Abstract
A eukaryotic plasmid DNA carrying the AACGTT CpG motif in its ampR gene is a ‘danger’ signal for mice and caused an increase in the specific antibody titres of fish and mice after immunization with β-galactosidase (β-gal). A second pUC-based plasmid, which is inactive in mice and contains the GACGTC CpG motif in its cytomegalovirus (CMV) promoter, had no effect on antibody responses to β-gal in either fish or mice. A synthetic oligonucleotide, which contains the GACGTT motif, potentiated antibody responses to co-administered β-gal protein in mice, but not in fish. This is early evidence that lower and higher vertebrates recognize different unmethylated CpG motifs as ‘danger’ signals. In addition, plasmid DNA expressing mouse granulocyte–macrophage colony-stimulating factor (GM-CSF) had a marked effect on cytotoxic T-cell-like activity in fish by reducing the average number of myofibres that expressed β-gal, 28 days after co-injection with plasmid DNA expressing β-gal. Although the mechanism by which the mouse GM-CSF exerted its biological effects in fish is unknown, this finding might have important implications for fish vaccination, particularly when cytotoxic T cells may play a critical role.
Plasmid DNAs encoding protective antigens of pathogens have shown promise as vaccines for the control of infectious diseases. Plasmid DNA, apart from encoding the antigen, has pronounced immunostimulatory properties (adjuvant activity) that have been attributed to specific single-stranded oligonucleotide sequences containing unmethylated CpG motifs.1 These CpG motifs have been shown to exert a mitogenic effect on B cells,2 and directly activate monocytes, macrophages, and dendritic cells leading to secretion of cytokines that favour the development of murine T helper 1 (Th1) effector cells.3,4 DNA from bacteria, insects and nematodes is immunostimulatory, whereas DNA from mammals and lower vertebrates, e.g. fish is not.5 Thus, bacterial DNA, but not eukaryotic DNA, prompts the vertebrate innate immune system to sense ‘danger’ by employing pattern recognition receptors with broad reactivity,6 as opposed to the antigen-specific receptors used by the adaptive immune system.
Fish seroconvert more readily than mice following intramuscular injection of plasmid DNA expressing β-galactosidase (β-gal).7 In this study we asked whether this increased immunogenicity of plasmid DNA in fish was because of their increased responsiveness to immunostimulatory CpG. To test this, a synthetic oligodeoxynucleotide (ODN) TCCATGACGTTCCTGACGTT (termed 1826) that increases murine antibody responses to proteins,8 was compared with an ODN in which the two CG in 1826 were changed to GG (termed GpG ODN), for their adjuvanticity to co-injected β-gal protein in goldfish and mice. A second CpG system, in which the entire pUC-based plasmid containing the CpG-ampR gene9 was compared with another pUC-based plasmid containing the non-CpG-kanR gene9 in fish.
Immune responses to DNA vaccines can be modulated by coinjecting plasmid DNA expressing various cytokines that play a critical regulatory role in immunity. GM-CSF in particular has been found to exert an adjuvant effect for antibody and cellular responses to DNA vaccines.10 In addition, a cytokine akin to GM-CSF has been described in fish.11 Therefore, we tested the adjuvanticity of plasmid DNA, expressing mouse GM-CSF on the immune responses to co-administered plasmid DNA expressing β-gal (lacZ) in mice and in fish.
Each group of six female BALB/c mice were injected into the middle of each anterior tibial muscle with one of following injections in 50 μl endotoxin-free saline/mouse by a standard method:7 (i) 10 μg/mouse recombinant β-gal (rβ-gal) protein (Sigma, Poole, UK) with 50 μg/mouse CpG ODN 1826 (purchased from Genosys Biotechnologies, Pampisford, UK) or 50 μg/mouse GpG ODN (purchased from Genosys Biotechnologies); (ii) 50 μg of plasmid DNA expressing the β-gal reporter gene (pcDNA 3·1/His/lacZ, Invitrogen, Leek, the Netherlands) (lacZ) with 50 μg of plasmid DNA expressing the mouse GM-CSF (pcMVi-mGM-CSF, a generous gift from Professor Stephen Johnston, University of Texas, Southminster Medical Centre, Dallas, TX) or with 50 μg of empty pcDNA3 (Invitrogen). Serum samples were collected 42 days after priming and stored at −20° until use.
