Abstract
A recombinant bacillus Calmette–Guérin (BCG) vaccine has been developed, which constitutively secretes interleukin (IL)-2. Groups of deer were immunized with either normal BCG (Pasteur 1173 P2 strain) or recombinant BCG (rBCG/IL-2) and their immune responses were monitored over 3 months. Animals gained weight over this period and showed no signs of adverse reactions to either vaccine. Lymphocyte transformation responses did not differ significantly between the two groups. No antibody that was specific for BCG was detected in any animal. Intradermal skin-test responses to BCG antigens showed that the rBCG/IL-2 induced a smaller delayed-type hypersensitivity response than the normal BCG. Cytokine transcription was determined by reverse transcription–polymerase chain reaction (RT–PCR). While IL-2 and interferon-γ (IFN-γ) levels did not differ significantly between the two groups, the level of IL-4 was found to be lower in the group given rBCG/IL-2. This resulted in a strong interferon-γ:IL-4 ratio, suggesting a skewing of the immune response towards a Type 1 response. The rate at which the vaccine was eliminated from the host was the same regardless of whether BCG or rBCG was used. At autopsy (3 months after vaccination) 99·99% of the organisms had been eliminated. The small number of organisms isolated from the draining lymph node of animals given rBCG/IL-2 were grown in antibiotic-containing media. They were shown to still contain the shuttle plasmid and to secrete biologically active IL-2, indicating that the plasmid was stably maintained despite the host's immune response and in the absence of antibiotic selection.
INTRODUCTION
Vaccination continues to represent the most efficient and cost-effective method of eradicating a wide range of diseases from humans. The members of the Mycobacterium tuberculosis complex, M. tuberculosis, M. bovis and M. africanum cause tuberculosis (TB). TB continues to be a major cause of morbidity and mortality in humans despite the advent, in 1948, of a vaccine campaign based on bacillus Calmette–Guérin (BCG). A number of countries also have problems with TB in their domesticated and feral animals.1 While BCG is acknowledged as an extremely safe and thoroughly tested vaccine, doubts as to its efficacy still remain.2 Results from efficacy trials have varied greatly and while BCG has been shown to offer up to 80% protection against TB, several trials have failed to show any protective effects. A number of hypotheses have been put forward to explain this variability, including genetic effects, presence of endogenous, cross-reacting environmental mycobacteria and the immuonocompetence of the host.3,4 It is likely that all these factors play some part in vaccine failure but whatever the cause it would seem that they have a common immunological impact. It has been well characterized that a strongly dominant Type 1 immune response is required for the containment and eradication of virulent tubercle bacilli by macrophage.5 The development of a Type 1 response can be adversely affected by a number of factors including malnutrition,6 stress7 and concurrent infection with human immunodeficiency virus (HIV).8,9 We have recently shown that immunosuppressive episodes at the time of vaccination can significantly reduce the protective efficacy of BCG.10
It would appear therefore that, unlike many of the more successful vaccines which rely on the induction of antibody (Type 2 responses) as the protective agent, a TB vaccine will require a high quality, Type 1, cell-mediated immune response to be effective. It is these responses that are most susceptible to immunosuppression.7 To ensure the efficacy of BCG, a new-generation vaccine will be needed that is capable of overcoming minor immunosuppressive influences. A recombinant BCG vaccine has been produced by O'Donnell et al.,11 which contains a shuttle plasmid that encodes a cytokine gene. This gene is constitutively expressed under the control of a mycobacterial heat-shock protein (hsp 60). Recombinants containing a number of different cytokines have been successfully tested for their ability to improve the immune response of mice to BCG antigens.12 We have used O'Donnell's construct to express cervine IL-2. Here we report an in vivo study using a large, naturally susceptible, animal model of tuberculosis13 to determine the influence of the vaccine on the immune response, its safety and its stability in vivo.
MATERIALS AND METHODS
Animals and vaccination
TB free, outbred red deer (Cervus elaphus) were used. Two groups of six deer were used. Animals in the first group were immunized with 2×106 colony-forming units (c.f.u.) of live BCG Pasteur strain 1173 P2 (provided by Dr M. Gheorghiu, Pasteur Institute, Paris) and animals in the second group were immunized with 2×106 c.f.u. of recombinant BCG (rBCG; Pasteur strain 1173 P2 containing the shuttle plasmid pRBD411) secreting interleukin (IL)-2. Blood samples were taken at vaccination and afterwards at 2, 4, 8, 12 and 14 (autopsy) weeks. A third group (n = 10) of ‘in-contact animals’ was monitored regularly for immune reactivity to BCG antigens. At no stage did these animals display in vitro lymphocyte reactivity consistent with sensitization to BCG.
Cell preparation and culture
Peripheral blood mononuclear cells (PBMC) were purified from whole blood on a buoyancy layer of Ficoll-Conray. The cells were washed in phosphate-buffered saline (PBS) and the pellet was resuspended, to give a concentration of 2·5×106 cells/ml, in RPMI-1640 (Sigma, St Louis, MO) supplemented with 10% normal deer serum, 4 mm l-glutamine (Sigma) and 5·5×10−6 m 2-mercaptoethanol (Gibco BRL, Auckland, NZ). Lymphocyte transformation assays were performed as previously described.14 Briefly, 100-μl samples of cells were aliquoted into 96-well tissue culture plates (Falcon, NJ). Fifty microlitres of media (negative control), concanavalin A (Con A) or M. bovis-derived purified-protein derivative (PPD) were added in triplicate to cells. The cells were then cultured for 4 days before being pulsed with 50 μl of tritiated thymidine (10 μCi/ml; Amersham, Little Chalfont, Bucks, UK) per well. After a further 18–20 hr of incubation, the cells were harvested onto glass-fibre filters, dried and counted in scintillation fluid using a liquid scintillation counter (Beta Plate; Wallac, Turku, Finland). Data are expressed as mean c.p.m. of three separate cultures.
