased the T-cell proliferative response to MPT64 (Fig. 1). The effect of multiple doses of GM-CSF on the immune response to DNA vaccines was investigated. Mice were co-immunized with DNA-64 and pGM-CSF three times, three weeks apart. Increased antigen-specific T-cell proliferation was observed with multiple doses of the plasmids compared to mice given DNA-64 only (Fig. 1).
Figure 1.
The effect of immunization with DNA-64 and pGM-CSF on T-cell proliferation. Proliferative responses of spleen-derived lymphocytes from mice immunized imi with 100 μg DNA-64, co-immunized with pGM-CSF, or the empty vector. Mice were immunized once or three times at three week intervals. The incorporation of 3H-thymidine (Δc.p.m.±SEM) in response to 10 μg/ml MPT64 was measured as described in the materials and methods. The statistical significance of the differences in the proliferative response was determined by Student's t-test, *P = 0·002, **P = 0·007.
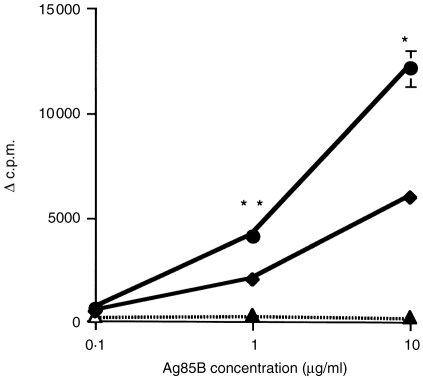
This enhanced proliferation was also investigated with another vaccine, DNA-85B, expressing the 30 000 MW mycobacterial secreted protein, Ag85B. Co-immunization of DNA-85B with plasmid encoding GM-CSF augmented the anti85B proliferative response of T-cells compared to mice immunized with the DNA-85B alone (Fig. 2).
Figure 2.
The effect of immunization with DNA-85B and pGM-CSF on T-cell proliferation. Proliferative responses of spleen-derived lymphocytes from mice immunized three times imi, with 100 μg DNA-85B alone (♦), co-immunized with pGM-CSF (•), or the empty vector (▴). The incorporation of 3H-thymidine (Δc.p.m.±SEM) in response to Ag85B was measured as described in the materials and methods. The statistical significance of differences in the proliferative responses was determined by Student's t-test, *P = 0·001, **P = 0·01.
Increased cytokine release by co-immunization with plasmid encoding GM-CSF
Lymphocytes from mice immunized with DNA vaccines released IFN-γ in recall to mycobacterial antigen. The production of IFN-γ by lymphocytes of mice immunized with either DNA-64 or DNA-85B was increased by co-immunization with pGM-CSF (Table 1). Immunization with the mycobacterial vectors did not induce cells to produce IL-4, with or without pGM-CSF (data not shown).
Increased antigen-specific IFN-γ production in response to co-immunization with DNA vaccines and a plasmid expressing GM-CSF
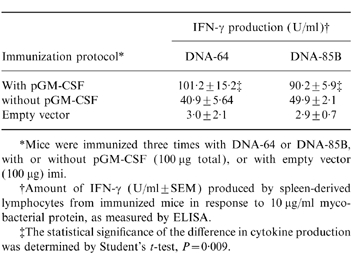
*Mice were immunized three times with DNA-64 or DNA-85B, with or without pGM-CSF (100 μg total), or with empty vector (100 μg) imi.
†Amount of IFN-γ (U/ml±SEM) produced by spleen-derived lymphocytes from immunized mice in response to 10 μg/ml mycobacterial protein, as measured by ELISA.
‡The statistical significance of the difference in cytokine production was determined by Student's t-test, P = 0·009.
Plasmid expressing GM-CSF did not affect antibody production
The production of antigen-specific antibody by DNA vaccines was analysed. Mice immunized by DNA-64 generated IgG antibodies against MPT64. Likewise, DNA-85B immunization generated anti-Ag85B antibodies. Unlike the cellular immune response, there was no enhancement of this antibody response in mice co-immunized with pGM-CSF and either DNA vaccine (Table 2).
Effect of co-immunization of plasmid expressing GM-CSF and DNA vaccines on generation of a humoral response against mycobacterial proteins
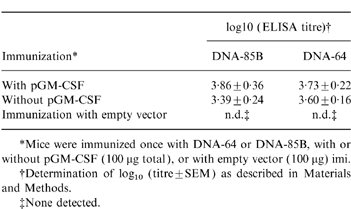
*Mice were immunized once with DNA-64 or DNA-85B, with or without pGM-CSF (100 μg total), or with empty vector (100 μg) imi.