Each group of 10 goldfish (Carassius auratus L.) were injected intramuscularly under anaesthesia into the epaxial muscle by a standard method7 with the following formulations; 50 μg lacZ +50 μg pcDNA3, 50 μg lacZ +50 μg plasmid expressing mouse GM-CSF, 10 μg rβ-gal+50 μg lacZ, 10 μg rβ-gal+50 μg empty pcDNA3, 10 μg rβ-gal+50 μg CpG ODN 1826, 10 μg rβ-gal+50 μg GpG ODN, 10 μg rβ-gal+50 μg empty VR1223 plasmid containing the kanR gene (donated by Vical, San Diego, CA, and emptied by removal of its luciferase gene), and finally 10 μg rβ-gal. Serum was collected 4 weeks after injection.7
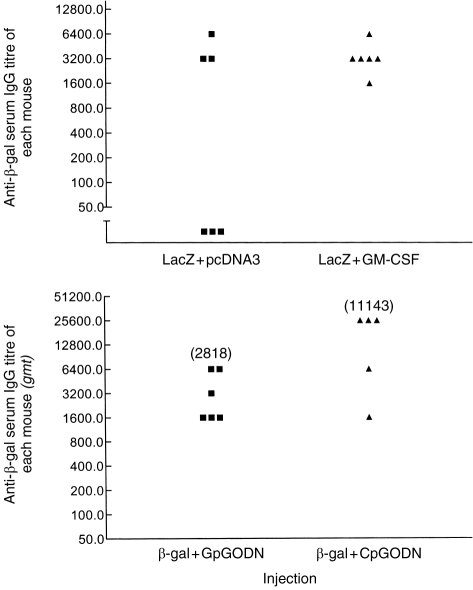
Mouse serum anti-β-gal immunoglobulin G (IgG) antibody responses, measured by enzyme-linked immunosorbent assay (ELISA) as previously described,7 were significantly increased (P < 0·05) in mice immunized with r-β-gal protein and CpG ODN 1826 when compared with mice immunized with the protein and the GpG ODN (Fig. 1). Similarly, plasmid DNA expressing mouse GM-CSF was shown to increase the number of responding mice to β-gal expressed by the co-administered lacZ plasmid (Fig. 1).
Figure 1.
Serum IgG antibody responses in BALB/c mice 42 days after a single injection by the intramuscular route with these injections: (a) lacZ +pcDNA3 or lacZ with plasmid DNA expressing mouse GM-CSF; (b) rβ-gal+GpG ODN or rβ-gal with the 1826 CpG motif. Antibody titres to β-gal were measured by ELISA as previously described.7 The titre was the last dilution to give a binding twice the conjugate control, i.e. > 0·228.
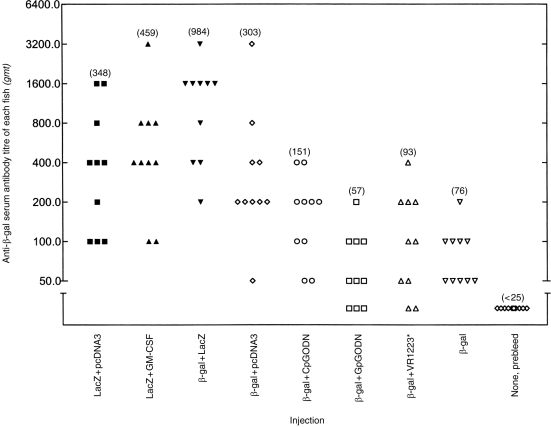
Coinjection of murine CpG ODN 1826 with rβ-gal in goldfish caused a small upward twofold shift in the scatter of serum anti-β-gal antibody titres [measured by ELISA as previously described7] when compared with the control groups immunized with the protein alone, or the protein with the GpG ODN (Fig. 2). The observed increase was not significant (P > 0·05). However, co-injection of r-β-gal protein with empty pcDNA3 or lacZ, both containing the CpG-ampR gene, increased the anti-β-gal antibody response by fourfold (P < 0·05), and 13-fold (P < 0·0001), respectively (Fig. 2). The empty VR1223 plasmid containing the non-CpG-kanR gene did not have any effect on the anti-β-gal antibody responses (Fig. 2).
