Abstract
Human placental trophoblast is critically involved in mediating maternal tolerance of the fetal semiallograft. Genes encoding highly polymorphic major histocompatibility complex (MHC) class I and class II antigens that could provoke maternal immune rejection responses are silenced in trophoblast. However, several MHC class I or class I-related products exhibiting reduced or negligible polymorphism are expressed and assumed to be functionally involved in maintaining pregnancy. The CD1 gene family encodes non-polymorphic MHC class I-like products that have the unusual ability to present non-peptide antigens to T cells. One member, CD1D, is expressed in certain epithelial cells and interacts with a specific T-cell subset that may promote the development of Th2-mediated responses believed to be associated with pregnancy. In this study we examined the expression of CD1D in human trophoblast cell lines and placentally derived trophoblast cells by reverse transcriptase–polymerase chain reaction using CD1D-specific oligonucleotide primers. We have found that CD1D mRNA transcripts are expressed in trophoblast cells and cell lines. We have also identified a novel alternatively spliced CD1D mRNA transcript lacking exon 4. Exon 4-intact and exon 4-deficient CD1D transcripts appear to be differentially expressed in different trophoblast and non-trophoblast cell populations. Our studies suggest that at least one member of the CD1 family is transcribed in human trophoblast.
INTRODUCTION
Throughout human pregnancy, contact between the mother and her semiallogeneic fetus is limited to a unique specialized epithelium called trophoblast. Derived entirely from the fetus, trophoblast is in direct and continuous contact with maternal immune effectors over the whole of the feto–maternal interface. For this reason the status of trophoblast for immune-related products, especially those encoded by major histocompatibility complex (MHC) and MHC-related genes is of interest for our understanding of how pregnancy is maintained. In part, trophoblast appears to avoid rejection responses characteristic of incompatible transplants by not expressing highly polymorphic MHC-encoded proteins. Thus, trophoblast appears to lack MHC class II antigen and fails to express the most polymorphic MHC class I antigens, human leucocyte antigen (HLA)-A, -B.1–3 Despite this, trophoblast is not inert with respect to the expression of class I and class I-related genes. Some trophoblast subpopulations are well known to express the relatively non-polymorphic MHC class Ib product HLA-G.4,5 Moreover, proteins with the characteristics of HLA-C, and the class I-like immunoglobulin G (IgG) transporter FcRn are also expressed.6–8 Selective expression of these genes by trophoblast suggests they are functionally involved in the maintenance of feto–maternal relations.
CD1 is a third lineage of antigen-presenting molecules, believed to have diverged from the MHC class I and class II lineages during the early stages of mammalian evolution. In humans, CD1 consists of a family of five closely linked genes, designated CD1 A–E and located on chromosome 1 (reviewed in ref. 9). Although outside the MHC, CD1 genes have a similar intron/exon organization to MHC class I genes. The second, third and fourth exons encode the three extracellular domains of mature CD1, which by analogy with MHC class I proteins are designated the α1, α2 and α3 domains, respectively. With the exception of CD1E, protein products for all the CD1 genes have been described. They are non-polymorphic, typically β2-microgobulin-associated cell surface proteins that appear to be expressed in a tissue- and developmentally restricted manner. CD1 proteins are classified into two groups based on comparison of their sequences. The group 1 CD1 proteins CD1a, CD1b and CD1c are expressed by professional antigen-presenting cells in a variety of different tissues. The more divergent group 2 CD1 protein, CD1d, may have a substantially different, albeit less well-defined, tissue distribution. To date, interest has focused mainly on the expression of CD1d by epithelial cells of the gastrointestinal tract.9,10
It has recently been demonstrated that some CD1 proteins have the remarkable ability to present lipids and glycolipids derived from microbial antigen to T cells.11,12 In addition, both CD1d and its murine homologue are recognized by an unusual T-cell subset that carries both T-cell markers and natural killer (NK) receptors. In the presence of CD1d these cells produce large quantities of interleukin-4 (IL-4), which can promote the development of T helper type 2 (Th2) over Th1 subsets.13–18 Interestingly, it has recently been suggested that normal pregnancy may be associated with a bias towards Th2 immune responses (reviewed in ref. 19). Although trophoblast is known to express several MHC class I or class-related genes whose products exhibit reduced or negligible polymorphism, the status of this epithelium for the expression of CD1 is currently unknown.
In this study we set out to investigate the expression of CD1 in human trophoblast. We focused specifically on CD1D since this is the only member of the CD1 family known to be expressed in epithelial cells. Using CD1D-specific oligonucleotide primers in the reverse transcriptase–polymerase chain reaction (RT-PCR), we present evidence that CD1D mRNA transcripts are expressed both in choriocarcinoma cell lines and in placentally derived trophoblast. We also identify a previously unreported CD1D mRNA transcript that is deficient in exon 4.