†Determination of log10 (titre±SEM) as described in Materials and Methods.
‡None detected.
Co-immunization of the plasmid encoding GM-CSF does not improve protection by DNA vaccines against challenge with M. tuberculosis
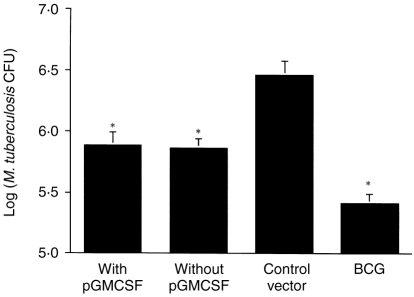
Having observed the enhancement of T-cell activation and cytokine release, the effect of codelivery of the GM-CSF gene on DNA-elicited protection against challenge of tuberculosis was investigated. Mice were immunized with DNA or BCG, then given an aerosol challenged with 100 c.f.u. of virulent M. tuberculosis. Four weeks after infection, the extent of the bacterial load in the lungs was determined. In mice immunized with DNA-64, DNA-85B (data not shown) or a combination of the mycobacterial vectors (Fig. 3), no increased protection in the lung was observe with co-immunization of the GM-CSF gene. Despite increasing the immune response elicited by DNA vaccines, co-immunization with a plasmid expressing GM-CSF did not improve protection against a mycobacterial challenge.
Figure 3.
The effect of codelivery of a vector expressing GM-CSF on the protective efficacy of DNA vaccines against challenge with M. tuberculosis. Mice were immunized (100 μg imi) with a combination of DNA-64 and DNA-85B, with or without pGM-CSF, three times, every three weeks. As positive control, mice were given a subcutaneous immunization with BCG (5 × 104 c.f.u.), and the empty vector was used as the negative control. Four weeks later, mice were challenged with aerosol-delivered M. tuberculosis H37Rv (100 c.f.u.). Four weeks postinfection, the bacteria in the lung were enumerated (±SEM), as described in the material and methods. The statistical significance of differences compared to empty vector were determined by the Mann–Whitney test (n = 5, *P < 0·009). There was no statistically significant difference between DNA vaccines with or without pGM-CSF.
DISCUSSION
The global problem of TB and the limitations of the vaccine, BCG, continue to drive the development of second-generation vaccines. A number of groups have demonstrated the potential of DNA vaccination in protecting against TB challenge.7–10 Encouragingly, a single antigen has been able to protect in some circumstances as effectively as BCG, but no DNA vaccine is better than BCG. We have shown that co-immunization with three DNA vectors expressing secreted antigens increased the protective efficacy against aerosol TB compared to a single DNA vaccine.8 Improved protection may be achieved as other mycobacterial antigens are investigated for inclusion in a protective panel of DNA vaccines. A different approach is to attempt to enhance the immune response elicited by DNA vaccination by cytokine modulation. We show here that codelivery of a vector expressing GM-CSF enhances the cellular immune response generated by antimycobacterial DNA vaccines.
The full mechanism by which intramuscular delivery of DNA elicits an immune response is not fully elucidated. Although, the DNA vector is ostensibly taken up by the myocytes, bone-marrow-derived APCs are responsible for presenting the encoded antigen to the immune system (reviewed by Tighe et al.6), and can be targeted for manipulation. T helper 1 (Th1)/Th2 cytokines may be used to drive the immune response down a particular path,11,23 while the same or other cytokines modulate APC function.18,29
GM-CSF is a pleotrophic cytokine. It is produced by T and B cells, endothelial cells, fibroblasts, and macrophages, and is involved in haemopoiesis of mononuclear phagocytes and granulocytes.30 In terms of APC function, GM-CSF recruits mononuclear cells and activates them to become potent immunostimulatory cells.13–15 GM-CSF increases expression of major histocompatibility complex (MHC) class II molecules and enhances antigen presentation.16,17 Therefore codelivery of a vector expressing GM-CSF may increase the numbers of APCs at the site of immunization and their functional potential for uptake and processing of mycobacterial proteins encoded by DNA vaccines, and to enhance the resulting anti-TB immune response and protective efficacy.
The cellular immune response, in terms of antigen-specific T-cell proliferative response and IFN-γ production, was augmented by GM-CSF (Figs 1 and 2, and Table 1). DNA itself is immunostimulatory, causing the production of IFN-α, IL-12 and IL-18 by macrophages and IFN-γ by natural killer (NK) cells (reviewed by Tighe et al.6). Therefore, response to multiple exposures of DNA may possibly mask the effect of GM-CSF. This was not the case, as co-immunization with DNA-64 and pGM-CSF three times still increased the proliferative response compared to DNA-64 delivered once or three times (Fig. 1). Codelivery of pGM-CSF not only maintained the production of IFN-γ, but enhanced it (Table 1).
