Abstract
Interleukin-4 (IL-4) is the prototypic type 2 immunoregulatory cytokine that can suppress the production of many monocyte and macrophage pro-inflammatory mediators. In this study we investigated the regulation by IL-4 of IL-12 and IL-10 production. While IL-4 suppressed lipopolysaccharide (LPS)-induced IL-12 and IL-10 production by human peripheral blood monocytes, IL-4 suppressed LPS-induced IL-12, but not IL-10, production by synovial fluid mononuclear cells from patients with rheumatoid arthritis. IL-4 also suppressed IL-12, but not IL-10 production, by LPS-stimulated in vitro monocyte-derived macrophages. Similarly, IL-4 cannot suppress LPS-induced tumour necrosis factor-α (TNF-α) production by synovial fluid cells and monocyte-derived macrophages. The failure of IL-4 to regulate IL-10 production is not due to the failure of IL-4 to suppress TNF-α, and vice versa. The data suggest that the IL-4 receptor subunit, γc, is essential for IL-4 regulation of LPS-induced IL-10 production and that a correlation exists between duration of monocyte culture, reduction in γc mRNA in cultured cells and hyporesponsiveness of monocyte-derived macrophages to IL-4 for regulation of LPS-induced IL-10 production. This study highlights the importance of investigating responses to IL-4, as a potential therapeutic anti-inflammatory agent, by cells isolated from inflammatory sites and not by the more easily accessible blood monocytes. This study emphasizes the involvement of signalling from γc in IL-4 regulation of LPS-induced IL-10 production by monocytes and macrophages.
INTRODUCTION
Interleukin-4 (IL-4), the prototypic type 2 immunoregulatory cytokine (reviewed in refs 1 and 2) can affect cells that contribute to the human inflammatory response. In addition to inhibiting phagocytic activity of neutrophils and being a chemotactic factor for eosinophils, IL-4 modulates cytokine production by endothelial cells, monocytes and macrophages.1,2 IL-4 can also reduce inflammation by stimulating the production of monocyte IL-1 receptor antagonist3 and soluble ‘decoy’ IL-1 receptor.4 The regulatory effects of IL-4 on monocyte activity suggest that it may be used as an anti-inflammatory agent. However, IL-4 does not have the same anti-inflammatory properties on synovial fluid cells in vitro as on blood monocytes,5 and a similar change in functional response to IL-4 can be obtained by culturing monocytes in vitro. 6 IL-4 regulation of IL-12 and IL-10 production by activated human monocytes has been previously studied.7,8 However, the effects of IL-4 on their production by human inflammatory cells, namely synovial fluid mononuclear cells (SFMC), the type of cells that must be targeted in therapy, have not.
In this study, we investigated the regulation by IL-4 of IL-12 and IL-10 production by human monocytes versus inflammatory macrophages. IL-12 has been generally characterized as a pro-inflammatory mediator9 because by rapid induction of interferon-γ (IFN-γ) synthesis it activates T helper type 1 (Th1) cells.7 IL-12 stimulates T-cell proliferation10 and induces the synthesis of tumour necrosis factor-α (TNF-α), granulocyte–macrophage colony-stimulating factor, macrophage colony-stimulating factor (M-CSF), IL-3, IL-8 and IL-2 by a variety of immune cells.11 IL-10 shows both positive and negative immunoregulatory functions.12 Originally named ‘cytokine synthesis inhibitory factor’13 due to its ability to suppress IFN-γ production by Th1 cells, IL-10 is produced by monocytes in response to TNF-α14 and IL-12.8 IFN-γ,15 IL-4,8 as well as IL-10 itself,8 suppress monocyte IL-10 production. As an anti-inflammatory agent, IL-10 suppresses TNF-α, IL-1β and IL-6 production, reduces major histocompatibility complex class II expression and induces IL-1 receptor antagonist production by monocytes and macrophages.8,16
In this study of peripheral blood mononuclear cells (PBMC) compared with SFMC from patients with rheumatoid arthritis (RA), we found IL-4 regulation of lipopolysaccharide (LPS) -induced IL-10, but not IL-12 production, differed for the two populations. Similar results were obtained with fresh, compared with cultured, monocytes. To help understand the altered responses to IL-4 at the cellular level, we observed that a functional IL-2 receptor (IL-2R) γ-chain (γc) was required for IL-4 regulation of monocyte and macrophage IL-10 production but not for IL-12 production.
