Abstract
Gangliosides are sialic acid-containing glycolipids and have various immunomodulatory effects. We previously reported that various gangliosides in vitro either inhibited or enhanced spontaneous immunoglobulin production by human peripheral blood mononuclear cells (PBMC). Among them, GT1b was the most inhibitory. In this study, we further examined the mechanism for the inhibitory effect of GT1b. The inhibitory effect of GT1b was apparent at 0·1 μm, increased dose dependently, and was maximal at 10 μm. In the presence of 10 μm GT1b, spontaneous production of immunoglobulin (Ig)G, IgM and IgA in human PBMC was reduced by 60%, 59·5% and 58%, respectively, compared with controls. GT1b did not affect the proliferation and viability of PBMC, and did not enhance their apoptosis. GT1b did not alter immunoglobulin production of B cells alone. Interleukin (IL)-6 and IL-10 each partially reversed the GT1b-induced inhibition of immunoglobulin production by PBMC, and the presence of both cytokines completely reversed the inhibition. GT1b inhibited IL-6 and IL-10 production in monocytes, without affecting that in T or B cells. When monocytes were preincubated with GT1b, washed and then cultured with B and T cells, the immunoglobulin production was also suppressed. These results suggest that GT1b may indirectly suppress immunoglobulin production of B cells in whole PBMC via reducing the production of IL-6 and IL-10 in monocytes. It is thus indicated that GT1b may act as an important inhibitor for human humoral immune responses.
INTRODUCTION
Gangliosides are sialic acid-containing glycosphingolipids, and are constituents of the plasma membranes of various cells.1 Gangliosides are also shed into the extracellular environment, and play important immunomodulatory roles in neoplastic and neurological diseases.2,3 Previous studies reported that various gangliosides either inhibited or enhanced cellular immune responses in mice and humans.2,4,5 Several studies also revealed the effects of gangliosides on human6–8 and murine9 humoral immune responses in vitro. However, the results of these studies are inconsistent with one another: ganglioside GM1 enhanced immunoglobulin production of human plasma cells,6 while it suppressed anti-sheep erythrocyte plaque-forming cell responses of murine splenocytes.9 GM2 and GM3 suppressed Staphylococcus aureus Cowan strain I plus interleukin (IL)-2-induced immunoglobulin production of human tonsillar small resting B cells,7 but had no effect on immunoglobulin production of human plasma cells.6 Presumably the effects of individual gangliosides may vary with species and type of immunoglobulin-producing cells. These previous investigators generally used mitogen- or cytokine-induced systems for immunoglobulin production or immortalized or neoplastic cells to test the effects of gangliosides.7,8 However, these experimental conditions might alter the cellular responses to gangliosides and thus mask the original effects of gangliosides on the constitutive immunoglobulin production of non-immortalized cells. Our first study therefore aimed to elucidate the in vitro effects of gangliosides on spontaneous immunoglobulin production by peripheral blood mononuclear cells (PBMC) from normal human subjects.10 Spontaneous IgG, IgM and IgA production was inhibited by GT1b and GD1b, while enhanced by GQ1b, GM2 and GD1a.10 Amongst these, the inhibitory effect of GT1b was strong and reproducible. In this study, we further examined the mechanism for the inhibitory effect of GT1b on immunoglobulin production by human PBMC. PBMC consist of various subpopulations: B cells, which differentiate into immunoglobulin-producing cells; and accessory cells, like T cells or monocytes, which help B-cell differentiation via the release of cytokines and/or direct contact with B cells.10 We thus aimed to identify whether GT1b suppresses B-cell activity directly, or helper functions of accessory cells, or both.
MATERIALS AND METHODS
Reagents
Highly purified bovine brain ganglioside GT1b was purchased from Sigma (St Louis, MO). Recombinant human IL-1α and IL-1β were from R&D Systems (Minneapolis, MN). Recombinant human IL-2, IL-4 and IL-6 were purchased from Boehringer Mannheim (Indianapolis, IN). Recombinant human IL-10 was from Bachem Bioscience (Philadelphia, PA).