MATERIALS AND METHODS
Cell lines
The human choriocarcinoma cell line JEG-3 and the human thymoma cell line MOLT-4 were obtained from the American Type Culture Collection (Rockville, MD). The human choriocarcinoma cell line JAR was a generous gift from Prof. C. F. Graham, Department of Zoology, Oxford University, UK. The human thymoma cell line MOLT-4 was maintained in RPMI-1640 containing 10% (v/v) fetal calf serum (FCS) and 2 mm l-glutamine (GibcoBRL, Paisley, UK). JEG-3 and JAR were maintained in Ham's F12K supplemented with 10% (v/v) FCS, 2 mm l-glutamine and 1% (v/v) minimal essential medium (MEM) non-essential amino acid solution (Sigma Chemical Co., Poole, UK).
Isolation and purification of placental trophoblast and amnion cells
Trophoblast cells were isolated from the placental membranes or amniochorion delivered at term by elective Caesarean section by a two-step enzyme disaggregation method as previously described.20,21 Briefly, the amnion was removed and the decidual aspect of the chorionic membrane was gently scraped with a rubber policeman to reduce decidual cell contamination. Pieces of membrane were incubated with 1 mg/ml protease type XIV (Sigma) or 0·5 mg/ml trypsin (Sigma) for 60 min at 37°. They were then washed and incubated with 1 mg/ml collagenase from Clostridium histolyticum (BCL, Lewes, UK) and 2 mg/ml hyaluronidase type 1-S (Sigma) for 90 min at 37°. Trophoblast cells were further enriched by subjecting the primary cell suspensions to both positive and negative selection using magnetic microspheres (Dynal, Wirral, UK) according to the manufacturer's instructions. Positive selection was achieved using the trophoblast-specific monoclonal antibody (mAb) NDOG2 to placental alkaline phosphatase.22 Negative selection was achieved using the mAb L227 against MHC class II antigens.23 The procedure was monitored on cytospin preparations and was found to generate trophoblast suspensions of more than 95% (positive selection) and 90% (negative selection) purity. A similar two-step enzyme disaggregation procedure was used to prepare amnion cell suspensions from washed amniotic membranes except that the enzyme incubation steps were allowed to proceed for only 30–45 min21 More than 90% of the cells in these amnion preparations were positive for cytokeratins and the trophoblast marker NDOG2 stained less than 1:500 cells, indicating that trophoblast cells were absent.
Amplification and sequencing of CD1D
Following comparison of all five CD1 sequences, CD1D-specific oligonucleotide primers were selected and either synthesized on a DuPont Coder 300 DNA synthesizer, or supplied by VHBio Ltd (Gosforth, UK). Three pairs of forward and reverse primers designated F1/R1, F2/R2, and F3/R3 were used. These were designed to amplify CD1D segments as indicated diagramatically in Fig. 1. The oligonucleotide sequences were as follows: F1/R1, 5′-GCTCAACCAGGACAAGTGGACGAG-3′ (forward) and 5′-AGTCCTGGCGTGCTTGCTGTTCCT-3′ (backward); F2/R2, 5′-GAAGCAGCTTCACCAGGGACG-3′ (forward) and 5′-GCAAGGTGGGAGCTACACCTC-3′ (backward); F3/R3, 5′-GCCCCACTTTGGGTAAACTTGGC-3′ (forward) and 5′-GGAGGTAAAGCCCACAATGAGGAG-3′ (backward).
Figure 1.
Diagrammatic representation of CD1D. Exons are indicated by boxes and introns by horizontal broken lines. The 5′- and 3′-untranslated regions (UT) are indicated as are the exons encoding the leader (L), α1–3, transmembrane (TM) and cytoplasmic (C) domains of CD1d. Horizontal arrows indicate the positions of forward (F) and reverse (R) primers F1/R1, F2/R2 and F3/R3 expected to give amplified fragments of 453, 507 and 349 bp, respectively. Diagonal lines linking exons 3 and 5 indicate the position of the exon 3–exon 5 junction spanned by the reverse primer R3.