MATERIALS AND METHODS
Antibodies and reagents
Reagents were obtained as gifts as indicated: human recombinant M-CSF (rM-CSF; Dr J. Schreurs, Chiron Corporation, Cetus Oncology Division, Emeryville, CA), human rIL-4 (Dr S. Gillis, formerly of Immunex, Seattle, WA), human rIL-10 (Dr R. de Waal Malefyt, DNAX, Palo Alto, CA) and mouse anti-human TNF-α antibody (2TNF-H22, Professor A. C. Allison, formerly of Syntex, Palo Alto, CA).
Patients
Synovial fluid was drained as part of clinical practice from eight RA patients. When possible, peripheral blood was taken at the same time. All patients were classified as having RA according to the criteria of the American College of Rheumatology. The patients were taking a variety of medications including methotrexate, azathioprine and steroidal and non-steroidal anti-inflammatory drugs.
Isolation of synovial fluid mononuclear cells, monocytes and development of monocyte-derived macrophages
Mononuclear cells from synovial fluid and peripheral blood were isolated on Lymphoprep density gradients (Nycomed, Oslo, Norway).17 Monocytes were isolated by countercurrent centrifugal elutriation from leucocyte-enriched buffy coats kindly provided by the Adelaide Red Cross Blood Bank (South Australia). Monocytes were enriched to >93% and cultured in RPMI-1640 medium (Cytosystems, Castle Hill, Australia) supplemented with 13·3 mm NaHCO3, 2 mm glutamine, 50 μm β-mercaptoethanol, 100 U/ml penicillin, 100 μg/ml streptomycin, and 2 nm 3-(N-morpholino)propane-sulphonic acid with an osmolality of 290 mmol/kg H2O (complete RPMI). Monocyte-derived macrophages (MDMac) were developed by culturing the elutriated monocytes for 7 days at 106/ml in 15–25 ml of complete RPMI supplemented with 10% fetal calf serum (FCS) (heat inactivated for 30 min at 56°) and M-CSF (50 ng/ml) in 40 ml Teflon pots (Savillex, Minnetonka, MN). During isolation and subsequent culture of all cells, care was taken to limit LPS contamination of buffers and culture media.
Cell activation and assessment of functional responses to IL-4
Unless otherwise indicated, the following reagents were added to cultures of SFMC, monocytes or MDMac to give the final concentrations: IL-4 (10 ng/ml); IL-10 (10 ng/ml); IFN-γ (10 ng/ml, PeproTech #300–02, Rocky Hill, NJ); M-CSF (50 ng/ml) and LPS from Escherichia coli 0111:B4 500 ng/ml (Sigma, St. Louis, MO). Replicate cultures for each test variable were incubated at 37° in 5% CO2. After 20 hr, mRNA was extraction from the cell pellets and culture supernatants were used to assess IL-12, IL-10 and TNF-α levels.
Mono Mac 6 and U937 cells
Mono Mac 6 cells were obtained from Dr H. W. L. Ziegler-Heitbrock (Institute of Immunology, Munich, Germany) and cultured as previously described.17,18 For testing of functional responses to IL-4, Mono Mac 6 cells were cultured at 106 cells/ml in RPMI-1640-supplemented media in 24-well plates and activated with LPS (10 ng/ml) and phorbol myristate acetate (PMA) (30 ng/ml) for 5 hr.17
U937 cells were cultured in complete RPMI supplemented with 10% FCS. For testing of functional responses to IL-4, U937 cells were cultured at 2×105 cells/0·2 ml in 96-well plates and activated with LPS (10 ng/ml) and PMA (20 ng/ml) for 48 hr.17
Assay of IL-12, IL-10 and TNF-α by enzyme-linked immunosorbent assay
Culture supernatants from monocytes, MDMac, SFMC, Mono Mac 6 and U937 cells were stored at −20° until used. IL-12, IL-10 and TNF-α were measured by sandwich enzyme-linked immunosorbent assay (ELISA) using monoclonal antibodies (mAb) to human IL-12 [p35/p70 capture antibody (#MO18037) and p40/p70 biotinylated detection antibody (#MO14976), PharMingen, San Diego, CA]; to human IL-10 [rat anti-human capture (#18551D) and biotinylated detection (#18562D) antibody, PharMingen]; and to human TNF-α [capture (#18631D) and biotinylated detection (#18642D) antibody, PharMingen]. The assays were sensitive to levels of >80 pg/ml for IL-12 and >40 pg/ml for both IL-10 and TNF-α.