Preparation of PBMC, monocytes, B cells and T cells
Blood was taken from consenting, healthy volunteers who had been informed of the objectives and methods of this study. PBMC were isolated by centrifugation over Ficoll–Hypaque (Pharmacia, Uppsala, Sweden) as previously described.11 PBMC were allowed to adhere to plastic dishes. From the dish-adherent cells, CD3−, CD19− and CD56− cells were isolated by negative selection using immunomagnetic beads (Dynal, Great Neck, NY), as previously described,12 and were used as monocytes. This monocyte population was >97% CD14+, and the contamination of CD3+, CD19+, or CD56+ cells was <1% by flow cytometry. From the non-adherent cells, CD56− cells were isolated using immunomagnetic negative selection, and were incubated with neuraminidase-treated sheep erythrocytes as previously described.13 From the rosette-forming cells, CD14− and CD19− cells were isolated using immunomagnetic negative selection, and were used as T cells. This T-cell population was >98% CD3+, and the contamination of CD14+, CD19+, or CD56+ cells was <2%. From the non-rosette-forming cells, CD3− and CD14− cells were isolated using immunomagnetic negative selection, and were used as B cells. This B-cell population was >97% CD19+, and the contamination of CD3+, CD14+ or CD56+ cells in this population was <1%.
Cell cultures
PBMC or B cells (2×105/200 μl/well) were cultured in triplicate in round-bottom 96-well tissue culture plates, with or without GT1b at indicated doses in the culture medium, at 37° in an atmosphere of 5% CO2 in air, for 5 days (unless otherwise indicated). As a high concentration of serum hinders ganglioside binding to the cell membrane,14–16 we used serum-free Hymedium 920 (Kohjin Bio, Tokyo, Japan) containing 250 mg/l human albumin, which is a concentration appropriate for cell maintenance and ganglioside binding.15,16 The composition of amino acids, minerals and vitamins in Hymedium 920 is similar to that in RPMI-1640 (Life Technologies, Grand Island, NY), except that the former medium contains 0·48 mg/l thymidine, 2 mg/l human transferrin, 0·2 mg/ml 3,3′,5-triiodo-l-thyronine, and 60 mg/l kanamycin. GT1b was dissolved in absolute ethanol and diluted at least 1000-fold to the required concentrations. Control cultures contained ethanol at the highest concentration used in the experimental cultures, and this level of ethanol was not toxic to PBMC. The culture supernatants were then harvested and stored at −70° until use. The levels of IgG, IgM and IgA in the supernatants were measured by an enzyme-linked immunosorbent assay (ELISA), as described previously.17 For the measurement of proliferation, PBMC or B cells were cultured as described above for 4 days (unless otherwise indicated), and pulsed with 1 μCi [3H]thymidine ([3H]TdR) for 8 hr. The cells were then harvested and [3H]TdR uptake was counted. For the measurement of cytokine production, PBMC, monocytes, T cells, or B cells (2×105/200 μl/well) were cultured, as described above, for 2 days. The level of IL-1α, IL-1β, IL-2, IL-4, IL-6 and IL-10 in the culture supernatants was measured using ELISA kits (Endogen, Cambridge, MA), according to the manufacturer's instructions. The detection limits for IL-1α, IL-1β, IL-2, IL-4, IL-6 and IL-10 were 2, 1, 6, 2, 1 and 3 pg/ml, respectively.
Statistical analyses
For the data in Fig. 1 and Fig. 4, a one-way analysis of variance (anova) with Dunnet's multiple comparison test was used. For the data in Fig. 5 and Table 1 and Table 2, a paired t-test was used. For the data in Fig. 3, a one-way anova with Scheffe's multiple comparison test was used. A value of P < 0·05 was considered to be significant.
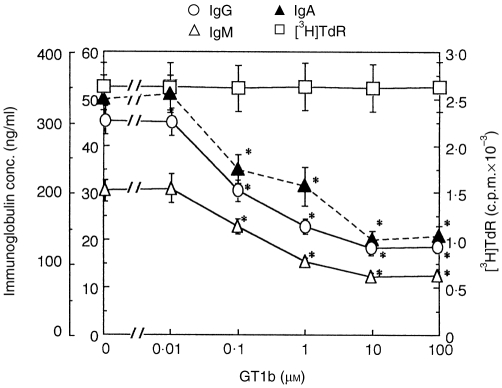
Figure 1.
Dose dependency of the effects of GT1b on human peripheral blood mononuclear cells (PBMC). PBMC from one healthy donor were cultured in triplicate in the presence of the indicated doses of GT1b. The production of IgG, IgM and IgA was determined by enzyme-linked immunosorbent assay (ELISA) on day 5, and [3H]TdR uptake was determined on day 4. Values are expressed as mean±SD of triplicate cultures. *P < 0·0001vs. control cultures. The data are representative of four separate experiments using PBMC from four different donors.