Augmentation of antigen responses by IL-2
In experiments to determine whether recombinant IL-2 was able to augment immune responses to suboptimal doses of antigen, blood lymphocytes were separated as described above. They were then exposed to 2 μg/ml PPD rather than to 20 μg/ml. In addition, either 50 μl of media or 50 μl of recombinant IL-2 (final concentration 10 ng/ml) were added to triplicate cultures as controls. Samples were tested from five animals shown to have TB at autopsy and five ‘in contact’, delayed-type hypersensitivity (DTH)-positive but disease-free animals.
RNA extraction and polymerase chain reaction amplification
One-millilitre aliquots of the cells were plated in a 24-well tissue culture plate (Falcon). For each animal, samples were stimulated with 20 μg/ml Con A, 20 μg M. bovis PPD (CSL, Melbourne, Australia) or media alone. The cells were cultured overnight at 37° in a humidified 5% CO2 atmosphere. Total cellular RNA was extracted by a previously described method.15 The RNA was reverse transcribed using SuperscriptTMII reverse transcriptase (Gibco BRL). The reaction mixture (final volume 20 μl) consisted of 50 mm Tris-HCl (pH 8·3), 75 mm KCl, 10 mm dithiothreitol (DTT), 3 mm MgCl2, 0·5 mm dNTPs, 200 U SuperscriptTMII reverse transcriptase, 50 pmol oligo d(T)15 (Boehringer Mannheim, Auckland, NZ), 40 U RNAsin (Boehringer Mannheim) and 5 μl of RNA sample. The reaction was incubated at 37° for 60 min. The polymerase chain reaction (PCR) amplification was carried out in a Techne PHC-3 thermal cycler. Cycling times and temperatures were as follows: 94° for 30 seconds, 56° for 1 min and 72° for 1 min for 28 or 30 cycles (interferon-γ (IFN-γ) and IL-4, respectively) followed by 1 cycle of 94° for 30 seconds, 42° for 1 min with a final extension at 72° for 7 min. Several dilutions of a plasmid containing the gene (IFN-γ, IL-4 or β-actin) were included to ensure the reactions were not saturated. The reaction consisted of cDNA, 10 mm Tris-HCl (pH 8·85), 25 mm KCl, 5 mm (NH4)2SO4, 3 mm MgSO4, 200 μm dNTPs, 20 μm primers and 2·5 U Taq DNA polymerase (Boehringer Mannheim). The IL-4 primers were designed using the published cervine IL-4 gene sequence16 and produced a PCR product of 224 bp. The primer sequences were as follows: sense 5′-AGTGCTGGTCTGCTTAC TGG-3′ and antisense 5′-TGTGGCTCCTGTAGATACGC-3′ (Macromolecular Resources, Fort Collins, CO, USA). The IFN-γ primers were designed from cervine sequence, GenBank accession number L07502, and were: sense 5′-AGGTCATTCAGAGGAG CGTGGAT-3′ and antisense 5′-TGAGGTTAGATTTT GGCGACAGG-3′ (Gibco BRL Life Technologies, NZ) producing a product of 189 bp. The β-actin primers were designed using the partially sequenced cervine β-actin gene and amplified a PCR product of ≈219 bp. The β-actin primers were as follows: sense 5′-CCCAAGGCCAACCGTGAGAAGATG-3′ and antisense 5′-ATCCCGG CCAGCCAAGTCCAG-3′ (Gibco BRL, Life Technologies, NZ). Amplification was carried out for 26 cycles with an annealing temperature of 62°. All sets of primers were designed to span an intron/exon boundary. Included with each set of PCR reactions were negative controls containing either no DNA or genomic DNA.
Imaging and analysis
PCR product was blotted onto Hybond N+ (Amersham) by capillary transfer. A 115-bp fragment of IL-4, a 98-bp fragment of IFN-γ and a 117-bp fragment of β-actin were labelled with 32P by random priming. The filters were hybridized with the probe overnight at 61° and were then washed for 1 minute in 2×SSC (300 mm Nacl2, 30 mm Nacitrate pH 7·0)/0·1% sodium dodecyl sulphate (SDS), for 15 min in 1×SSC/0·1% SDS and twice for 15 min in 0·1×SSC/0·1% SDS.
The filters and digitized images were analysed using the BIO-RAD Model GS-670 Densitometer in combination with Macintosh Molecular AnalystTM Image Analysis software (BIORAD, Hercules, CA). All data was standardized to β-actin.
Intradermal skin test
Midcervical skin testing (MCST) was carried out by injecting 0·1 ml of M. bovis-derived PPD (PPD-B) (1 mg/ml) and M. avium-derived PPD (PPD-A) (0·5 mg/ml) into separate, closely shaved areas (10 cm×10 cm) in the midcervical region of the neck. Results were read after 72 hr using Digimatic Calipers (Mitutoyo Corporation, Tokyo, Japan), at which time the increase in skin thickness was measured at each skin test site, respectively. Preinjection skin thickness (mm) readings were recorded and subtracted from the post-test reading.
Culture of BCG and estimation of numbers (c.f.u.)
BCG was grown in 7H9 broth (Difco Laboratories, Detroit, MI) supplemented with 10% OADC (3·83 g NaCl, 25 g bovine serum albumin (BSA), 15 ml of sodium oleate and 20 ml of 50% glucose in 465 ml of distilled water) and 0·05% Tween-80. Cultures were grown at 37°, without shaking, to an optical density of 0·5. The cultures were then washed three times before the bacteria were enumerated under a phase-contrast microscope and retrospectively by culturing on 7H11 agar (Difco Laboratories). Samples from lymph nodes taken at autopsy were plated onto 7H11 agar containing 20 μg/ml kanamycin. These were grown for 1–3 months before the number of colonies were enumerated.