Poly(A)+ RNA was prepared from cultured cell lines using a Poly(A)Tract mRNA isolation kit (Promega, Madison, WI) and from placental trophoblast and amnion cell suspensions using the guanidinium thiocyanate method. A total of 1–5 μg of mRNA was copied into cDNA using a First Strand Synthesis kit (Pharmacia Biotech, Uppsala, Sweden). CD1D was amplified by PCR from 2·5 μl template cDNA in a total reaction volume of 50 μl containing 0·1 μg of primer and 350 μm of each dNTP in a reaction buffer consisting of 50 mm Tris–HCl (pH 9·2), 16 mm (NH4)2SO4, 1·75 mm MgCl2 and 100 μm dithiothreitol. Following an initial denaturing step at 94° for 10 min, 2·5 U Expand Taq/pwo DNA polymerase (Boehringer Mannheim plc, Lewes, UK) was added to each reaction. Unless otherwise stated, amplification was for 35 cycles at 94° for 15 seconds, 58° for 1 min 30 seconds, and 68° for 2 min 30 seconds. Amplification products were separated on 1·2% (w/v) agarose gels with 1×TAE [0·4 m Tris–HCl pH 8, 0·05 m sodium acetate, 0·01 m ethylenediaminetetraacetic acid (EDTA)] containing 5 μg/ml ethidium bromide. For negative controls, amplifications were conducted in the absence of cDNA template. For positive controls, ubiquitous actin-specific primers were substituted for the CD1D-specific primers.
Complementary DNA cloning and sequencing
For cloning and sequencing, products amplified by PCR were separated by electrophoresis on 2% (w/v) low melting point (LMP) agarose gels, excised, and recovered by electro-elution for 2 hr at 150 V in 0·2× TAE. The recovered DNA was ligated into pUC18 phage vector DNA and sequenced on an Applied Biosystems 377 Automated DNA sequencer (Cetus, CA). Alternatively, sequencing was performed directly from PCR products.
RESULTS
Choriocarcinoma cell lines are widely used as models to study trophoblast. In the first instance therefore, cDNA were prepared from the choriocarcinoma cell lines JEG-3 and JAR and used as templates in PCR with CD1D-specific primers to examine CD1D expression. Because previous studies suggest that CD1D mRNA is expressed at low abundance,24,25 all PCR experiments were performed using 35 cycles of amplification. Primers designated F1/R1 were designed to prime within exon 3 and exon 5, respectively, of CD1D (Fig. 1). These primers are predicted to amplify a 453-base pair (bp) product containing part of exon 3, all of exon 4, and the start of exon 5. As a positive control, the primers were also used to amplify a cDNA template made from the human thymoma cell line MOLT-4 which is known to express CD1D.26 Prominent bands of apparent sizes 453, 346, 196 and 174 bp were consistently obtained from MOLT-4 (Fig. 2, lane 1). By contrast, only two products were identified using F1/R1 in JEG-3. These apparently comigrated with the 196-bp and 174-bp bands observed in MOLT-4 (Fig. 2, lane 2). Bands of 196 and 174 bp were also weakly detected in JAR (data not shown). Interestingly, products comigrating with the 453- and 346-bp products observed in MOLT-4 were not detected in the choriocarcinoma cell lines (compare Fig. 2, lanes 1 and 2, respectively).
Figure 2.
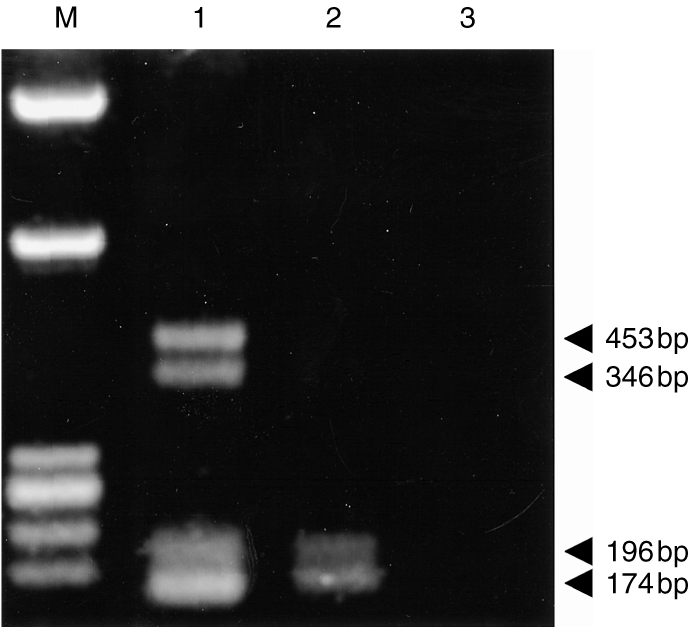
Results of RT-PCR amplification using CD1D-specific oligonucleotide primers F1/R1 on cDNA from MOLT-4 (lane 1), JEG-3 (lane 2) and, as a negative control, in the absence of template cDNA (lane 3). The products were analysed by 1·2% (w/v) agarose gel electrophoresis. Lane M shows molecular size markers, φX174 DNA/Hae III and λDNA/Hin dIII digest. The size of the fragments observed in MOLT-4 and JEG-3 is indicated on the right.