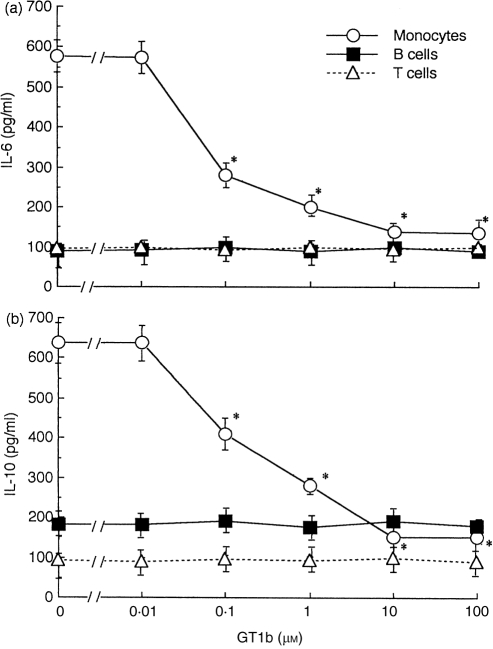
Figure 4.
Dose dependency of the effects of GT1b on production of interleukin (IL)-6 (a) and IL-10 (b). Monocytes, T cells and B cells from one healthy donor were cultured in triplicate in the presence or absence of the indicated doses of GT1b for 2 days, and the culture supernatants were analysed for cytokines by enzyme-linked immunosorbent assay (ELISA). Values are expressed as mean±SD of triplicate cultures. *P < 0·05 vs. control cultures. The data are representative of four separate experiments using peripheral blood cells from four different donors.
Figure 5.
The effect of preincubation of monocytes with GT1b on production of immunoglobulins (a) and cytokines (b). Monocytes (1×106 cells) from one healthy donor were preincubated with 1 ml of medium alone or with medium containing GT1b (10 μm) for 24 hr. After washing, the monocytes (2×104 cells) were added to B cells (2×104 cells) and T cells (15×104 cells) from the same donor, and cultured, in triplicate, in 200 μl of medium, for 5 days. IgG, IgM and IgA production in the culture supernatants were measured by ELISA. Simultaneously, the preincubated and washed monocytes (2×105 cells) were cultured alone with 200 μl of medium in triplicate for 5 days, and the supernatants were analysed for IL-6 and IL-10 by ELISA. Values are expressed as mean±SD of triplicate cultures. *P < 0·05vs. control cultures of preincubation with medium alone. The data are representative of three separate experiments using peripheral blood cells from three different donors.
Table 1.
The effects of GT1b on B cells
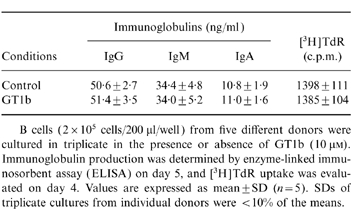
B cells (2×105 cells/200 μl/well) from five different donors were cultured in triplicate in the presence or absence of GT1b (10 μM).Immunoglobulin production was determined by enzyme-linked immunosorbent assay (ELISA) on day 5, and [3H]TdR uptake was evaluated on day 4. Values are expressed as mean±SD (n = 5). SDs of triplicate cultures from individual donors were <10% of the means.
Table 2.
The effects of GT1b on cytokine production by peripheral blood mononuclear cells (PBMC)
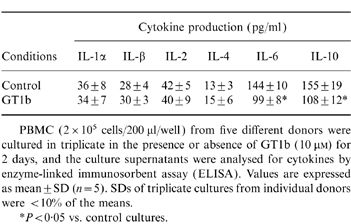
PBMC (2×105 cells/200 μl/well) from five different donors were cultured in triplicate in the presence or absence of GT1b (10 μM) for 2 days, and the culture supernatants were analysed for cytokines by enzyme–linked immunosorbent assay (ELISA). Values are expressed as mean±SD (n = 5). SDs of triplicate cultures from individual donors were <10% of the means.
*P < 0·05 vs. control cultures.
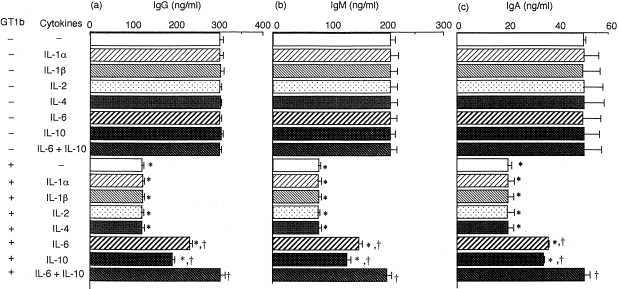
Figure 3.