Bioassay of rBCG/IL-2 recovered from lymph nodes
Lymphoblasts were prepared from cervine mononuclear leucocytes according to a previously described method.17 Briefly, mononuclear leucocytes were incubated with 5 μg/ml Con A (Sigma). After 72 hr of incubation, the lymphoblasts were washed twice in PBS and resuspended at 1×106 cells/ml in RPMI-1640 supplemented with 10% normal deer serum. Kanamycin-resistant BCG colonies grown from the lymph nodes of vaccinated animals were transferred to broth cultures containing kanamycin (20 μg/ml) and grown to stationary phase. The supernatants were then removed and filter sterilized. Fifty microlitres of these supernatants were serially diluted in RPMI-1640, in triplicate, and added to 100 μl of the Con A blasts in a 96-well microtitre plate. A positive control of recombinant bovine IL-2 (Cyanamid, Princeton, NJ) was used. A supernatant derived from a culture of recombinant BCG containing the shuttle plasmid pRBD4 minus the IL-2 gene was used as the negative control. The control culture was grown to the same density as the test culture before assaying. After 40 hr of incubation at 37° in 5% CO2, 50 μl of tritiated thymidine (10 μCi/ml; Amersham) was added to each well and the plate was incubated for a further 6 hr. The cells were then harvested onto glass fibre filters and the radioactive incorporation measured using a beta counter. Results are expressed as the mean c.p.m.±the standard error of each triplicate.
RESULTS
Potentiation of lymphocyte responses to suboptimal doses of IL-2
The ability of exogenous cytokines to potentiate the immune response was determined in a limiting antigen experiment. Lymphocytes from M. bovis-infected deer usually respond optimally, in vitro, to PPD at 20 μg/ml. We therefore investigated whether recombinant IL-2 could potentiate a suboptimal antigen dose (2 μg/ml). The data in Fig. 1 shows that the immune response to PPD of both non-diseased and diseased animals was significantly up-regulated by the use of recombinant IL-2. We therefore decided to use IL-2 as the prototype cytokine to be expressed from the recombinant BCG.
Figure 1.
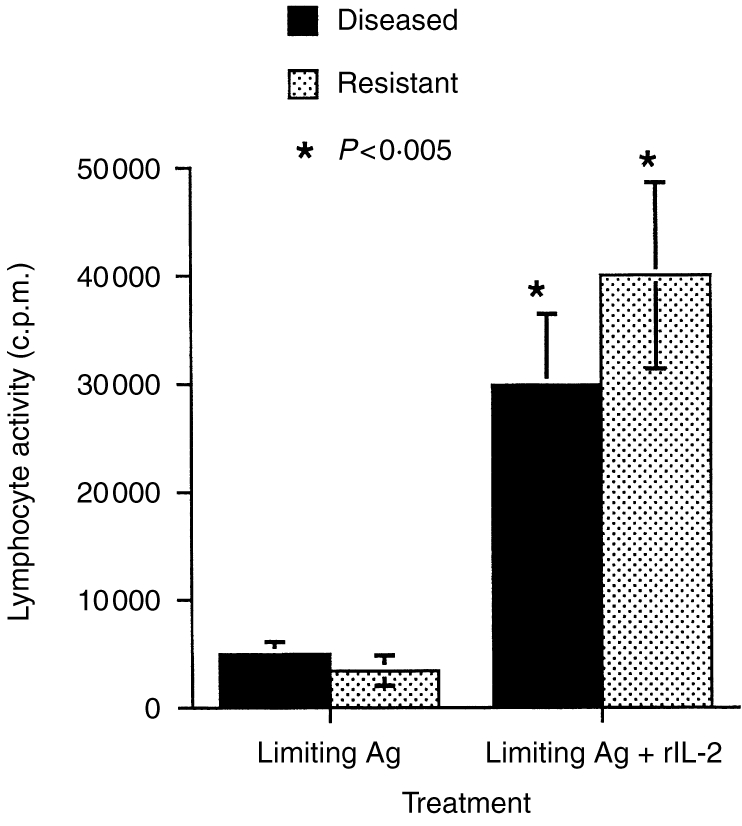
The immunopotentiating effect of recombinant interleukin 2 (rIL-2) on in vitro antigen-specific lymphocyte transformation responses. Peripheral blood lymphocytes from tuberculous (diseased) or non-diseased, skin-test positive, in-contact (resistant) animals were cultured with antigen from Mycobacterium bovis. An optimal concentration (10 μg/ml) of rIL-2 was given to one set of cultures, while media alone was given to the control cultures.
Immune response to BCG and recombinant BCG
To determine the immunoregulatory properties of the rBCG/IL-2 vaccine, we carried out a number of immunoassays after vaccinating groups of animals with either BCG or rBCG/IL-2. Figure 2 shows that the DTH response generated by vaccination with rBCG/IL-2 was less than that for normal BCG. The DTH induced by rBCG/IL-2 seemed to be more antigen specific than that induced by normal BCG. This was suggested by the proportionally larger responsiveness to M. bovis- than M. avium-derived PPD in animals given rBCG/IL-2 (2·7±0·6) compared with normal BCG (1·5±0·25). When lymphocyte activation was measured by lymphocyte transformation, the data showed that the response kinetics to both vaccines were similar (Fig. 3). The peak response appeared to be lower in the rBCG/IL-2 vaccinates but this was not statistically significant. Cytokine transcription in PBMCs after in vitro restimulation was also measured. Figure 4(a) shows that 8 weeks after vaccination, animals vaccinated with BCG were producing large amounts of IL-2, IL-4 and IFN-γ mRNA. In comparison, animals vaccinated with the rBCG were producing similar amounts of IL-2 and IFN-γ but no IL-4. By 14 weeks after vaccination, the levels of IFN-γ and IL-4 had decreased in animals given BCG although IL-2 transcription continued to be high. In animals given rBCG the IL-2 and IFN-γ levels remained high and some IL-4 message was detectable.