Expression of several members of the CD1 gene family is known to be associated with considerable mRNA splicing complexity.27 However, Calabi et al. previously detected only one CD1D mRNA species in MOLT-4 by Northern analysis using a CD1D-specific probe.28 The amplification of multiple fragments from MOLT-4 using CD1D-specific primers was therefore unexpected. Several approaches were used to investigate these products further. In the first instance, an additional set of CD1D-specific primers was designed. Designated F2/R2, these are located 5′ of F1 and 3′ of R1, respectively (Fig. 1) and are predicted to amplify a 507-bp CD1D product. F2/R2 amplified four prominent bands of apparent sizes 507, 436, 245 and 228 bp from MOLT-4 (Fig. 3, lane 1). When JEG-3 were examined, a single band of apparent size 228 bp was amplified using F2/R2 (Fig. 3, lane 2): a single 228-bp band was also amplified from JAR (not shown). Thus, two sets of CD1D primers located within a similar region of the CD1D gene amplified multiple products from MOLT-4. In the case of the choriocarcinoma cell lines, both primer pairs amplified fragments that were smaller in size than the expected PCR product.
Figure 3.

Results of RT-PCR amplification using CD1D-specific oligonucleotide primers F2/R2 on cDNAs from MOLT-4 (lane 1), JEG-3 (lane 2) and, as a negative control, in the absence of template cDNA (lane 3). The products were analysed by 1·2% (w/v) agarose gel electrophoresis. The size of the fragments observed is indicated on the right.
To examine the nature of these products further, each of the four fragments amplified by F1/R1 in MOLT-4 was excised and re-amplified. The 453-bp band yielded 453 and, in trace amounts, 174-bp fragments. The 346-bp fragment yielded only 196- and 174-bp products. The 196-bp product generated 196- and 174-bp fragments, while the 174-bp product generated only 174-bp fragments. Thus the 346-bp fragment appeared to represent a PCR artefact and was not investigated further. The effect of increasing the primer annealing temperature on the fragments generated by F1/R1 in MOLT-4 was also examined. As expected, there was an apparent decrease in the amount of the 346-bp band when the annealing temperature was raised from 58° to 64°. In addition, increasing the annealing temperature also resulted in a marked reduction in the amount of the 196-bp fragment suggesting that this too represented a PCR artefact. Similar results were obtained for the 436- and 245-bp fragments observed in MOLT-4 using F2/R2.
On the basis of the above results the 453- and 174-bp products obtained in MOLT-4 after amplification with F1/R1 were cloned and sequenced. The 453-bp fragment was found to be identical to the sequence expected to be amplified from CD1D (Fig. 4). There were no intron sequences indicating that amplification of mRNA and not genomic DNA had occurred. The 174-bp fragment was identical to a CD1D transcript in which the 279 bp encoding exon 4 were absent: as shown in Fig. 4, the sequence presented by this fragment clearly reveals a junction formed between exon 3 and exon 5.
Figure 4.
Alignment of the CD1D nucleotide sequence (upper sequence) against nucleotide sequences obtained from the 453-bp (middle sequence) and 174-bp (lower sequence) fragments amplified from MOLT-4 using the oligonucleotide primers F1/R1. Boundaries between exons 3/4 and 4/5 are indicated by arrowheads in the upper sequence. Nucleotide agreement with the consensus CD1D sequence is indicated by a hyphen (-). Sequence absent from the 174-bp fragment is indicated by the dots (·). Diagrams of the CD1D transcripts generated from the 453- and 174-bp fragments are also shown.
Attention was next turned to the products observed in the choriocarcinoma cell lines. As in MOLT-4, the amount of the 196-bp fragment amplified in JEG-3 by F1/R1 was found to decrease with increasing annealing temperature and this fragment was therefore not investigated further. The 174-bp fragment generated from JEG-3 by F1/R1 amplification was cloned and sequenced. As for MOLT-4, the sequence of this fragment was found to be identical to CD1D lacking the 279 bp encoding exon 4. Thus, both MOLT-4 and JEG-3 appear to express a CD1D mRNA species in which exon 4 is absent.
A third set of oligonucleotide primers, designated F3/R3, was constructed to confirm that the exon 4-deficient CD1D products were authentic amplimers. The reverse primer was designed to limit F3/R3 amplification to exon 4-deficient products by spanning the exon 3/exon 5 junction (Fig. 1). These primers were predicted to amplify a 349-bp product comprising part of exon 2, all of exon 3, and the exon 3–5 junction itself. The expected fragment was indeed detected in MOLT-4 using these primers (Fig. 5, lane 1). Similar products were also detected in the choriocarcinoma cell lines JEG-3 (Fig. 5, lane 2) and JAR (Fig. 5, lane 3). Direct nucleotide sequencing of the amplified products confirmed that the 349-bp products amplified from MOLT-4, JEG-3 and JAR were genuine exon 4-deficient CD1D amplimers.