Cytokine-induced recovery from the GT1b-induced inhibition of immunoglobulin production by peripheral blood mononuclear cells (PBMC). PBMC from five different donors were cultured in triplicate for 5 days with (+) or without (−) GT1b (10 μm) in the presence or absence of various cytokines (each 50 pg/ml). IgG (a), IgM (b) and IgA (c) production in each culture were measured by enzyme-linked immunosorbent assay (ELISA) on day 5. Values are expressed as mean±SD (n = 5). SDs of triplicate cultures from individual donors were <10% of the means. *P < 0·001 vs. control cultures. †P < 0·001 vs. cultures with GT1b alone.
RESULTS
Dose dependency for the inhibitory effect of GT1b on immunoglobulin production by human PBMC
First, GT1b was added to the culture medium at different doses, and its effect was examined on spontaneous production of IgG, IgM and IgA by human PBMC. As shown in Fig. 1, GT1b significantly inhibited immunoglobulin production. The inhibitory effect of GT1b was first evident at 0·1 μm, increased dose dependently, and was maximal at 10 μm, where IgG, IgM and IgA production was reduced by 60%, 59·5% and 58%, respectively, compared with controls. On the other hand, the proliferation of PBMC, as determined by [3H]TdR uptake, was not altered by GT1b at any dose tested. Moreover, GT1b at doses ≤ 100 μm did not reduce the viability of PBMC; the cell viability was always 90–95% as deterimined by Trypan Blue dye exclusion (data not shown). GT1b did not enhance apoptosis of PBMC; the level of apoptotic cells after 48 hr of culture were: (mean±SD) 5·8±1·7% (n = 5) in GT1b (10 μm)-treated PBMC compared with 5·9±2·0% in controls, as determined by propidium iodide staining. These results suggest that GT1b inhibits production of IgG, IgM and IgA by human PBMC without altering proliferation or viability of the PBMC. As 10 μm seemed to be an optimal concentration for the inhibitory effects of GT1b, and was non-toxic, this dose was used in subsequent experiments.
Kinetics of the inhibitory effect of GT1b
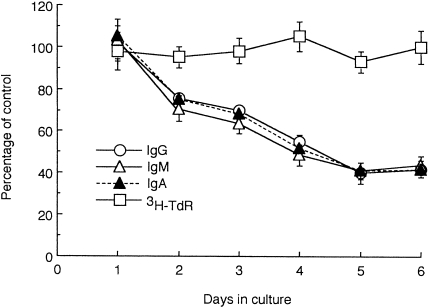
As shown in Fig. 2, GT1b-induced inhibition of IgG, IgM and IgA production by PBMC was first detected on day 2 of culture, with the greatest inhibition occurring on day 5. In contrast, GT1b did not affect the proliferation of PBMC on any day examined.
Figure 2.
Kinetics of the effects of GT1b on peripheral blood mononuclear cells (PBMC). PBMC from three different donors were cultured in triplicate in the presence of GT1b (10 μm) for 1–6 days. The production of IgG, IgM and IgA, and [3H]TdR uptake, were evaluated on each day. Results are expressed as the percentage vs. control cultures without GT1b. Values are expressed as mean±SD (n = 3). SDs of triplicate cultures from individual donors were <10% of the mean. The mean values of the controls (n = 3) on days 1, 2, 3, 4, 5 and 6 were: 118, 151, 181, 269, 311 and 324 ng/ml for IgG; 97, 114, 147, 180, 205 and 212 ng/ml for IgM; 10, 19, 22, 35, 49 and 61 ng/ml for IgA; and 1074, 1387, 1922, 2382, 2506 and 2378 c.p.m. for [3H]TdR uptake, respectively.
The direct effect of GT1b on B cells
To examine the direct effect of GT1b on spontaneous immunoglobulin production by B cells, B cells were isolated, for use, from PBMC. As it was anticipated that normal B cells may produce a very low amount of immunoglobulins without any stimuli or accessory cells, the absolute B-cell number was highly elevated up to 1×106/ml, which is approximately 10 times the B-cell number in 1×106/ml PBMC. As shown in Table 1, immunoglobulin amounts produced by B cells were still low but measurable. The immunoglobulin production of B cells was not significantly altered by GT1b. Neither was the proliferation of B cells affected by GT1b. These results suggest that GT1b may not directly affect B-cell activity but may act on accessory cells like monocytes or T cells, and suppress their function to help immunoglobulin production of B cells via the release of cytokines.