Figure 2.
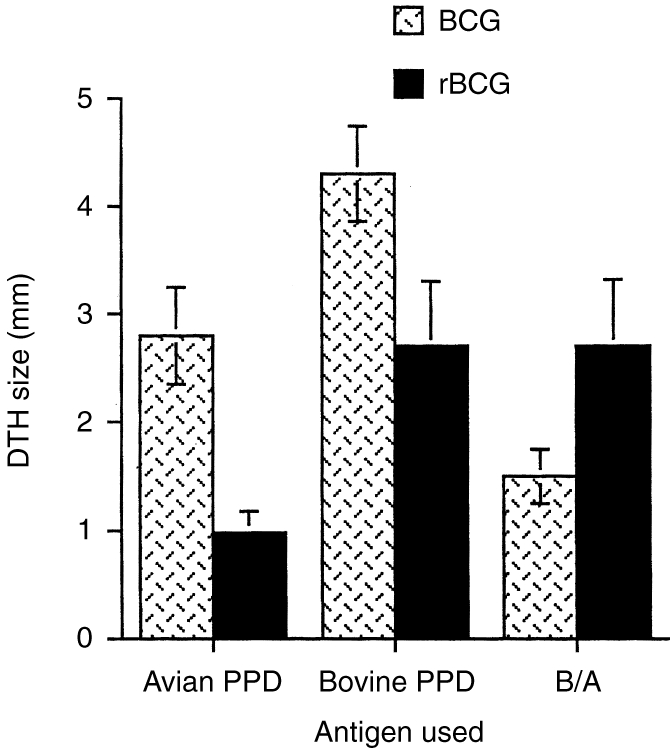
The delayed-type hypersensitivity (DTH) responses to specific and non-specific antigens were measured in animals 3 months after vaccination with either bacillus Calmette–Guérin (BCG) or recombinant BCG secreting interleukin-2(rBCG). DTH responses to intradermal injection of antigens from either Mycobacterium bovis (bovine purified-protein derivative) or M. avium (avian purified-protein derivative) were measured as skin thickening (mm). The data represents the mean±SE of the group (n = 6).
Figure 3.
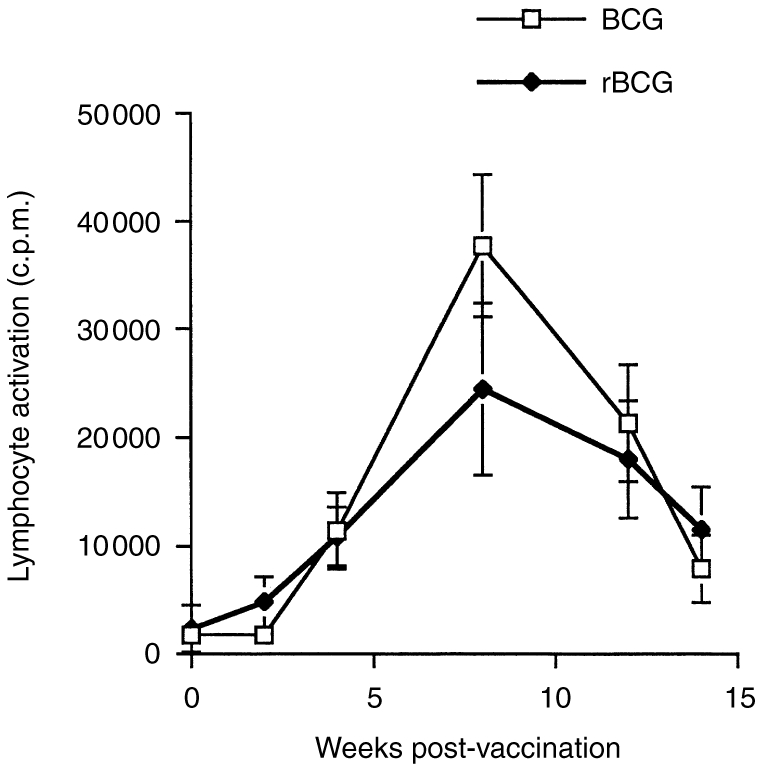
The peripheral blood lymphocyte responses to Mycobacterium bovis (PPD) antigens were measured in animals vaccinated with either bacillus Calmette–Guérin (BCG) or recombinant BCG (rBCG). Blood samples were taken at 2, 4, 8, 12 and 14 weeks. The lymphocytes were assayed for antigen-specific lymphocyte transformation. The data represents the mean±SE of the group (n = 6).
Figure 4.