Figure 5.

Results of RT-PCR amplification using CD1D-specific oligonucleotide primers F3/R3 on cDNAs from MOLT-4 (lane 1), JEG-3 (lane 2) and JAR (lane 3). In the negative control (lane 4), amplification was conducted in the absence of template cDNA. The products were analysed by 1·2% (w/v) agarose gel electrophoresis. The size of the amplified fragments is indicated on the right.
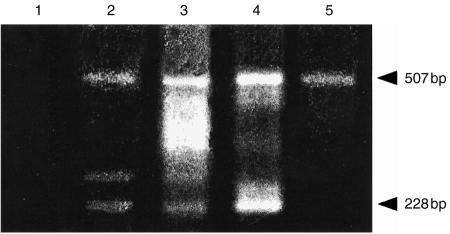
Finally, the expression of CD1D was examined in cDNA prepared from freshly isolated human placental trophoblast cells. For this, cytotrophoblast cells were isolated from term placental membranes (amniochorion) by enzyme disaggregation. The initial cell suspension generated by this procedure contained 70–80% cytotrophoblast cells. Further enrichment was achieved either by positive or negative selection using magnetic microbeads coated with appropriate mAb. Negative selection was achieved by removing the majority of contaminating maternal decidual cells using beads coated with the anti-class II mAb L227. Positive selection was achieved using beads coated with mAb NDOG2 to placental alkaline phosphatase, a trophoblast-specific protein expressed by most, but not all, cytotrophoblast cells in the amniochorion. cDNA prepared from freshly isolated human amnion cells was also included in these studies. F1/R1 and F2/R2 primers amplified prominent bands comigrating with both exon 4-intact and exon 4-deficient CD1D products from placental trophoblast cells isolated by positive and negative selection. The 507-bp and 228-bp products amplified by F2/R2 are shown in Fig. 6 for both negatively (track 2) and positively (track 3) selected trophoblast cells. The identity of additional products observed in the positively selected trophoblast cells, including one of 280 bp which was also observed in negatively selected trophoblast cells, is currently unknown. Taken together, these studies suggest that in common with MOLT-4 but unlike choriocarcinoma cell lines, freshly isolated placental trophoblast cells express both exon 4-intact and exon 4-deficient CD1D mRNAs. By contrast, freshly isolated human amnion cells appear to express only the exon 4-intact 507-bp CD1D product: the 228-bp band that comigrates with the exon 4-deficient CD1D product was not detected in these cells (Fig. 6, track 5).
Figure 6.
Results of RT-PCR amplification using CD1D-specific oligonucleotide primers F2/R2 on cDNAs from human placental trophoblast cells isolated by negative selection (lane 2) or positive selection (lane 3), from MOLT-4 (lane 4), and from freshly isolated human amnion cells (lane 5). In the negative control (lane 1), amplification was conducted in the absence of template cDNA. The products were analysed by 1·2% (w/v) agarose gel electrophoresis and the size of the amplified products is indicated on the right.
DISCUSSION
The objective of the present study was to determine if CD1D is expressed in human trophoblast. RT-PCR coupled with the use of different CD1D-specific oligonucleotide primers has shown that CD1D mRNA transcripts are indeed expressed in trophoblast-derived cell lines and in placentally derived trophoblast cell preparations. These studies have also identified an apparently novel, alternatively spliced CD1D mRNA transcript lacking exon 4. Moreover, our results suggest that the exon 4-intact and exon 4-deficient CD1D transcripts are variably expressed in different trophoblastic and non-trophoblastic cell populations.
The demonstration of a novel CD1D transcript deficient in exon 4 was unexpected. Milstein and colleagues have previously conducted extensive analysis of alternative splicing among the CD1 gene family and found multiple splice variants for CD1A, CD1C and CD1E, but not for CD1D. For example, Northern analysis of MOLT-4 using probes derived from the 3′-untranslated region revealed several transcripts for all of the CD1 genes except CD1D which displayed only a single transcript.28 More recently, RT-PCR using oligonucleotides designed to amplify segments located between exon 4 and the 3′ untranslated region identified a major product encoding the membrane isoform of CD1D in freshly isolated human thymocytes.27 Our own studies have used different primers to those used by Milstein and colleagues to demonstrate the alternatively spliced CD1D transcript. We were also able to confirm the existence of this product by using a primer designed to span the exon 3–exon 5 boundary and by direct nucleotide sequencing of the product amplified from MOLT-4 and choriocarcinoma cell lines. Our results therefore suggest that the expression of CD1D, as well as other CD1 genes, can be associated with alternative splicing. It remains to be determined whether oligonucleotides designed to probe elsewhere within the CD1D sequence can amplify additional alternatively spliced CD1D transcripts.