Cytokine-induced recovery from the inhibitory effect of GT1b
To examine the involvement of cytokines in the inhibitory effect of GT1b, cytokines that seemed relevant to immunoglobulin production were added to PBMC, together with GT1b, and their effect was tested on GT1b-induced inhibition. As shown in Fig. 3, these cytokines were used at doses that did not influence immunoglobulin production without GT1b. Among various cytokines, IL-6 and IL-10 each partially reversed the GT1b-induced inhibition of IgG, IgM and IgA production by PBMC. When both IL-6 and IL-10 were used together, additive effects were obtained and the GT1b-induced inhibition was completely reversed. These results suggest that both IL-6 and IL-10 may be involved in the GT1b-induced inhibition of PBMC. This also indicates that GT1b may reduce IL-6 and IL-10 production in PBMC.
The inhibitory effects of GT1b on cytokine production
To examine the effects of GT1b on cytokine production by PBMC, culture supernatants, with or without GT1b, were assayed for the activity of various cytokines. After 2 days of culture, GT1b reduced both IL-6 and IL-10 production in PBMC by 31·4% and 30·5%, respectively, compared with controls (Table 2). On the other hand, GT1b did not significantly alter the production of IL-1α, IL-1β, IL-2, and IL-4. Similar results were obtained when the culture period was changed to 1, 3 and 4 days (data not shown). PBMC were then subfractionated into B cells, T cells and monocytes, and GT1b effects on IL-6 and IL-10 production by each fraction were examined. As shown in Fig. 4, GT1b did not affect IL-6 and IL-10 production in monocytes by 75·7% and 76·5%, respectively, compared with controls. The dose–response curves of the GT1b effects on cytokine production were similar to those shown for immunoglobulin production (Fig. 1). Similarly to whole PBMC, GT1b did not alter IL-1α, IL-1β, IL-2 and IL-4 production by any fraction (data not shown). Thus, GT1b suppressed IL-6 and IL-10 production in monocytes. It is suggested that those effects of GT1b on monocytes may contribute to the inhibition of immunoglobulin production by B cells in whole PBMC culture.
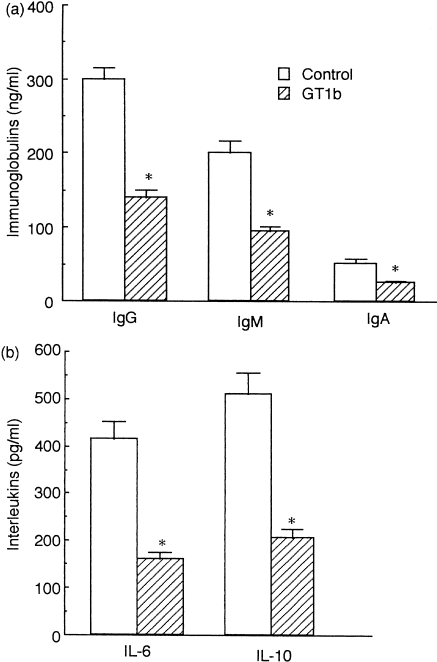
To confirm this, we performed the following experiments: we preincubated monocytes with GT1b for 24 hr, washed them and then added them to B cells and T cells, cultured for 5 days and then examined if immunoglobulin production was suppressed by the preincubation. As shown in Fig. 5(a), preincubation of monocytes with GT1b reduced IgG, IgM and IgA production by 50·3%, 51·7% and 48·7%, respectively, compared with controls. This suggests that the inhibitory effects of GT1b on immunoglobulin production may be manifested by the GT1b molecules incorporated into monocytes. Production of IL-6 and IL-10 by GT1b-preincubated monocytes, as examined in parallel, was also reduced by 59·8% and 59·2%, respectively, compared with controls (Fig. 5b). It is thus suggested that GT1b may indirectly inhibit the immunoglobulin production of B cells by reducing the paracrine IL-6 and IL-10 production of monocytes in whole PBMC.
DISCUSSION
This study demonstrated that GT1b inhibited spontaneous IgG, IgM and IgA production by human PBMC. GT1b did not act directly on B cells but decreased IL-6 and IL-10 production of monocytes, and thus seemed to indirectly inhibit immunoglobulin production of B cells in whole PBMC. Previous studies have also reported that GT1b did not directly affect immunoglobulin production of human plasma cells, lymphoblastoid B-cell lines or tonsillar small resting B cells.6–8 Other gangliosides have also been shown to regulate immunoglobulin production of B cells, indirectly, by modulating the cytokine production of accessory cells: the stimulatory effect of GQ1b was mediated by promoting IL-6 and IL-10 production in T cells.10
Both IL-6 and IL-10 are known to differentiate B cells into immunoglobulin-secreting cells.18,19 We also previously revealed that exogenous IL-6 and IL-10 additively enhanced IgG, IgM and IgA production in B cells through autocrine and paracrine mechanisms. Our present results suggest that GT1b may not influence the autocrine loops of IL-6 and IL-10 but may suppress the paracrine cytokine production of monocytes. IL-10 also enhances the proliferation of B cells.19 However, GT1b did not inhibit the proliferation of PBMC, including B cells (Fig. 1), although it did reduce the level of IL-10 in PBMC (Table 2). Some reasons are proposed for this result; B cells are numerically a small population, 10% of PBMC, and thus the reduction of B-cell proliferation by decrease of IL-10 may easily be obscured in whole PBMC. Alternatively, GT1b-induced IL-10 decrease in PBMC may be too small to reduce the proliferation of B cells although it did reduce their production of immunoglobulin.