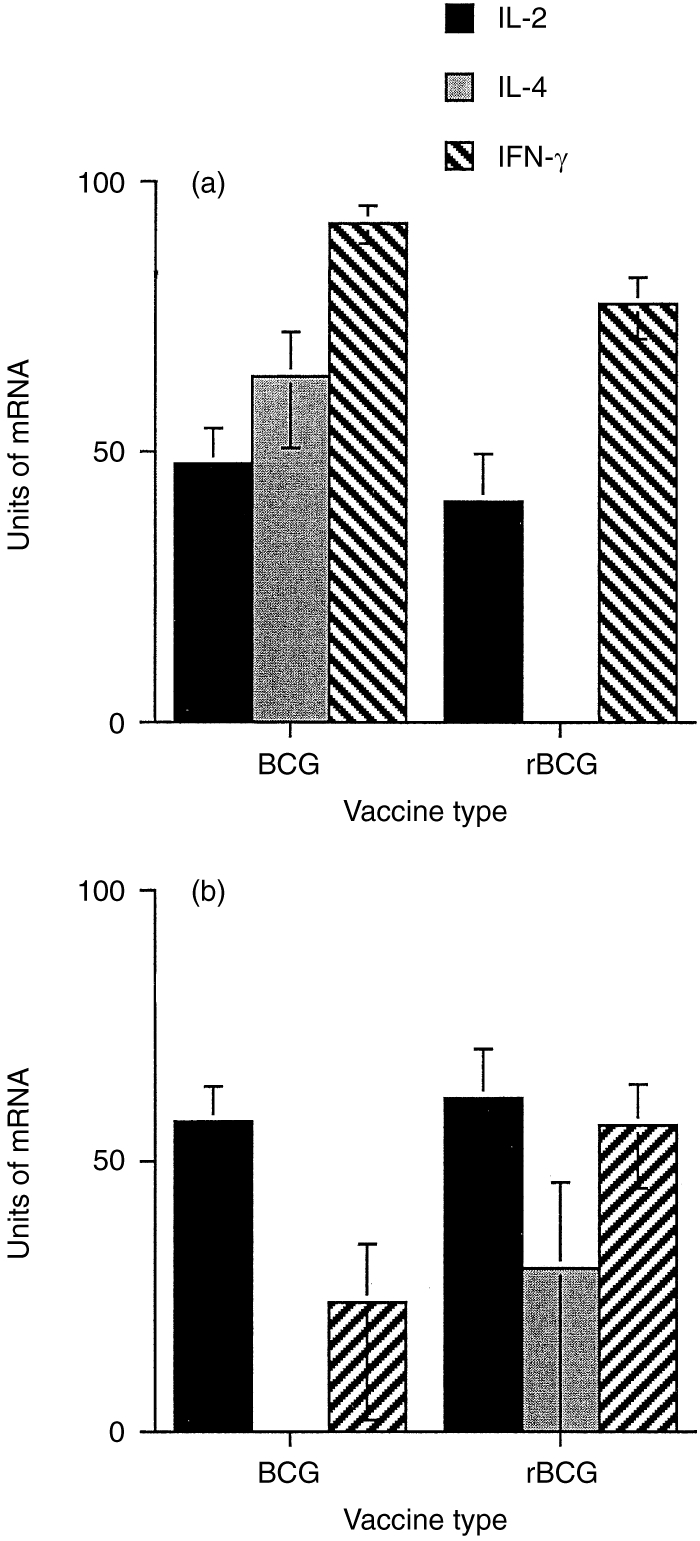
The transcription of interleukin-2 (IL-2), interleukin-4 (IL-4) and interferon-γ (IFN-γ) mRNA was measured after antigen restimulation of lymphocytes from animals vaccinated 8 weeks (a) and 14weeks (b) previously with either bacillus Calmette–Guérin (BCG) or recombinant BCG (rBCG). Units of mRNA were calculated from autoradiographs of probed blots of electrophoresed PCR product. The optical density of the product was then standardized to a control mRNA (β-actin). The data represents the mean±SE of the results from each group (n = 6).
Persistence of vaccine in vivo
To determine whether the rBCG/IL-2 was eliminated more rapidly or persisted for longer in the lymph nodes of animals after vaccination, we cultured a variety of different lymph nodes from animals after autopsy (14 weeks postvaccination). The numbers of culturable BCG in each lymph node was estimated from the c.f.u. (Table 1). The data shows that even although 50% of animals tested in each group continued to harbour culturable BCG 3 months after vaccination, the number of organisms had been reduced by 4 logs of magnitude (i.e. 99·99% of the vaccine dose had been removed). There was no statistically significant difference between the number of culturable organisms obtained from the lymphoid tissues of animals vaccinated with either BCG or rBCG/IL-2. Of some interest was the finding that one animal vaccinated with normal BCG had culturable organisms in lymph nodes of the head and gut. There were no measurable differences between the immune responses of this animal and animals that had either eradicated the vaccine completely or contained the vaccine to the draining lymph node.
Numbers of viable bacillus Calmette–Guérin (BCG) recovered from lymphoid tissues of animals 3 months after vaccination
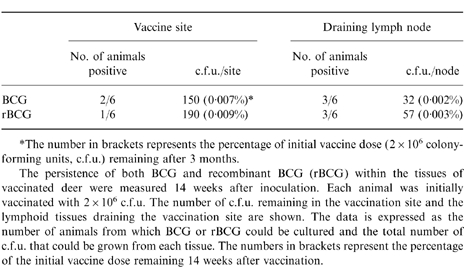
*The number in brackets represents the percentage of initial vaccine dose (2×106 colonyforming units, c.f.u.) remaining after 3 months.
The persistence of both BCG and recombinant BCG (rBCG) within the tissues of vaccinated deer were measured 14 weeks after inoculation. Each animal was initially vaccinated with 2×106 c.f.u. The number of c.f.u. remaining in the vaccination site and the lymphoid tissues draining the vaccination site are shown. The data is expressed as the number of animals from which BCG or rBCG could be cultured and the total number of c.f.u. that could be grown from each tissue. The numbers in brackets represent the percentage of the initial vaccine dose remaining 14 weeks after vaccination.