Figure 2.
Serum antibody titres to β-gal 28 days after intramuscular injection of fish with different formulations. Antibody titres to β-gal were measured by ELISA as previously been described.7 The titre was the last dilution to give a binding twice the conjugate control, i.e. > 0·336.15 *VR1223 was empty (see text). Values in parenthesis represent geometric mean titres.
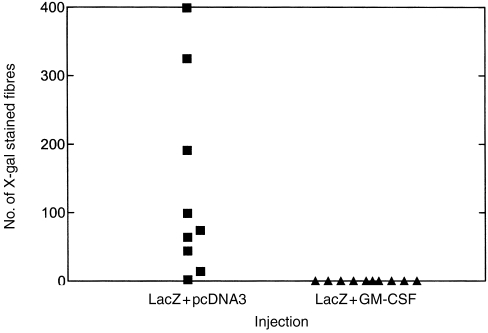
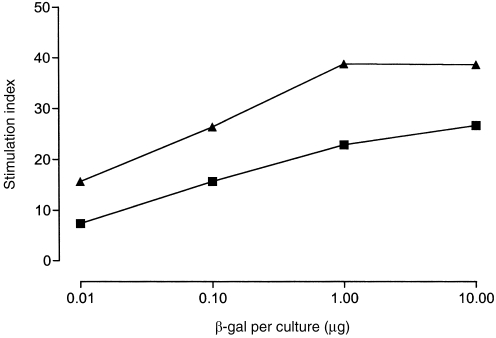
Plasmid DNA-expressing mouse GM-CSF coinjected with lacZ in fish made no appreciable difference to the serum anti-β-gal antibody response to lacZ (Fig. 2). However, the number of β-gal-expressing muscle fibres was dramatically reduced 28 days after co-injection with lacZ (Fig. 3). In addition, GM-CSF increased the proliferative T-cell responses of mouse derived splenocytes after in vitro restimulation with various concentrations of rβ-gal (Fig. 4). In a separate control group where pcDNA3 was injected alone, the stimulation indices (SIs) of splenocytes after in vitro restimulation with β-gal were less than 2 (data not shown).
Figure 3.
Number of β-gal positive muscle fibres 28 days after intramuscular injection of goldfish with lacZ +pcDNA3or lacZ with plasmid DNA expressing mouse GM-CSF. Serial 10 μm transverse sections were cut in a cryostat at 1-mm intervals over the site of injection. The total number of X-gal-stained myofibres was determined microscopically from six sections per fish at regular intervals across the tissue block between gills and dorsal fin.15
Figure 4.
Proliferative T-cell responses of splenocytes from groups of mice immunized (on days 0 and 56) by the intramuscular route with lacZ +pcDNA3 (▪) or with lacZ and plasmid DNA-expressing mouse GM-CSF (▴), 70 days after priming by standard technique.17 Results were expressed as stimulation indices (SI) of the mean counts per minute (c.p.m.) from triplicate cultures in the presence of β-gal protein divided by mean c.p.m. of triplicate cultures with medium only. The counts per minute for triplicate unstimulated cultures averaged 777 (▪) or 888 (▴).