Messenger RNA extraction, reverse transcription, polymerase chain reaction and semiquantification of the product
Freshly isolated and cultured monocytes (3×106) were lysed in 800 μl Total RNA Isolation Reagent (Advanced Biotechnologies, Leatherhead, UK). RNA was isolated at 4° using the manufacturer's protocol and complementary DNA was synthesized as previously described.17 For polymerase chain reaction (PCR), deoxynucleotide triphosphate and Mg2+ concentrations were 200 μm and 1·5 mm, respectively. Primer sequences (5′ then 3′) and cycle number were as follows:
Glyceraldehyde 3-phosphate dehydrogenase (GAPDH): ACCACCATGGAGAAGACTGG, CTCAGTGTAGCCCAGGATGC, 20 cycles; IL-10: ATGCCCCAAGCTGAGAACCAAGACCCAG, TGGGATAGCTGACCCAGCCCCTTGAGA, 35 cycles.
Cycling parameters were 94° for 1 min, 60° for 1 min and 72° for 1 min for both GAPDH and IL-10. The PCR product was electrophoresed, denatured, neutralized and transferred to a nylon membrane (Hybond N+, Amersham, North Ryde, Australia) by Southern blotting, probed with an oligonucleotide internal to the PCR primers (GAPDH: GTGGAAGGACTCATGACCACAGTCCATGCC; IL-10: ATCAGGGTGGCGACTCTATAGACTCTAGGA) and end-labelled with 32P as previously reported.17
Bound 32P label was measured by a Storage PhosphorScreen (Molecular Dynamics, Sunnyvale, CA), which was scanned on a series 400 PhosphorImager (Molecular Dynamics) and data were calculated using the ImageQuant program (Molecular Dynamics). To ensure that the variations in mRNA expression were not due to variations in the amount of cDNA starting material, all values were standardized according to GAPDH mRNA expression by the same sample. Within all samples from a particular donor there was <20% variation in GAPDH mRNA. In addition, to show that the amount of PCR product measured under the conditions chosen was a function of the number of target molecules, cDNA from monocytes was serially diluted, resulting in proportionally less amplified product (data not shown).
Expression of results and statistical analyses
Cytokine measurements were performed on samples from replicate cultures; the mean values from each set of replicates were used to determine the mean +SEM for n donors or experiments. For comparison of differences in the responses by cell populations from a number of different donors or experiments and for comparison of ratios, Student's paired t-test was used. For comparison of results within an experiment, an unpaired t-test was used. A value of P < 0·05 was considered significant.
RESULTS
IL-4 regulation of IL-12 production by SFMC, monocytes and MDMac
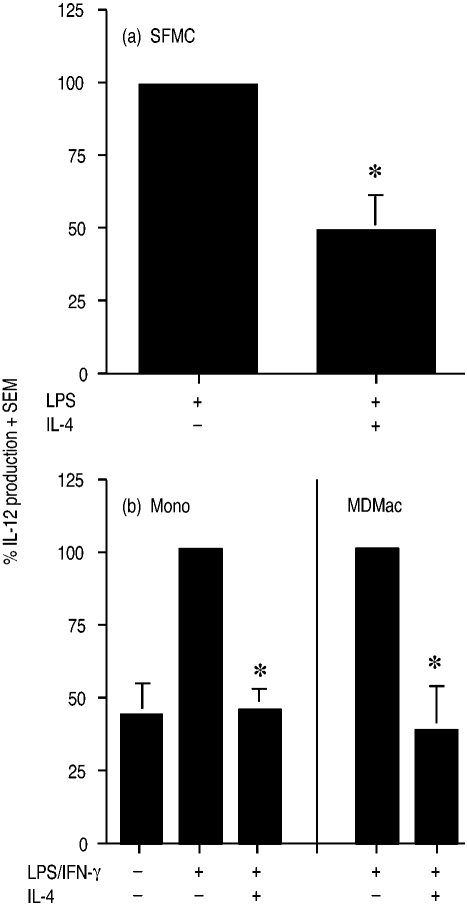
For eight patients with RA, synovial fluid was aspirated, the mononuclear cells were isolated and then cultured at 106 cells/ml for 20 hr with LPS (500 ng/ml) with or without IL-4 (10 ng/ml). LPS-induced IL-12 levels from SFMC ranged from 230 to 730 pg/ml. IL-4 consistently suppressed LPS-induced IL-12 production by SFMC by a mean of 47% (Fig. 1a); LPS-induced values here, and hereafter, were normalized to 100% to accommodate interindividual variation. IL-4 also suppressed LPS-induced IL-12 production from peripheral blood mononuclear cells (PBMC) isolated from two patients at the same time as joint aspiration by 36% and 45%, respectively (data not shown).