Stability of the rBCG/IL-2 in vivo
Colonies from animals vaccinated with rBCG/IL-2 were isolated on kanamycin-containing plates and grown in broth culture to stationary phase. The supernatants from these cultures were assayed for T-cell growth factor activity. Figure 5 shows that after 14 weeks in vivo, the rBCG/IL-2 were still capable of expressing biologically active IL-2. The levels detected were equivalent to those produced by organisms used in the original inoculum. In other experiments we have calculated this to be approximately 20–40 ng/ml.
Figure 5.
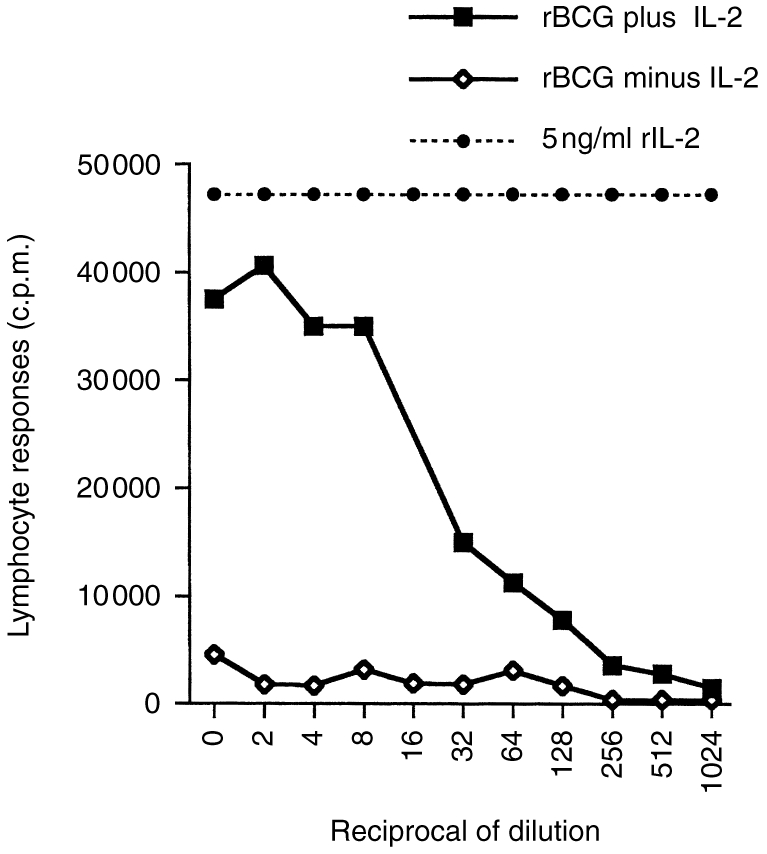
Expression of interleukin-2 (IL-2) was measured from recombinant bacillus Calmette–Guérin (BCG) (rBCG+IL-2) that had been isolated from the lymph nodes of animals vaccinated 14 weeks previously. Levels of IL-2 activity were measured in the culture supernatants of colonies of rBCG grown to OD600 = 1. Supernatants were tested for their ability to induce proliferation in peripheral blood lymphocytes suboptimally stimulated with concanavalin A (Con A blast assay). Doubling dilutions of supernatants from rBCG plus IL-2 were compared to recombinant BCG with the IL-2 gene deleted (BCG minus IL-2) and purified recombinant IL-2 protein (rIL-2).
Animal well-being
Animals were weighed monthly throughout the trial. No animal lost weight and most gained weight despite the trial being conducted during winter. On average, BCG vaccinates gained 2·1 kg over the period of the trial while those given rBCG/IL-2 gained 2·3 kg.
DISCUSSION
We have recently reported that the efficacy of BCG can be improved by giving two or more doses of the live vaccine.10 Despite being given an optimal dose of live BCG, however, a proportion of animals, 0–20%, remain unprotected by the vaccine. We believe that this is the result of a number of host factors that are independent of the vaccine. Nutritional deficiencies,6 prior exposure to certain strains of saprophytic mycobacteria,18 stress7 and genetic factors19 are all known to be capable of adversely influencing the efficacy of BCG. If such factors are responsible for the low efficacy of BCG in some field trials, then any conventional vaccine will suffer the same fate. To optimize the efficacy of an antituberculous vaccine under these conditions it will be necessary to overcome a pre-existing inadequate or inappropriate immune response in some animals using an immunotherapeutic vaccine.
IL-2 is a well-characterized cytokine with wide-ranging effects. Indeed, it is pivotal in the induction of an immune response, enhancing the expansion and activation of lymphocytes, including CD4+, CD8+ and natural killer (NK) cells.20 IL-2 has been shown to reverse unresponsiveness to M. bovis antigens.21 Recombinant IL-2 has also been successfully used as an immunotherapeutic agent in the treatment of Listeria monocytogenes infections.22 If immunosuppression is a factor in the variable efficacy of BCG, we hypothesized that the co-delivery of antigen (BCG) with an immunopotentiating cytokine, such as IL-2, should help the host respond to the vaccine and hence develop a protective immune response. Initial experiments confirmed that exogenous IL-2 was able to up-regulate the in vitro responses of lymphocytes from naturally infected animals to suboptimal doses of BCG antigens. A recombinant BCG has been developed11 that has been engineered to constituitively secrete a variety of cytokines, including cervine IL-2. We were able to show that our recombinant BCG/IL-2 produced low but biologically active levels of IL-2, which up-regulated the proliferative activity of cervine lymphocytes in vitro. The shuttle plasmid could be stably maintained in culture for long periods (months to years).