The exon 4-deficient CD1D transcript was found along with its exon 4-intact counterpart in the human thymoma cell line MOLT-4 used as a positive control in these studies. Similarly, both exon 4-intact and exon 4-deficient CD1D transcripts were detected in freshly isolated and enriched human placental trophoblast cell preparations. We cannot rule out the possibility that the CD1D products observed in these latter preparations were derived from contaminating cells rather than from trophoblast cells. However, this seems unlikely. In the first place, the CD1D transcripts were observed not only in highly enriched trophoblast preparations depleted of MHC class II-expressing contaminants, but also in trophoblast populations isolated by positive selection using the trophoblast-specific marker placental alkaline phosphatase. In addition, our studies provide clear evidence for the expression of an albeit alternatively spliced CD1D mRNA transcript in choriocarcinoma cell lines, which are frequently used as models to study placental trophoblast.
Interestingly, and in contrast to placentally derived trophoblast and MOLT-4, the choriocarcinoma cell lines JEG-3 and JAR expressed the exon 4-deficient CD1D transcript in the apparent absence of the exon 4-intact product. Conversely, freshly isolated human amnion cells expressed the exon 4-intact CD1D product in the apparent absence of the exon 4-deficient transcript. While malignant transformation or adaptation to tissue culture conditions could influence the expression of CD1D transcripts, it is also possible that CD1D mRNAs are under the influence of tissue-specific splicing. Woolfson and Milstein have already shown that there are differences in CD1A, CD1B and CD1C mRNA splicing patterns between mouse myeloma transfectants and freshly isolated human thymocytes.27 Such tissue-specific splicing could also account for the observed differences in expression of CD1D transcripts between amnion cells on the one hand, and trophoblast or MOLT-4 cells on the other. However, tissue-specific splicing is less likely to account for the observed differences in the CD1D mRNAs observed between trophoblast cell lines and freshly isolated placental trophoblast cells. It might be speculated that expression of the exon 4-intact and exon 4-deficient CD1D transcripts is influenced by trophoblast differentiation or by gestational age. In this respect, the choriocarcinoma cell lines might be more characteristic of villous or extravillous trophoblast populations in the developing human placenta, rather than the trophoblast cells of the amniochorion investigated here. The expression of CD1D mRNA transcripts in these different trophoblast cell populations deserves further investigation.
The protein sequence deduced from the exon 4-deficient CD1D transcript identified in the present study is devoid of the α3 domain which, in MHC class I molecules, is responsible for β2-microglobulin binding. Thus, protein products arising from the exon 4-deficient CD1D transcript would be unlikely to associate with β2-microglobulin. Moreover, while β2-microglobulin has recently been shown to be essential for the processing and surface transport of CD1a, CD1b and CD1c molecules,29 there is evidence that CD1d expression may be less dependent on β2-microglobulin. In particular, Balk et al. have reported that a 37 000 MW non-glycosylated CD1d protein is expressed in the absence of β2-microglobulin at the surface of human intestinal epithelial cells.30 Interestingly, a novel protein C/activated protein C receptor that shares close homology with CD1d but which is devoid of an α3 domain has been identified in human endothelial cells where it has been implicated in the control of inflammatory responses.31 Similarly, Kirszenbaum et al. have also recently identified a novel HLA-G mRNA transcript, designated HLA-G4, which lacks exon 4.32 The HLA-G4 transcript was expressed along with its exon 4-containing counterpart in first-trimester placental trophoblast. Although protein products for HLA-G4 have not been reported, it has been suggested that such a product could also be expressed at the cell surface where it may bind peptides differently.32
Taken together, our studies provide evidence for the expression of a new addition to the repertoire of immune-related genes expressed by human trophoblast. Information emerging from other systems suggests that the murine homologue of CD1d, in common with human CD1a, CD1b and CD1c, can present glycolipids.33,34 In addition both murine and human CD1d can interact with a population of T cells bearing both NK and T-cell markers.13–18 Such functional activities could have considerable relevance to our understanding of antigen presentation and immunoregulation at the human feto–maternal interface during pregnancy. In order to explore further the implications of our results, it will now be of considerable interest to determine if the CD1D mRNA transcripts identified in the present study are translated into proteins. This in turn will depend upon the availability of antibody reagents capable of specifically detecting CD1d proteins.