Figure 1.
Effect of IL-4 on production of IL-12 by (a) LPS-stimulated SFMC, and (b) LPS with IFN-γ-stimulated monocytes (Mono) and MDMac. In (a), SFMC from eight RA patients were incubated for 20 hr with LPS (500 ng/ml)±IL-4 (10 ng/ml), and in (b) monocytes and MDMac from seven and three donors were incubated for 20 hr with LPS (1 ng/ml)+IFN-γ (10 ng/ml)±IL-4 (10 ng/ml). IL-12 levels (+SEM) have been expressed as a percentage of the level induced by LPS or LPS/IFN-γ. An asterisk indicates a significant suppression by IL-4 of LPS- or LPS/IFN-γ-stimulated cells.
For measurement of IL-12 from more enriched populations of monocytes and macrophages, elutriated monocytes and their derived MDMac were studied. Monocytes and MDMac were less responsive to LPS alone and required IFN-γ (10 ng/ml) with LPS (1 ng/ml) for optimal IL-12 production.19 Cells were incubated and assayed as above with IL-12 levels from stimulated cells ranging from 376 to 842 pg/ml for monocytes (n = 7) and from 312 to 771 pg/ml for MDMac (n = 3), with the mean suppression of IL-12 production by IL-4 being by 55% and 63%, respectively (Fig. 1b).
IL-4 regulation of IL-10 production by SFMC, monocytes and MDMac
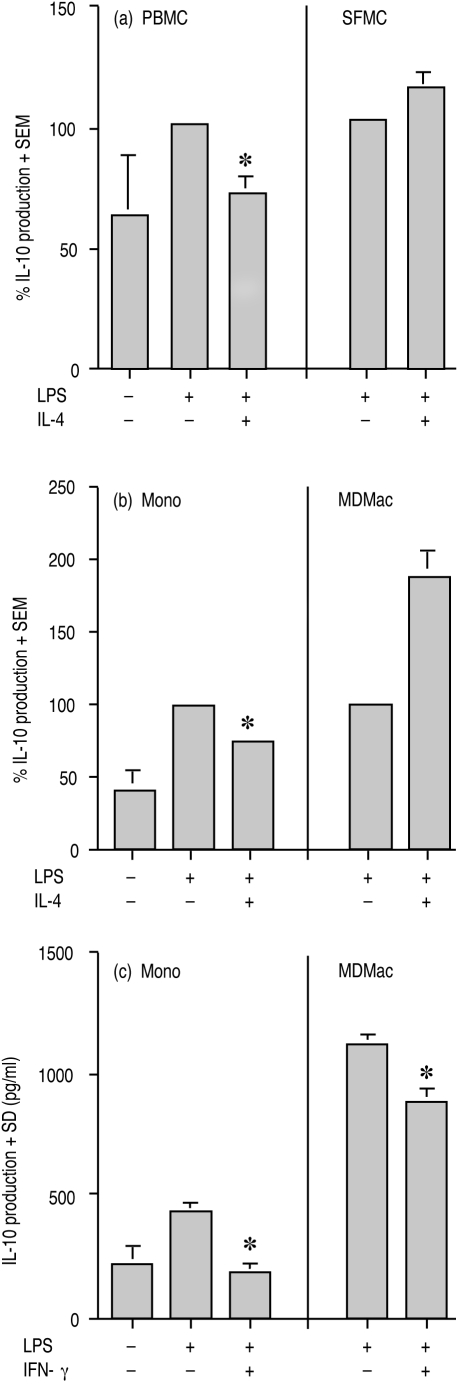
As shown in Fig. 2(a), IL-4 suppressed LPS-induced IL-10 production by PBMC from five patients with RA by a mean of 28%, with LPS-induced IL-10 production ranging from 40 to 348 pg/ml. In contrast, IL-4 (10 ng/ml) did not suppress LPS-induced IL-10 production by SFMC; LPS-induced IL-10 levels from 10 RA patients ranged from 72 to 1034 pg/ml. Even with IL-4 at 100 ng/ml, there was no reduced production of IL-10 by these cells (data not shown). A similar contrast in IL-4 regulation of IL-10 production was observed between monocytes and MDMac. IL-4 significantly suppressed LPS-induced IL-10 production by monocytes from nine donors by a mean of 27% (P < 0·0001). However, IL-4 could not suppress, and in fact significantly induced (P < 0·0001) LPS-induced IL-10 production by MDMac from 11 donors (Fig. 2b). LPS induced IL-10 production by monocytes and MDMac ranged from 42 to 155 pg/ml and from 166 to 2126 pg/ml, respectively. Although IL-4 was unable to reduce LPS-induced IL-10 production by MDMac, these cells were responsive to IFN-γ (10 ng/ml) for suppression of LPS-induced IL-10 levels by 22% (Fig. 2c), suggesting that the changed responses to IL-4 by MDMac were not generalized. This result was repeated and supports published data.15
Figure 2.