Prior to testing its protective efficacy, we conducted a contained field trial of this recombinant BCG/IL-2 vaccine to confirm its safety and ability to alter the immune response of a naturally susceptible large animal model of TB. The data suggests that IL-2 secreted by the rBCG does alter the immune response to the BCG components of the vaccine. DTH responses, as measured by intradermal skin tests, are reduced and more antigen specific. This may have implications for disease diagnosis where there is concern that vaccination with BCG will compromise the use of the skin test as a diagnostic tool. In these studies we used a vaccine dose of 2×106 c.f.u. whereas in recent studies10 we have shown that doses as low as 104 c.f.u. are protective if a booster vaccine is used. At this dose the DTH response is negligible with normal BCG and this trend would be accentuated if rBCG/IL-2 were used.
The recombinant vaccine did not significantly improve the ability of peripheral blood lymphocytes to respond to antigen restimulation. This is consistent with data that others have derived in the mouse model using DNA vaccines.23 As none of the animals used showed any signs of immunosuppression, it is unlikely that we would be able to demonstrate an improvement in the quantity of the immune response. We therefore looked at the quality of the immune response. As a strong Type 1 response is considered to be optimal for protection against intracellular pathogens, we compared the transcription of three immunoregulatory cytokines by lymphocytes from animals vaccinated with normal and recombinant BCG. While the levels of IL-2 and IFN-γ were similar, our data suggests that IL-4 is suppressed early in the response to rBCG/IL-2. We have previously proposed that whereas IFN-γ is necessary for the development of a protective immune response, it is by no means sufficient.24 The concomitant production of IL-4 seems to mask the positive influence of IFN-γ and predispose to a disease-related immune response. Thus, the suppression of IL-4 transcription by the rBCG/IL-2 suggests a skewing of the response towards a Type 1 response and therefore an improvement in the quality of the response. Fourteen weeks after vaccination, IL-2 mRNA levels were still high in both groups. While IL-4 transcription was no longer detectable in the BCG group, it started to appear in the group given rBCG/IL-2. This late burst of IL-4 activity at the end of an immune response to BCG has been reported by Orme et al.25 While by 14 weeks the IFN-γ response in the group vaccinated with BCG had waned 23-fold, the IFN-γ response in the rBCG/IL-2 group had dropped only 2·5-fold. Data we have derived in the mouse model (manuscript in preparation) confirms this response, showing that low doses of rBCG/IL-2 stimulate a much stronger IFN-γ response, which persists for longer, than equivalent doses of normal BCG. This has important implications for the efficacy of recombinant vaccine, suggesting that it induces a better quality (Type 1) of response that should enhance its protective efficacy.
It could be argued that the changes in cytokine patterns observed could be caused by differences in persistence of the vaccine within the host. Data derived by O'Donnell, in the mouse, suggests that this is unlikely because the rBCG/IL-2 was eliminated faster than the normal BCG.11 Our data also shows that there is little difference in the rates of vaccine elimination. By 14 weeks, 99·99% of the normal and recombinant BCG had been eliminated with 50% of animals in each group having no detectable vaccine remaining in their lymphoid tissues. How long BCG can persist in the host is a contentious issue but our data suggests that the observed effects are unlikely to be caused by the low level of residual organisms. There was no correlation between the animals that still harboured viable rBCG/IL-2 at 14 weeks and those with high levels of IFN-γ transcription. It is interesting to note from the results of Murray et al. that, despite being removed faster from the host, the immune response generated by the rBCG/IL-2 is superior to that generated by normal BCG.12
The stability of the plasmid inside the BCG is important. Here we show that in the absence of antibiotic selection pressures the plasmid is stably maintained for several months. It is likely that immunocompetent individuals will eliminate the rBCG/IL-2 quickly whereas immunocompromised individuals will achieve this more slowly. It is the latter group therefore that will derive the most benefit from the vaccine as it will persist for longer and express more cytokine. The vaccine will, in a sense, help those who are unable to help themselves.
As suggested above, the rBCG/IL-2 does not secrete large amounts of IL-2 by comparison with other expression systems, such as Escherichia coli. This may be an advantage in that the levels are more physiological and the chances of either local or systemic toxicity are minimized. The production of the cytokine in the microenvironment in which the antigen is being recognized, processed and presented, will maximize its effectiveness. The details of how the secreted IL-2 has its effect when it is supposedly inside a phagocyte, such as a macrophage, is unknown at this time.
While this was only a small pilot study, it shows that the rBCG/IL-2 is a safe vaccine that can improve the quality of the immune response to antigens from tubercle bacilli. By manipulating the dose and type of cytokine expressed by the BCG we will be able to optimize the protective immune response generated. The true test of this vaccine will be in its ability to induce a protective immune response in immunosuppressed animals.
Acknowledgments
This work was supported by the New Zealand Foundation for Research, Science and Technology.