It is reported that exogenous gangliosides, including GT1b, are incorporated into the plasma membranes of various cells14 and modulate transmembrane signal transduction.20 In this study, GT1b inhibited only IL-6 and IL-10 production, and not that of other cytokines, from monocytes. This indicates that GT1b may block certain signalling pathways that mainly act in IL-6 and IL-10 production and are not essential to the production of other cytokines. The cyclic AMP (cAMP)-dependent signalling pathway is involved in IL-6 and IL-10 production of human monocytes,21,22 and thus may be related to the inhibitory effects of GT1b. It is reported that GT1b down-regulates the cAMP-dependent protein kinase in neuronal cells, directly by inhibiting this enzyme activity, and indirectly by decreasing the intracellular level of cAMP through the activation of cyclic nucleotide phosphodiesterase.23 GT1b may inhibit the cAMP-dependent signalling pathway similarly in monocytes and thus reduce their production of IL-6 and IL-10. Another possible mechanism may be the inhibition of the Ca2+/calmodulin-dependent pathway; GT1b inhibits various Ca2+/calmodulin-activated enzymes, such as Ca2+/calmodulin-dependent protein kinase II, by binding to Ca2+/calmodulin complexes and/or to the catalytic domains of the enzymes.24 As it has been reported that a calmodulin antagonist suppresses IL-6 and IL-10 production from human monocytes,25,26 GT1b may also suppress a certain Ca2+/calmodulin-activated enzyme involved in the production of these cytokines.
It is unknown why GT1b inhibits IL-6 and IL-10 production only in monocytes, and not in T or B cells. Possibly in monocytes, putative GT1b target signalling molecules, as described above, may be associated with plasma membranes in a greater number, and thus be more susceptible to GT1b effects, compared with other cell types. Alternatively, signalling systems regulating IL-6 and IL-10 production may vary with cell types; in T and B cells, GT1b target signalling molecules may not be necessary for the production of these cytokines.
Our results in vitro suggest that GT1b may also in vivo inhibit immunoglobulin production in humans. The human serum GT1b level is 0·4–0·5 μm,27 which is higher than the threshold for GT1b effects in vitro (0·1 μm) although lower than its optimal dose (10 μm). Thus, GT1b may systemically down-regulate immunoglobulin production, although its contribution may be weak. Furthermore, GT1b is enriched in the brain: its concentration in the human occipital lobe is 103 μmol/kg wet tissue weight.28 This indicates that monocytes in the brain may be at least locally exposed to GT1b at high concentrations, which would block IL-6 and IL-10 production, and that GT1b may act as an inhibitor for intrathecal immunoglobulin synthesis. The inhibitory effect of GT1b may, however, be compensated for with other gangliosides, such as GQ1b or GD1a, which were shown to enhance in vitro PBMC immunoglobulin production in our previous study.10 Thus, intrathecal immunoglobulin synthesis may be totally balanced by these various gangliosides' effects. Although gangliosides have only been tested for the treatment of neuropathies,1 the inhibitory effects of GT1b also indicate that this ganglioside can be used as a therapeutic immunosuppressive agent. GT1b may normalize abnormally increased humoral immune responses in some autoimmune diseases, such as systemic lupus erythematosus (SLE).28,29 In patients with SLE, production of IL-6 and IL-10 in monocytes are extremely high, which may induce the abnormal enhancement of immunoglobulin production in these patients.29,30 It is thus indicated that GT1b may contribute to the treatment of SLE patients through the inhibition of IL-6 and IL-10 production. We are currently investigating whether GT1b reduces IL-6 and IL-10 production, and also that of immunoglobulins, in PBMC from SLE patients.