Effect of IL-4 on production of IL-10 by LPS-stimulated (a) PBMC and SFMC, and (b) and (c) monocytes (Mono) and MDMac. In (a), PBMC and SFMC from five and 10 RA patients, respectively; in (b), monocytes and MDMac from nine and 11 donors, respectively, were incubated with LPS±IL-4 (10 ng/ml) for 20 hr. In (c), monocytes and MDMac from one donor were incubated with LPS±IFN-γ (10 ng/ml). For (a) and (b), IL-10 levels (+SEM) have been expressed as a percentage of the level induced by LPS. For (c), the mean (+SD) IL-10 level for triplicate cultures is shown. An asterisk indicates a significant suppression by IL-4 or IFN-γ of LPS-stimulated cells.
IL-4 regulation of IL-10 in monocytes and MDMac is at the mRNA level
While LPS-induced IL-10 levels from MDMac were not down-regulated with the addition of IL-4 (Fig. 3), we questioned whether this altered response to IL-4 was determined at the mRNA level. Reverse transcription (RT)-PCR was used to investigate IL-4 regulation of IL-10 mRNA extracted from matched pairs of monocytes and MDMac from five donors that had been stimulated with LPS, with or without IL-4, for 20 hr. Binding of a radiolabelled internal oligonucleotide to the IL-10 PCR product, as well as to the GAPDH PCR product (a housekeeping gene control) allowed for semiquantification of the IL-10 mRNA. As shown in Fig. 3, when expressed as a ratio of IL-10 mRNA to GAPDH mRNA, IL-4 suppressed LPS-induced IL-10 mRNA production from monocytes but not MDMac. While the ratio of IL-10 mRNA to GAPDH mRNA for monocytes fell from a mean of 7·3 to 3·9 with the addition of IL-4, for MDMac relative values for IL-10 mRNA stimulated by LPS did not deviate significantly from the mean of 1·8 when IL-4 was added. In light of these results we hypothesize that control of monocyte and macrophage IL-10 production by IL-4 is regulated, at least in part, at the mRNA level.
Figure 3.
The ratio of the mRNA levels for IL-10 to GAPDH mRNA levels for monocytes (Mono) and MDMac. Total RNA was extracted from matched pairs of monocytes and MDMac from five donors after stimulation with LPS±IL-4 (10 ng/ml) for 20 hr. Messenger RNA levels were quantified by measurement of the RT-PCR product and expressed relative to the GAPDH mRNA level for the same cDNA preparation. The mean ratio +SEM is shown. An asterisk indicates significant suppression by IL-4 of LPS-induced IL-10 mRNA levels.
IL-4 regulation of IL-10 production in the presence of an anti-TNF-α antibody
It has been previously reported that TNF-α can induce monocyte IL-10 production which in turn, by an autocrine or paracrine process, can suppress further monocyte TNF-α and IL-10 synthesis.14 Given the different abilities of IL-4 to regulate TNF-α5 and IL-10 production by LPS-stimulated monocytes, SFMC and MDMac, we investigated the possibility of an interaction between IL-10 and TNF-α and the effects of IL-4. Blocking studies were performed to investigate whether the hyporesponsiveness of MDMac and SFMC to IL-4 for suppression of TNF-α production affected levels of IL-10 such that IL-4 down-regulation of IL-10 by SFMC and MDMac was falsely masked.