REFERENCES
- 1.Griffin JFT, Buchan GS. Aetiology, pathogenesis and diagnosis of Mycobacterium bovis infection in deer. Vet Microbiol. 1994;40:193. doi: 10.1016/0378-1135(94)90055-8. [DOI] [PubMed] [Google Scholar]
- 2.Comstock GW. Field trials of tuberculosis vaccines: how could we have done them better? Contr Clin Trials. 1994;15:247. doi: 10.1016/0197-2456(94)90042-6. [DOI] [PubMed] [Google Scholar]
- 3.Fine PE. Variation in protection by BCG: implications of and for heterologous immunity. Lancet. 1995;346:1339. doi: 10.1016/s0140-6736(95)92348-9. [DOI] [PubMed] [Google Scholar]
- 4.Griffin JFT, MacKintosh CG, Buchan GS. Animal models of protective immunity in tuberculosis to evaluate candidate vaccines. Trends Microbiol. 1995;3:418. doi: 10.1016/s0966-842x(00)88994-5. [DOI] [PubMed] [Google Scholar]
- 5.Bretscher PA. A strategy to improve the efficacy of vaccination against tuberculosis and leprosy. Immunol Today. 1992;13:342. doi: 10.1016/0167-5699(92)90168-7. [DOI] [PubMed] [Google Scholar]
- 6.McMurray DN, Mintzer CL, Bartow RA, Parr RL. Dietary protein deficiency and Mycobacterium bovis BCG affect interleukin-2 activity in experimental pulmonary tuberculosis. Infect Immun. 1989;57:2606. doi: 10.1128/iai.57.9.2606-2611.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rook GAW, Hernandez-Pando R, Lightman SL. Hormones, peripherally activated prohormones and regulation of the Th1/Th2 balance. Immunol Today. 1994;15:301. doi: 10.1016/0167-5699(94)90075-2. [DOI] [PubMed] [Google Scholar]
- 8.Silveira H, Ordway D, Dockrell H, Jackson M, Ventura F. Cell-mediated immune responses to mycobacterial antigens in patients with pulmonary tuberculosis and HIV infection. Clin Exp Immunol. 1997;110:26. doi: 10.1046/j.1365-2249.1997.5091407.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bloom BR, Murray CJL. Tuberculosis: Commentary on a reemergent killer. Science. 1992;257:1055. doi: 10.1126/science.257.5073.1055. [DOI] [PubMed] [Google Scholar]
- 10.Griffin JFT, MacKintosh CG, Slobbe L, Thomson AJ, Buchan GS. Vaccine protocols to optimise the protective efficacy of BCG. Tubercle. 1999 doi: 10.1054/tuld.1998.0202. in press. [DOI] [PubMed] [Google Scholar]
- 11.O'Donnell MA, Aldovini ADRB, Yang H, Szilvasi A, Young RA, De Wolf WC. Recombinant Mycobacterium bovis BCG secreting functional interleukin-2 enhances gamma interferon production by splenocytes. Infect Immun. 1994;62:2508. doi: 10.1128/iai.62.6.2508-2514.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Murray PJ, Aldovini A, Young RA. Manipulation and potentiation of antimycobacterial immunity using recombinant bacille Calmette–Guerin strains that secrete cytokines. PNAS. 1996;93:934. doi: 10.1073/pnas.93.2.934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Buchan GS, Griffin JFT. Tuberculosis in domesticated deer (Cervus elaphus): a large animal model for human tuberculosis. J Comp Path. 1990;103:11. doi: 10.1016/s0021-9975(08)80131-4. [DOI] [PubMed] [Google Scholar]
- 14.Griffin JFT, Hesketh JB, MacKintosh CG, You-en S, Buchan GS. BCG vaccination in deer: distinctions between delayed type hypersensitivity (DTH) and laboratory parameters of immunity. Immunol Cell Biol. 1993;71:559. doi: 10.1038/icb.1993.62. [DOI] [PubMed] [Google Scholar]
- 15.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate–phenol–chloroform extraction. Anal Biochem. 1987;162:156. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 16.Hook SM, Crawford AM, Chinn DN, Griffin JFT, Buchan GS. Cloning and expression of the cervine Interleukin 4 gene. Scand J Immunol. 1994;40:71. doi: 10.1111/j.1365-3083.1994.tb03435.x. [DOI] [PubMed] [Google Scholar]
- 17.Buchan GS, Grimmett DJ, Griffin JFT. Cervine T-lymphocyte growth factors and their measurement in tuberculosis. Vet Immunol Immunopathol. 1991;29:115. doi: 10.1016/0165-2427(91)90057-j. [DOI] [PubMed] [Google Scholar]
- 18.Hernandez-Pando R, Pavon L, Arriaga K, Orozco H, Madrid-Marina V, Rook G. Pathogenesis of tuberculosis in mice exposed to low and high doses of an environmental mycobacterial saprophyte before infection. Infect Immun. 1997;65:3317. doi: 10.1128/iai.65.8.3317-3327.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Apt AS, Avdienko VG, Nikonenko BV, Kramnik IB, Moroz AM, Skamene E. Distinct H-2 complex control of mortality and immune responses to tuberculosis infection in virgin and BCG-vaccinated mice. Clin Exp Immunol. 1993;94:322. doi: 10.1111/j.1365-2249.1993.tb03451.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smith KA. Interleukin-2, inception, impact and implications. Science. 1988;240:1169. doi: 10.1126/science.3131876. [DOI] [PubMed] [Google Scholar]
- 21.Colizzi V, Malkovsky M. Augmentation of interleukin-2 production and delayed hypersensitivity in mice infected with Mycobacterium bovis and fed a diet supplemented with vitamin A acetate. Infect Immun. 1985;48:581. doi: 10.1128/iai.48.2.581-583.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Haak-Frendscho M, Czuprynski CJ. Use of recombinant interleukin-2 to enhance adoptive transfer of resistance to Listeria monocytogenes infection. Infect Immun. 1992;60:1406. doi: 10.1128/iai.60.4.1406-1414.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lim YS, Kang BY, Kim E, Kim S, Hwang S, Kim TS. Potentiation of antigen-specific, Th1 immune responses by multiple DNA vaccination with an ovalbumin/interferon-γ hybrid construct. Immunology. 1998;94:135. doi: 10.1046/j.1365-2567.1998.00510.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hook S, Griffin F, MacKintosh C, Buchan G. Activation of an interleukin-4 mRNA-producing population of peripheral blood mononuclear cells after infection with Mycobacterium bovis or vaccination with killed, but not live, BCG. Immunology. 1996;88:269. doi: 10.1111/j.1365-2567.1996.tb00014.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Orme I, Roberts A, Griffen J, Abrams J. Cytokine secretion by CD4+ T lymphocytes acquired in response to M. tuberculosis infection. J Immunol. 1993;151:518. [PubMed] [Google Scholar]