Codelivery of GM-CSF did not improve the humoral immune response elicited by the mycobacterial DNA vaccine. There are differences in the reported effects of GM-CSF on antibody responses to DNA vaccines, with some having no improvement,20,25 but in other studies codelivery of GM-CSF enhanced antibody production.18,21,23 The particularly high titre antibody response by DNA-64 and DNA-85B may have masked any effect of GM-CSF on antibody production.
Protective immunity against M. tuberculosis is dependent on the recruitment of antigen-specific T cells to the lung and the release of cytokines, particularly IFN-γ, to activate macrophage killing mechanisms. The principal T-cell subset required are CD4+ T cells, although CD8+ T cells and γδ T-cell receptor (TCR)-bearing T cells may also play a role (reviewed by Orme et al.3). As codelivery of GM-CSF enhanced the proliferative response of T cells and their production of IFN-γ, the critical mediator of anti-TB immunity, the protective efficacy of this vaccine strategy was investigated in an aerosol model of TB. Mice immunized with mycobacterial DNA vaccines demonstrated partial protection against TB challenge, however, codelivery of GM-CSF did not augment the protective efficacy of mycobacterial vectors alone or in combination (Fig. 3). Despite in vitro enhancement of IFN-γ production by T cells of GM-CSF co-immunized mice, the generation of IFN-γ-secreting cells in vivo may not have occurred with the right timing or in the necessary location to increase the protective efficacy of DNA vaccination. This is the first study to investigate in a bacterial model of disease the effect of codelivery of GM-CSF, a strategy which had significantly increased protection against viral disease18,26 and tumours.19
Thomson and coworkers31 constructed a DNA vaccine encoding a number of CD8+ cytotoxic T lymphocytes (CTL) epitopes and found mice co-immunized with this vector and GM-CSF generated an enhanced cytotoxic T-cell response to each epitope. Despite the absence of a CD4+ T helper cell epitope, this polytope vaccine was protective against virus and tumour challenge. This and other studies22–25 have confirmed that codelivery of GM-CSF enhances the generation of CD8+ CTLs. The role of the CD8+ T cells in protective immunity against TB is not fully elucidated. MHC class I-restricted T cells appear to protect against TB in mice,32,33 but this effect is though a perforin- or granzyme-independent mechanism.34,35 However, any expansion of CD8+ cytotoxic T cells associated with codelivery of GM-CSF and mycobacterial plasmids did not increase protection against TB, as it did for viral infection and tumours.
DNA immunization against TB does show some promise as a future vaccine strategy. Various manipulations of DNA vaccines may enhance their immunogenicity; however, their effect on protective efficacy must be tested to determine any potential benefit of the modified DNA vaccines.
Acknowledgments
This work was supported financially by the National Health and Medical Research Council of Australia and the Immunology of Mycobacteria (IMMMYC) Program of the World Health Organisation. Arun Kamath is a recipient of an Australian Post-Graduate Research Award. We are grateful to Dr A. Bean and Dr J. Triccas for helpful discussions.
Glossary
Abbreviations
- APC
antigen-presenting cell
- BCG
Mycobacterium bovis bacille Calmette–Guérin
- CTL
cytotoxic T lymphocytes
- GM-CSF
granulocyte–macrophage colony-stimulating factor
- IFN-γ
interferon-γ IL-4, interleukin-4
- imi
intramuscular injection
- TB
tuberculosis
- M. tuberculosis
Mycobacterium tuberculosis
- PBS
phosphate-buffered saline
- pGM-CSF
plasmid expressing GM-CSF
REFERENCES
- 1.Bloom BR, Murray CJL. Tuberculosis: commentary on a reemergent killer. Science. 1992;257:1055. doi: 10.1126/science.257.5073.1055. [DOI] [PubMed] [Google Scholar]
- 2.Colditz GA, Brewer TF, Berkley CS, et al. Efficacy of BCG vaccine in the prevention of tuberculosis. Meta analysis of the published literature. JAMA. 1994;271:698. [PubMed] [Google Scholar]
- 3.Orme IM, Andersen P, Boom WH. T cell response to Mycobacterium tuberculosis. J Infect Dis. 1993;167:1481. doi: 10.1093/infdis/167.6.1481. [DOI] [PubMed] [Google Scholar]