Immunostimulatory CpG motifs are one of the main features of DNA vaccines.12 The finding in this study that ODN 1826 CpG motif increases antibody responses to β-gal in mice, but not in fish, was unexpected and implies that fish are not sensitive to the same immunostimulatory CpG motifs as mammals. Fish do, however, respond to some CpG motifs that are immunostimulatory in mammals because pcDNA3, which contains the ampR gene with two repeats of 5′-AACGTT-3′,12 potentiated anti-β-gal antibody responses in fish as well as mice. In contrast, co-administration of the non-coding kanR-based vector with β-gal, induced no higher level of antibody response than β-gal alone. This finding is in agreement and extends previous observations on the lack of immunostimulation by the non-coding kanR-pUC-based vector in mice.9 The effect of pcDNA3 in fish could not be explained by the CpG motif GACGTC, which is present in its CMV promoter, because the same promoter is in the empty VR1223 which is inactive in fish. Thus, it appears that the switch from GACGTC as in CMV to GACGTT as in ODN 1826, enables CpG motifs to stimulate murine immune responses. A further switch to AACGTT as in ampR may be crucial for immunostimulation in fish. pcDNA3 obviously contains other CpG in addition to AACGTT, and so further work is required to identify new CpG motifs with specific fish immunostimulatory properties. The findings that CpG ‘danger’ signals have evolved during phylogeny is of great significance with regard to vaccine and adjuvant design, because CpG motifs that are optimal for mice may not be optimal for fish, chickens or man.
It is also interesting to note that co-injection of rβ-gal with lacZ in fish resulted in a very significant enhancement of antibody responses compared with rβ-gal alone. This may be as a result of long-term antigen production by the lacZ insert and by non-specific immunostimulation by CpG in its vector DNA.
Murine GM-CSF enhanced both antibody and proliferative responses in mice, confirming earlier observations on its adjuvanticity.10,13 However, when coinjected with lacZ intramuscularly in fish, it did have a marked effect on the rate of disappearance of muscle fibres, which is an indirect measure of T-cell cytotoxicity.14,15 This cross-species activity seen in fish was rather unexpected because mouse GM-CSF does not activate human cells16 and did not induce an increased antibody response in the same fish. Studies in mice have shown that the adjuvanticity of plasmid DNA-expressing mouse GM-CSF is exerted by the cytokine molecule itself, rather than by CpG motifs present in the GM-CSF plasmid.13 However, the GM-CSF plasmid might have immunostimulatory sequences specific for fish, or goldfish may have a receptor for mouse GM-CSF which humans lack. GM-CSF has been shown to increase the frequency of CD8+ T-cells in mice,13 and the observation that this cytokine increased the rate of disappearance of transfected muscle fibers, suggest it may have a similar function in fish. The mechanism by which the plasmid-expressing mouse GM-CSF exerted its biological effects in fish is unknown. However, this finding might have important implications for fish vaccination, particularly when CD8+ T cells play a critical role in controlling viral infections of mammals.
In conclusion, in this study we presented the first evidence to suggest that: (i) CpG motifs are phyla-specific, and (ii) a mouse cytokine, such as GM-CSF, can modulate cell-mediated immune responses to DNA vaccines in fish.
Acknowledgments
This work was in part funded by Wellcome Trust. Professor A. G. Ambali has a visiting Wellcome Trust Fellowship, and V. L. Butler was supported by a Wellcome Trust vacation scholarship.