A mAb to TNF-α (2TNF-H22) was added to monocytes, MDMac and SFMC (for four, five and five donors, respectively) at a concentration of 1 μg/106 cells prior to the addition of LPS, with or without IL-4. IL-10 levels in the culture supernatants were measured after incubation for 20 hr. As shown in Fig. 4, while addition of a TNF-α antibody significantly suppressed LPS-induced levels of IL-10 by an average of 20–30%, the antibody did not alter the inability of IL-4 to regulate IL-10 production by MDMac and SFMC. This suggests that although LPS-induced IL-10 production is due, in small part, to LPS stimulation of TNF-α, the inability of IL-4 to suppress IL-10 production by MDMac and SFMC is not due indirectly to the presence of TNF-α.
Figure 4.
Effect of a TNF-α antibody on the regulation by IL-4 of monocyte and macrophage IL-10 production. Monocytes (Mono), MDMac and SFMC from four, five and five donors, respectively, were incubated for 20 hr with LPS±IL-4 (10 ng/ml) with or without the TNF-α antibody. Cytokine levels (+SEM) have been expressed as a percentage of the level induced by LPS alone. An asterisk indicates a significant suppression by IL-4of LPS-induced IL-10 production.
IL-4 regulation of TNF-α in the presence of a soluble IL-10 receptor
We next examined whether the inability of IL-4 to down-regulate IL-10 affected IL-4 regulation of LPS-induced TNF-α production. SFMC from four RA patients and monocytes from four control donors were incubated for 20 hr at 106 cells/ml with LPS, with or without 1 μg/ml soluble IL-10 receptor (sIL-10R, R & D Systems #274-R1-050, Minneapolis, MN), with or without IL-4. Secreted TNF-α from monocytes ranged from 9·9 to 53·6 ng/ml, and from 2·7 to 8·8 ng/ml for SFMC. Specificity of the sIL-10R was shown by its ability to block the effects of IL-10, but not IL-4, on LPS-induced TNF-α production by monocytes (Fig. 5). For SFMC from four RA patients, the addition of the sIL-10R did not alter the inability of IL-4 to suppress LPS-induced TNF-α production (Fig. 5).
Figure 5.
Effect of a soluble IL-10 receptor on the regulation by IL-4 of TNF-α production by monocytes (Mono) and SFMC. Monocytes from four donors and SFMC from four RA patients were incubated for 20 hr with LPS±IL-4 (10 ng/ml) with or without sIL-10R. TNF-α levels (+SEM) have been expressed as a percentage of the level induced by LPS alone. An asterisk indicates a significant suppression by IL-4 of LPS-induced TNF-α production; a hash indicates a significant effect of sIL-10R on IL-10 regulation of TNF-α production.
Reduction in γc mRNA correlates with the reduced ability of IL-4 to down-regulate LPS-induced IL-10 production
We have previously published that γc mRNA levels in monocytes decline over time in culture and that this fall correlates with a change in IL-4 suppression of LPS-induced TNF-α.17 In this study we examined whether there was a similar correlation between γc mRNA levels and IL-4 regulation of IL-10 production. We published previously that from four donors, the γc mRNA level (expressed as a ratio to the GAPDH mRNA level) for cultured monocytes fell from the normalized value of 100% for monocytes cultured for 1 day, to a mean of 60% and 20% after 3 and 5 days in culture, respectively.17 Figure 6 shows the data for cells from a representative normal donor for IL-4 regulation of LPS-induced IL-10 production. After 1 day of culture, IL-4 suppressed LPS-induced IL-10 production by 65%, but by day 3 of culture, IL-4 could no longer suppress LPS-induced IL-10. Similar results were obtained in a repeat experiment using a different donor.
Figure 6.
Relative changes in IL-4 regulation of LPS-induced IL-10 production by monocytes with increasing time in culture. Monocytes from a single donor were cultured under non-adherent conditions for 1, 3 and 5 days with M-CSF and then incubated with medium alone (complete RPMI with 1% FCS) or with LPS±IL-4 (10 ng/ml), for 20 hr. IL-10 levels are represented as mean (+SD) for triplicate cultures. An asterisk indicates a significant suppression by IL-4 of IL-10 production from LPS-stimulated cells.