Acknowledgments
H.J.J. and P.F. are supported by University of Bristol Postgraduate Research Scholarships. S.D.W. and K.L.S. are supported by the Medical Research Council (grant numbers G9403577 and G9631136). We are grateful to Dr Richard Blumberg of Harvard Medical School for his help and advice.
REFERENCES
- 1.Goodfellow PN, Barnstable CJ, Bodmer WF, Snary DE, Crumpton MJ. Expression of HLA system antigens on placentae. Transplantation. 1976;22:595. doi: 10.1097/00007890-197612000-00009. [DOI] [PubMed] [Google Scholar]
- 2.Hunt J, Orr H. HLA and maternal-fetal recognition. FASEB J. 1992;6:2344. doi: 10.1096/fasebj.6.6.1544544. [DOI] [PubMed] [Google Scholar]
- 3.Schmidt CM, Orr HT. Maternal/fetal interactions: the role of the MHC class I molecule HLA-G. Crit Rev Immunol. 1993;13:207. [PubMed] [Google Scholar]
- 4.Ellis SA, Palmer MS, McMichael AJ. Human trophoblast and the choriocarcinoma cell line BeWo express a truncated HLA class I molecule. J Immunol. 1990;144:731. [PubMed] [Google Scholar]
- 5.Kovats S, Main EK, Librach C, Stubblebine M, Fisher SJ, Demars R. A class I antigen, HLA-G, expressed in human trophoblasts. Science. 1990;248:220. doi: 10.1126/science.2326636. [DOI] [PubMed] [Google Scholar]
- 6.King A, Boocock C, Sharkey AM, et al. Evidence for the expression of HLA-C class I mRNA and protein by human first trimester trophoblast. J Immunol. 1996;156:2068. [PubMed] [Google Scholar]
- 7.Simister NE, Story CM, Chen H-L, Hunt JS. An IgG-transporting Fc receptor expressed in the syncytiotrophoblast of human placenta. Eur J Immunol. 1996;26:1527. doi: 10.1002/eji.1830260718. [DOI] [PubMed] [Google Scholar]
- 8.Leach JL, Sedmak DD, Osborne JM, Rahill B, Lairmore MD, Anderson CL. Isolation from human placenta of the IgG transporter, FcRn and localization to the syncytiotrophoblast. Implications for maternal-fetal antibody transport. J Immunol. 1996;157:3317. [PubMed] [Google Scholar]
- 9.Porcelli SA. The CD1 family: a third lineage of antigen presenting molecules. Adv Immunol. 1995;59:1. doi: 10.1016/s0065-2776(08)60629-x. [DOI] [PubMed] [Google Scholar]
- 10.Blumberg RS, Terhorst C, Bleicher P, et al. Expression of a non-polymorphic MHC class I-like molecule, CD1D, by human intestinal epithelial cells. J Immunol. 1991;147:2518. [PubMed] [Google Scholar]
- 11.Beckman EB, Porcelli SA, Furlong S, Morita CT, Behar S, Brenner MB. Recognition of a lipid antigen by CD1-restricted alpha beta+ T cells. Nature. 1994;372:891. doi: 10.1038/372691a0. [DOI] [PubMed] [Google Scholar]
- 12.Seiling P, Chatterjee D, Porcelli SA, et al. CD1 restricted T cell recognition of lipoglycan antigens. Science. 1995;269:227. doi: 10.1126/science.7542404. [DOI] [PubMed] [Google Scholar]
- 13.Bendelac A, Lantz O, Quimby ME, Yewdell JW, Bennink JR, Brutkiewicz RR. CD1 recognition by mouse NK1+ T lymphocytes. Science. 1995;268:863. doi: 10.1126/science.7538697. [DOI] [PubMed] [Google Scholar]
- 14.Mendiratta SK, Martin WD, Hong S, Boesteanu A, Joyce S, Van Kaer L. CD1d1 mutant mice are deficient in natural T cells that promptly produce IL-4. Immunity. 1997;6:469. doi: 10.1016/s1074-7613(00)80290-3. [DOI] [PubMed] [Google Scholar]
- 15.Chen Y-H, Chiu NM, Mandal M, Wang N, Wang C-R. Impaired NK1+ T cell development and early IL-4 production in CD1-deficient mice. Immunity. 1997;6:459. doi: 10.1016/s1074-7613(00)80289-7. [DOI] [PubMed] [Google Scholar]
- 16.Prussin C, Foster B. TCR Vα24 and Vβ11 coexpression defines a human NK1 T cell analog containing a unique Th0 subpopulation. J Immunol. 1997;159:5862. [PubMed] [Google Scholar]