Acknowledgments
This work was supported, in part, by a grant from the Japanese Ministry of Education (10770389). The nomenclature for gangliosides follows the system of Svennerholm.31
REFERENCES
- 1.Rodden FR, Wiegandt H, Bauer BL. Gangliosides: the relevance of current research to neurosurgery. J Neurosurg. 1991;74:606. doi: 10.3171/jns.1991.74.4.0606. [DOI] [PubMed] [Google Scholar]
- 2.Hoon DSB, Irie RF, Cochran AJ. Gangliosides from human melanoma immunomodulate response of T cells to interleukin-2. Cell Immunol. 1988;111:410. doi: 10.1016/0008-8749(88)90104-9. [DOI] [PubMed] [Google Scholar]
- 3.Chiba A, Kusunoki S, Obata H, MacHinami R, Kanazawa I. Serum anti-GQ1b IgG antibody is associated with ophthalmoplegia in Miller Fisher syndrome and Guillain–Barre syndrome: clinical and immunohistochemical studies. Neurology. 1993;43:1911. doi: 10.1212/wnl.43.10.1911. [DOI] [PubMed] [Google Scholar]
- 4.Sela BA. Lymphocyte transformation induced by autologous splenocytes incorporated with the tetrasialoganglioside GQ1b. Eur J Immunol. 1981;11:347. doi: 10.1002/eji.1830110417. [DOI] [PubMed] [Google Scholar]
- 5.Ryan JL, Shinitzky M. Possible role for glycosphingolipids in the control of immune responses. Eur J Immunol. 1979;9:171. doi: 10.1002/eji.1830090215. [DOI] [PubMed] [Google Scholar]
- 6.Kimata H. GM1, a ganglioside that specifically enhances immunoglobulin production and proliferation in human plasma cells. Eur J Immunol. 1994;24:2910. doi: 10.1002/eji.1830241149. [DOI] [PubMed] [Google Scholar]
- 7.Kimata H, Yoshida A. Differential effects of gangliosides on Ig production and proliferation by human B cells. Blood. 1994;84:1193. [PubMed] [Google Scholar]
- 8.Kimata H. Differential effects of gangliosides on human IgE and IgG4 production. Eur J Immunol. 1995;25:302. doi: 10.1002/eji.1830250151. [DOI] [PubMed] [Google Scholar]
- 9.Esselman WJ, Miller HC. Modulation of B cell responses by glycolipid release from antigen-stimulated T cells. J Immunol. 1977;119:1994. [PubMed] [Google Scholar]
- 10.Kanda N, Tamaki K. Ganglioside GQ1b enhances Ig production by human PBMCs. J Allergy Clin Immunol. 1998;102:813. doi: 10.1016/s0091-6749(98)70022-3. [DOI] [PubMed] [Google Scholar]
- 11.Boyum A. Separation of leukocytes from peripheral blood and bone marrow. Scand J Clin Lab Invest. 1968;21(Suppl 97):77. [PubMed] [Google Scholar]
- 12.Gee AP, Lee C, Sleasman JW, Madden M, Ugelstad J, Barret DJ. T lymphocyte depletion of human peripheral blood and bone marrow using monoclonal antibodies and magnetic microspheres. Bone Marrow Transplant. 1987;2:155. [PubMed] [Google Scholar]
- 13.Farrant J, Bryant AE, Lever AML, Edwards AJ, Knight SC, Webster ADB. Defective low-density cells of dendritic morphology from the blood of patients with common variable hypogammaglobulinaemia: low immunoglobulin production on stimulation of normal B cells. Clin Exp Immunol. 1985;61:189. [PMC free article] [PubMed] [Google Scholar]
- 14.Saqr HE, Pearl DK, Yates AJ. A review and predictive models of ganglioside uptake by biological membranes. J Neurochem. 1993;61:395. doi: 10.1111/j.1471-4159.1993.tb02140.x. [DOI] [PubMed] [Google Scholar]
- 15.Ladisch S, Becker H, Ulsh L. Immunosuppression by human gangliosides: I. relationship of carbohydrate structure to the inhibition of T cell responses. Biochem Biophys Acta. 1992;1125:180. doi: 10.1016/0005-2760(92)90043-u. [DOI] [PubMed] [Google Scholar]
- 16.Facci L, Leon A, Toffano G, Sonnino S, Ghidoni R, Tettamanti G. Promotion of neuritogenesis in mouse neuroblastoma cells by exogenous gangliosides. Relationship between the effect and the cell association of ganglioside GM1. J Neurochem. 1984;42:299. doi: 10.1111/j.1471-4159.1984.tb02678.x. [DOI] [PubMed] [Google Scholar]