Effect of IL-4 on IL-10 production by Mono Mac 6 and U937 cells
With evidence suggesting the involvement of the γc chain in IL-4 regulation of TNF-α production,17 we investigated further the role of γc in IL-4 regulation of IL-10 production. Two myeloid cell lines were chosen that express varying levels of γc. Mono Mac 6 cells express little, if any, γc18 while significant γc protein can be detected on the surface of U937 cells,17 thereby mimicking MDMac and SFMC, and monocytes, respectively.
In four separate experiments, Mono Mac 6 cells were incubated for 5 hr at 106/ml with control medium alone or LPS (10 ng/ml) and PMA (30 ng/ml), with and without IL-4. In response to LPS and PMA, IL-10 (range: 90–453 pg/ml) was detected but IL-4 failed to suppress these IL-10 levels (Fig. 7). Increased levels of IL-4 (100 ng/ml) were still unable to suppress LPS/PMA-induced IL-10 production (data not shown).
Figure 7.
Effect of IL-4 on the production of IL-10 by Mono Mac 6 and U937 cells. Mono Mac 6 and U937cells were incubated for 5 hr and 48 hr, respectively, with LPS/PMA±IL-4 (10 ng/ml). All results show the mean (+SEM) for four and three experiments, respectively. Cytokine levels have been expressed as a percentage of the level induced by LPS/PMA. An asterisk indicates a significant suppression by IL-4 on LPS/PMA-induced IL-10 production.
In three experiments, U937 cells were incubated with control medium alone or LPS (10 ng/ml) and PMA (20 ng/ml), with or without IL-4. IL-4 significantly suppressed LPS/PMA-induced IL-10 production (IL-10 range: 110–307 pg/ml) by a mean of 37±13% (Fig. 7). Thus, U937 cells are similar to monocytes, whilst Mono Mac 6 cells are more like SFMC and MDMac, both in their expression of a functional γc17 and in their ability to respond to IL-4 for regulation of IL-10 production.
DISCUSSION
This is the first study to examine the regulation by IL-4 of LPS-induced IL-12 and IL-10 production by human inflammatory macrophages ex vivo and to compare the responses with those of blood monocytes. We have shown that while IL-4 suppresses LPS-induced IL-12 production by both blood monocytes and SFMC (Fig. 1) and LPS-induced IL-10 production by PBMC, IL-4 fails to suppress LPS-induced IL-10 production by SFMC (Fig. 2). Results using freshly isolated versus cultured monocytes were similar to those of PBMC versus SFMC. In fact, IL-4 significantly increased IL-10 levels of LPS-stimulated MDMac. These findings support our hypothesis that activation and differentiation of monocytes leads to altered IL-4 response profiles.
This extends our earlier observations of different responses to IL-4 by monocytes and macrophages.5 While IL-4 does not suppress LPS-induced TNF-α5 or IL-10 production by SFMC or MDMac, IL-4 can efficiently suppress LPS-induced IL-1β5 and IL-12 production by monocytes, SFMC and MDMac. Thus, we hypothesize that signalling pathways in monocytes and macrophages separate IL-4-regulated monokines into two groups: those such as IL-1β and IL-12 which are suppressed by IL-4 in both monocytes and macrophages, and those such as TNF-α and IL-10 which are down-regulated efficiently by IL-4 in monocytes only. This division varies from that described by D'Andrea and colleagues who linked IL-1β with IL-10 and TNF-α with IL-12.20 That group found that PBMC pretreated with IL-4 for 20 hr prior to LPS or Staphylococcus aureus stimulation were primed for increased IL-12 and TNF-α, but reduced IL-1β or IL-10 production. Given our findings with cells exposed to LPS and IL-4 at the same time, we suggest that such IL-4 priming does not critically involve a functional γc. However, their observations emphasize the complexity involved in IL-4 regulation of monokines in inflammatory responses. Given the similarity of altered IL-4 regulation of TNF-α and IL-10 production by SFMC and MDMac, we investigated the possibility of an interrelationship between regulation of TNF-α and IL-10. Experiments using a mAb to TNF-α and a soluble receptor to IL-10 excluded this possibility (Figs 4 and 5).