- 17.Exley M, Garcia J, Balk SP, Porcelli S. Requirements for CD1d recognition by human invariant Vα24+ CD4− CD8+ T cells. J Exp Med. 1997;186:109. doi: 10.1084/jem.186.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Davodeau F, Peyrat MA, Necker A, et al. Close phenotypic and functional similarities between human and murine alpha beta T cells expressing invariant TCR alpha-chains. J Immunol. 1997;158:5603. [PubMed] [Google Scholar]
- 19.Raghupathy R. Th1-type immunity is incompatible with successful pregnancy. Immunol Today. 1997;18:478. doi: 10.1016/s0167-5699(97)01127-4. [DOI] [PubMed] [Google Scholar]
- 20.Holmes CH, Simpson KL, Wainwright SD, et al. Preferential expression of the complement regulatory protein decay accelerating factor at the fetomaternal interface during human pregnancy. J Immunol. 1990;144:3099. [PubMed] [Google Scholar]
- 21.Houlihan JM, Biro PA, Harper HM, Jenkinson HJ, Holmes CH. The human amnion is a site of MHC class Ib expression: evidence for the expression of HLA-E and HLA-G. J Immunol. 1995;154:5665. [PubMed] [Google Scholar]
- 22.Sunderland CA, Davies JO, Stirrat GM. Immunohistology of normal and ovarian cancer tissue with a monoclonal antibody to placental alkaline phosphatase. Cancer Res. 1984;44:4496. [PubMed] [Google Scholar]
- 23.Lampson LA, Levy R. Two populations of Ia molecules on B cells. J Immunol. 1980;125:293. [PubMed] [Google Scholar]
- 24.Balk SP, Bleicher PA, Terhorst C. Isolation and characterization of a cDNA and gene coding for a fourth CD1 molecule. Proc Natl Acad Sci USA. 1989;86:252. doi: 10.1073/pnas.86.1.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Calabi F, Jarvis JM, Martin L, Milstein C. Two classes of CD1 genes. Eur J Immunol. 1989;19:285. doi: 10.1002/eji.1830190211. [DOI] [PubMed] [Google Scholar]
- 26.Bilsland CAG, Milstein C. The identification of the β2-microglobulin binding antigen encoded by the human CD1D gene. Eur J Immunol. 1991;21:71. doi: 10.1002/eji.1830210112. [DOI] [PubMed] [Google Scholar]
- 27.Woolfson A, Milstein C. Alternative splicing generates secretory isoforms of human CD1. Proc Natl Acad Sci USA. 1994;91:668314. doi: 10.1073/pnas.91.14.6683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Calabi F, Bilsland CAG, Yui CY. In: Leukocyte Typing IV. Knapp W, Dorkin B, Gilks WR, Reiber EP, Schmidt RE, von dem Brone AEGK, et al., editors. Oxford: Oxford University Press; 1989. p. 254. [Google Scholar]
- 29.Bauer A, Huttinger R, Staffler G, et al. Analysis of the requirement for β2-microglobulin for expression and formation of CD1 antigens. Eur J Immunol. 1997;27:1366. doi: 10.1002/eji.1830270611. [DOI] [PubMed] [Google Scholar]
- 30.Balk SP, Burke S, Polischuk JE, et al. Beta-2-microglobulin-independent MHC class Ib molecule expressed by human intestinal epithelium. Science. 1994;265:259. doi: 10.1126/science.7517575. [DOI] [PubMed] [Google Scholar]
- 31.Fukudome K, Esmon CT. Identification, cloning and regulation of a novel endothelial cell protein C/activated protein C receptor. J Biol Chem. 1994;269:26486. [PubMed] [Google Scholar]
- 32.Kirszenbaum M, Moreau P, Gluckman E, Dausset J, Carosella E. An alternatively spliced form of HLA-G mRNA in human trophoblasts and evidence for the presence of HLA-G transcript in adult lymphocytes. Proc Natl Acad Sci USA. 1994;91:4209. doi: 10.1073/pnas.91.10.4209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kawano T, Cui JQ, Koezuka Y, et al. CD1d-restricted and TCR-mediated activation of V (alpha) 14 NK T cells by glycosylceramides. Science. 1997;278:1626. doi: 10.1126/science.278.5343.1626. [DOI] [PubMed] [Google Scholar]
- 34.Joyce S, Woods AS, Yewdell JW, et al. Natural ligand of mouse CD1d1: cellular glycosylphosphatidylinositol. Science. 1998;279:1541. doi: 10.1126/science.279.5356.1541. [DOI] [PubMed] [Google Scholar]