REFERENCES
- 1.Tokunaga T, Yamamoto H, Shimada S, et al. Antitumor activity of deoxyribonucleic acid fraction from mycobacterium bovis BCG. I. Isolation, physicochemical characterization and antitumor activity. J Natl Cancer Inst. 1984;72:955. [PubMed] [Google Scholar]
- 2.Krieg AK, Yi AK, Matson S, et al. CpG motifs in bacterial DNA trigger direct B-cell activation. Nature. 1995;374:546. doi: 10.1038/374546a0. [DOI] [PubMed] [Google Scholar]
- 3.Klinman D, Yi AK, Beaucage SL, Conover J, Krieg AM. CpG motifs expressed by bacterial DNA rapidly induce lymphocytes to secrete IL-6, IL-12 and INF. Proc Natl Acad Sci USA. 1996;93:2879. doi: 10.1073/pnas.93.7.2879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chu RS, Targoni OS, Krieg AM, Lehmann PV, Harding CV. CpG oligodeoxynucleotides act as adjuvants that switch on T helper 1 (Th1) immunity. J Exp Med. 1997;186:1623. doi: 10.1084/jem.186.10.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sun S, Kishimoto H, Sprent J. DNA as an adjuvant: capacity of insect DNA and synthetic oligodeoxynucleotides to augment T-cell responses to specific antigen. J Exp Med. 1998;187:1145. doi: 10.1084/jem.187.7.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Krieg AM. An innate immune defence mechanism based on the recognition of CpG motifs in microbial DNA. J Lab Clin Med. 1996;128:128. doi: 10.1016/s0022-2143(96)90004-9. [DOI] [PubMed] [Google Scholar]
- 7.Russell PH, Kanellos T, Sylvester ID, Chang KC, Howard CR. Nucleic acid immunization with a reporter gene results in antibody production in goldfish (Carassius auratus L.) Fish Shellfish Immunol. 1998;8:121. [Google Scholar]
- 8.Davis HL, Weeranta R, Waldschmidt TJ, Tygrett L, Schorr J, Krieg AM. CpG DNA is a potent enhancer of specific immunity in mice immunized with recombinant hepatitis B surface antigen. J Immunol. 1998;160:870. [PubMed] [Google Scholar]
- 9.Roman M, Martin-Orozco E, Goodman JS, et al. Immunostimulatory DNA sequences function as T helper-1 promoting adjuvants. Nature Med. 1997;3:849. doi: 10.1038/nm0897-849. [DOI] [PubMed] [Google Scholar]
- 10.Xiang Z, Ertl HC. Manipulation of the immune response to a plasmid-encoded viral antigen by coimmunization with plasmids expressing cytokines. Immunity. 1995;2:129. doi: 10.1016/s1074-7613(95)80001-8. [DOI] [PubMed] [Google Scholar]
- 11.Kodama H, Mukamoto M, Baba T, Mule DM. Macrophage-colony stimulating activity in rainbow trout (Oncorhynchus mykiss) serum. Modulators Fish Immune Responses. 1994;1:59. [Google Scholar]
- 12.Sato Y, Roman M, Tighe H, et al. Immunostimulatory DNA sequences necessary for effective intradermal gene immunization. Science. 1996;273:352. doi: 10.1126/science.273.5273.352. [DOI] [PubMed] [Google Scholar]
- 13.Weiss WR, Ishii KJ, Hedstrom RC, et al. A plasmid encoding murine granulocyte-macrophage colony stimulating factor increases protection conferred by malaria DNA vaccine. J Immunol. 1998;161:2325. [PubMed] [Google Scholar]
- 14.Davis HL, Brazolot Millan CL, Watkins SC. Immune-mediated destruction of transfected muscle fibres after direct gene transfer with antigen-expressing plasmid DNA. Gene Ther. 1997;4:181. doi: 10.1038/sj.gt.3300380. [DOI] [PubMed] [Google Scholar]
- 15.Kanellos T, Sylvester ID, Ambali AG, Howard CR, Russell PH. The safety and longevity of DNA vaccines for fish. Immunology. 1999;96:307. doi: 10.1046/j.1365-2567.1999.00688.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hamblin AS. Cytokines and Cytokine Receptors. New York: Oxford University Press Inc; 1993. p. 35. [Google Scholar]
- 17.Partidos CD, Obeid OE, Steward MW. Antibody responses to non-immunogenic synthetic peptides induced by co-immunization with immunogenic peptides. Immunology. 1992;77:262. [PMC free article] [PubMed] [Google Scholar]