- 4.Andersen P. Effective vaccination of mice against Mycobacterium tuberculosis infection with a soluble mixture of secreted mycobacterial proteins. Infect Immun. 1994;62:2536. doi: 10.1128/iai.62.6.2536-2544.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Horwitz MA, Esther Lee B-W, Dillon BJ, Harth G. Protective immunity against tuberculosis induced by vaccination with major extracellular proteins of Mycobacterium tuberculosis. Proc Natl Acad Sci USA. 1995;92:1530. doi: 10.1073/pnas.92.5.1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tighe H, Corr M, Roman M, Raz E. Gene vaccination: plasmid DNA is more than just a blueprint. Immunol Today. 1998;19:89. doi: 10.1016/s0167-5699(97)01201-2. [DOI] [PubMed] [Google Scholar]
- 7.Zhu X, Venkataprasad N, Thangaraj HS, et al. Functions and specificity of T cells following nucleic acid vaccination of mice against Mycobacterium tuberculosis infection. J Immunol. 1997;158:5921. [PubMed] [Google Scholar]
- 8.Kamath AT, Feng CG, MacDonald M, Briscoe H, Britton WJ. Differential protective efficacy of DNA vaccines expressing secreted proteins of Mycobacterium tuberculosis. Infect Immun. 1999 doi: 10.1128/iai.67.4.1702-1707.1999. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huygen K, Content J, Denis O, et al. Immunogenicity and protective efficacy of a tuberculosis DNA vaccine. Nature Med. 1996;2:893. doi: 10.1038/nm0896-893. [DOI] [PubMed] [Google Scholar]
- 10.Tascon RE, Colston MJ, Ragno S, Stavropoulos E, Gregory D, Lowrie DB. Vaccination against tuberculosis by DNA injection. Nature Med. 1996;2:888. doi: 10.1038/nm0896-888. [DOI] [PubMed] [Google Scholar]
- 11.Kim JJ, Trivedi NN, Nottingham LK, et al. Modulation of amplitude and direction of in vivo immune responses by co-administration of cytokine gene expression cassettes with DNA immunogens. Eur J Immunol. 1998;28:1089. doi: 10.1002/(SICI)1521-4141(199803)28:03<1089::AID-IMMU1089>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 12.Corr M, Tighe H, Lee D, et al. Costimulation provided by DNA immunization enhances antitumor immunity. J Immunol. 1997;159:4999. [PubMed] [Google Scholar]
- 13.Heufler C, Koch F, Schuler G. Granulocyte/macrophage colony-stimulating factor and interleukin 1 mediate the maturation of murine epidermal Langerhans cells into potent immunostimulatory dendritic cells. J Exp Med. 1988;167:700. doi: 10.1084/jem.167.2.700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Elliott MJ, Strasser A, Metcalf D. Selective up-regulation of macrophage function in granulocyte–macrophage colony-stimulating factor transgenic mice. J Immunol. 1991;147:2957. [PubMed] [Google Scholar]
- 15.Tazi A, Bouchonnet F, Grandsaigne M, Boumsell L, Hance AJ, Soler P. Evidence that granulocyte macrophage-colony-stimulating factor regulates the distribution and differentiated state of dendritic cells/Langerhans cells in human lung and lung cancers. J Clin Invest. 1993;91:566. doi: 10.1172/JCI116236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fischer H-G, Opel B, Reske K, Reske-Kunz AB. Granulocyte–macrophage colony-stimulating factor-cultured marrow-derived macrophages reveal acessory cell function and synthesis of MHC class II determinants in the absence of external stimuli. Eur J Immunol. 1988;18:1151. doi: 10.1002/eji.1830180802. [DOI] [PubMed] [Google Scholar]
- 17.Fischer H-G, Frosch S, Reske K, Reske-Kunz AB. Granulocyte–macrophage colony-stimulating factor activates macrophages derived from bone marrow cultures to synthesis MHC class II molecules and to augment antigen presentation function. J Immunol. 1988;141:3882. [PubMed] [Google Scholar]
- 18.Xiang Z, Ertl HC. Manipulation of the immune response to a plasmid-encoded viral antigen by coinoculation with plasmids expressing cytokines. Immunity. 1995;2:129. doi: 10.1016/s1074-7613(95)80001-8. [DOI] [PubMed] [Google Scholar]
- 19.Bueler H, Mulligan RC. Induction of antigen-specific tumor immunity by genetic and cellular vaccines against MAGE: enhanced tumor protection by coexpression of granulocyte-macrophage colony-stimulating factor and B7. Mol Med. 1996;2:545. [PMC free article] [PubMed] [Google Scholar]