- 17.Linker-Israeli M, Quismorio FP, Horwitz DA. CD8+ lymphocytes from patients with systemic lupus erythematosus sustain, rather than suppress, spontaneous polyclonal IgG production and synergize with CD4+ cells to support autoantibody synthesis. Arthritis Rheum. 1990;33:1216. doi: 10.1002/art.1780330823. [DOI] [PubMed] [Google Scholar]
- 18.Muraguchi A, Hirano T, Tang B, et al. The essential role of B cell stimulatory factor 2 (BSF-2/IL-6) for the terminal differentiation of B cells. J Exp Med. 1988;167:332. doi: 10.1084/jem.167.2.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rousset F, Garacia E, Defrance T, et al. Interleukin 10 is a potent growth and differentiation factor for activated human B lymphocytes. Proc Natl Acad Sci USA. 1992;89:1890. doi: 10.1073/pnas.89.5.1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hakomori S. Bifunctional role of glycosphyngolipids. J Biol Chem. 1990;265:18713. [PubMed] [Google Scholar]
- 21.Meisel C, Vogt K, Platzer C, Randow F, Liebenthal C, Volk HD. Differential regulation of monocytic tumor necrosis factor-α and interleukin-10 expression. Eur J Immunol. 1996;26:1580. doi: 10.1002/eji.1830260726. [DOI] [PubMed] [Google Scholar]
- 22.Geng Y, Zhang B, Lotz M. Protein tyrosine kinase activation is required for lipopolysaccharide induction of cytokines in human blood monocytes. J Immunol. 1993;151:6692. [PubMed] [Google Scholar]
- 23.Yates AJ, Walters JD, Wood CL, Johnson DJ. Ganglioside modulation of cyclic AMP-dependent protein kinase and cyclic nucleotide phosphodiesterase in vitro. J Neurochem. 1989;53:162. doi: 10.1111/j.1471-4159.1989.tb07308.x. [DOI] [PubMed] [Google Scholar]
- 24.Higashi H, Yoshida S, Sato K, Yamagata T. Interaction of ganglioside with specific peptide sequences as a mechanism for the modulation of calmodulin-dependent enzymes. J Biochem. 1996;120:66. doi: 10.1093/oxfordjournals.jbchem.a021395. [DOI] [PubMed] [Google Scholar]
- 25.Lopez-Zalbalza MJ, Martinez-Lausin S, Bengoechea-Alonso MT, Lopez-Mortalla N, Gonzalez A, Santiago E. Signaling pathway triggered by a short immunomodulating peptide on human monocytes. Arch Biochem Biophys. 1997;338:136. doi: 10.1006/abbi.1996.9832. [DOI] [PubMed] [Google Scholar]
- 26.Brigino E, Haraguchi S, Koutsonikolis A, et al. Interleukin 10 is induced by recombinant HIV-1 Nef protein involving the calcium/calmodulin-dependent phosphodiesterase signal transduction pathway. Proc Natl Acad Sci USA. 1997;94:3178. doi: 10.1073/pnas.94.7.3178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Senn H-J, Orth M, Fitzke E, Wieland H, Gerok W. Gangliosides in normal human serum. Concentration, pattern and transport by lipoproteins. Eur J Biochem. 1989;181:657. doi: 10.1111/j.1432-1033.1989.tb14773.x. [DOI] [PubMed] [Google Scholar]
- 28.Yoshino H, Maeda Y, King M, et al. Sulfated glucuronyl glycolipids and gangliosides in the optic nerve of humans. Neurology. 1993;43:408. doi: 10.1212/wnl.43.2.408. [DOI] [PubMed] [Google Scholar]
- 29.Hagiwara E, Gourley MF, Lee S, Kinman DM. Disease severity in patients with systemic lupus erythematosus correlates with an increased ratio of interleukin-10: interferon-γ-secreting cells in the peripheral blood. Arthritis Rheum. 1996;39:379. doi: 10.1002/art.1780390305. [DOI] [PubMed] [Google Scholar]
- 30.Llorente L, Richaud-Patin Y, Fior R, et al. In vivo production of interleukin-10 by non-T cells in rheumatoid arthritis, Sjogren's syndrome, and systemic lupus erythematosus: a potential mechanism of B lymphocyte hyperactitivy and autoimmunity. Arthritis Rheum. 1994;37:305. doi: 10.1002/art.1780371114. [DOI] [PubMed] [Google Scholar]
- 31.Svennerholm L. Chromatographic separation of human brain gangliosides. J Neurochem. 1963;10:613. doi: 10.1111/j.1471-4159.1963.tb08933.x. [DOI] [PubMed] [Google Scholar]