- 20.Geissler M, Gesien A, Tokushige K, Wands JR. Enhancement of cellular and humoral immune responses to hepatitis C virus core protein using DNA-based vaccines augmented with cytokine-expressing plasmids. J Immunol. 1997;158:1231. [PubMed] [Google Scholar]
- 21.Svanholm C, Lowenadler B, Wigzell H. Amplification of T-cell and antibody responses in DNA-based immunization with HIV-1 Nef by co-injection with a GM-CSF expression vector. Scand J Immunol. 1997;46:298. doi: 10.1046/j.1365-3083.1997.d01-130.x. [DOI] [PubMed] [Google Scholar]
- 22.Iwasaki A, Stiernholm BJ, Chan AK, Berinstein NL, Barber BH. Enhanced CTL responses mediated by plasmid DNA immunogens encoding costimulatory molecules and cytokines. J Immunol. 1997;158:4591. [PubMed] [Google Scholar]
- 23.Chow YH, Chiang BL, Lee YL, et al. Development of Th1 and Th2 populations and the nature of immune responses to hepatitis B virus DNA vaccines can be modulated by co-delivery of various cytokine genes. J Immunol. 1998;160:1320. [PubMed] [Google Scholar]
- 24.Geissler M, Gesien A, Wands JR. Inhibitory effects of chronic ethanol consumption on cellular immune responses to hepatitis C virus core protein are reversed by genetic immunizations augmented with cytokine-expressing plasmids. J Immunol. 1997;159:5107. [PubMed] [Google Scholar]
- 25.Maecker HT, Umetsu DT, Dekruyff RH, Levy S. DNA vaccination with cytokine fusion constructs biases the immune response to ovalbumin. Vaccine. 1997;15:1687. doi: 10.1016/s0264-410x(97)00088-1. [DOI] [PubMed] [Google Scholar]
- 26.Sin JI, Sung JH, Suh YS, Lee AH, Chung JH, Sung YC. Protective immunity against heterologous challenge with Encephalomyocarditis virus by Vp1 DNA vaccination – effect of coinjection with a granulocyte-macrophage colony stimulating factor gene. Vaccine. 1997;15:1827. doi: 10.1016/s0264-410x(97)88856-1. [DOI] [PubMed] [Google Scholar]
- 27.Hanke T, Schneider J, Gilbert SC, Hill AVS, McMichael A. DNA multi-CTL epitope vaccines for HIV and Plasmodium falciparum – immunogenicity in mice. Vaccine. 1998;16:426. doi: 10.1016/s0264-410x(97)00296-x. [DOI] [PubMed] [Google Scholar]
- 28.Roche PW, Peake PW, Billman-Jacobe H, Doran T, Britton WJ. T-cell determinants and antibody binding sites on the major mycobacterial secretory protein MPB59 of Mycobacterium bovis. Infect Immun. 1994;62:5319. doi: 10.1128/iai.62.12.5319-5326.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hakim I, Levy S, Levy R. A nine-amino acid peptide from IL-1β augments antitumor immune responses induced by protein and DNA vaccines. J Immunol. 1996;157:5503. [PubMed] [Google Scholar]
- 30.Caux C, Dezutter-Dambuyant C, Schmitt D, Banchereau J. GM-CSF and TNF-α cooperate in the generation of dendritic Langerhans cells. Nature. 1992;360:258. doi: 10.1038/360258a0. [DOI] [PubMed] [Google Scholar]
- 31.Thomson SA, Sherritt MA, Medveczky J, et al. Delivery of multiple CD8 cytotoxic T cell epitopes by DNA vaccination. J Immunol. 1998;160:1717. [PubMed] [Google Scholar]
- 32.Flynn JL, Goldstein MM, Triebold KJ, Koller B, Bloom BR. Major histocompatibility complex class I-restricted T-cells are required for resistance to Mycobacterium tuberculosis infection. Proc Nat Acad Sci USA. 1992;89:12013. doi: 10.1073/pnas.89.24.12013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Orme IM. The kinetics of emergence and loss of mediator T lymphocytes acquired in response to infection with Mycobacterium tuberculosis. J Immunol. 1987;138:293. [PubMed] [Google Scholar]
- 34.Laochumroonvorapong P, Wang J, Liu CC, et al. Perforin, a cytotoxic molecule which mediates cell necrosis, is not required for the early control of mycobacterial infection in mice. Infect Immun. 1997;65:127. doi: 10.1128/iai.65.1.127-132.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cooper AM, D'Souza C, Frank AA, Orme IM. The course of Mycobacterium tuberculosis infection in the lungs of mice lacking expression of either perforin- or granzyme-mediated cytolytic mechanisms. Infect Immun. 1997;65:1317. doi: 10.1128/iai.65.4.1317-1320.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]