The IL-4 receptor is traditionally thought to comprise two chains, the 140 000 MW IL-4Rα chain (CDw124) and the IL-2Rγ chain (γc; common to receptors for IL-2, IL-4, IL-7, IL-9 and IL-15) that dimerize 1:1 upon ligand binding.21,22 We have previously reported a reduction in γc mRNA expression for MDMac17 and that a direct correlation exists between time of monocytes in culture, reduction in γc mRNA and hyporesponsiveness to IL-4 for LPS-induced TNF-α regulation. In this study we found that as levels of γc mRNA fall over time in culture, so does the ability of IL-4 to suppress LPS-induced IL-10 production (Fig. 6) and that this control is, at least in part, at the mRNA level (Fig. 3). Our hypothesis of γc involvement in IL-4 regulation of LPS-induced IL-10, but not IL-12 production, was supported by studies conducted using myeloid cell lines that express different levels of γc.
We have reported that in MDMac, signalling through the IL-4 receptor is significantly diminished with only low levels of STAT6 activation detected upon IL-4 exposure.17 Although the relationship between STAT6 levels and modulation of IL-10 and IL-12 gene expression is not known, our study with MDMac suggests that low levels of STAT6 may be sufficient for regulation of LPS-induced IL-12, but not IL-10 production. Alternatively, IL-10 but not IL-12 gene regulation by STAT6 and LPS-induced NF-κB may involve promoter element competition.23
The consistent suppression of LPS-induced IL-1β and IL-12 production by all cells studied implies the existence of a functional receptor for IL-4 on MDMac and SFMC. B cells from X-linked severe combined immunodeficient patients,24 endothelial cells25 and renal and colon carcinoma cells26,27 all respond to IL-4 for a variety of functions, yet they do not express γc. Several suggestions have been made for IL-4 receptor conformations other than the traditional receptor of one IL-4Rα-chain and γc. While there have been reports of possible homodimerization of the IL-4Rα-chain,28 others have supported the involvement of a γc-like protein, IL-13Rα1, from the IL-13 receptor (also known as IL-13Rα′ or γ′)29,30 and other laboratories have suggested a heterotrimeric configuration.27 The elucidation of a receptor conformation for functional activity of IL-4 on differentiated macrophages and its downstream signalling is part of an ongoing study. This study also highlights the importance of studying responses to potential anti-inflammatory cytokines by cells isolated from inflammatory sites and not by the more easily accessible blood monocytes.
Acknowledgments
We thank Drs R. Vandenberg, M. Ahern and M. Smith for collection of the synovial fluids, Drs J. Schreurs, S. Gillis, R. de Wall Malefyt and A. C. Allison for their generous gifts and Dr K. Williams for critical reading of the manuscript. This work was supported by grants to PHH and JJFJ from the National Health and Medical Research Council of Australia, the Arthritis Foundation of Australia, the Flinders Medical Centre Foundation and a Dora Lush National Health and Medical Research Council Postgraduate Award to CSB.
Glossary
Abbreviations
- IFN-γ
interferon-γ
- IL
interleukin
- LPS
lipopolysaccharide
- M-CSF
macrophage-colony stimulating factor
- MDMac
monocyte-derived macrophages
- PBMC
peripheral blood mononuclear cells
- PMA
phorbol myristate acetate
- RA
rheumatoid arthritis
- R
receptor
- SFMC
synovial fluid mononuclear cells
- STAT
signal transducers and activators of transcription
- TNF-α
tumour necrosis factor-α
REFERENCES
- 1.Brown MA, Hural J. Functions of IL-4 and control of its expression. Immunology. 1997;17:1. doi: 10.1615/critrevimmunol.v17.i1.10. [DOI] [PubMed] [Google Scholar]
- 2.Chomarat P, Banchereau J. An update on IL-4 and its receptor. Eur Cyt Netw. 1997;8:333. [PubMed] [Google Scholar]
- 3.Chomarat P, Vannier E, Dechanet J, et al. Balance of IL-1 receptor antagonist/IL-1β in rheumatoid synovium and its regulation by IL-4 and IL-10. J Immunol. 1995;154:1432. [PubMed] [Google Scholar]
- 4.Colotta F, Re F, Muzio M, et al. Interleukin-1 type II receptor: a decoy target for IL-1 that is regulated by IL-4. Nature. 1993;161:472. doi: 10.1126/science.8332913. [DOI] [PubMed] [Google Scholar]
- 5.Hart PH, Ahern MJ, Jones CA, Jones KA, Smith MD, Finlay-Jones JJ. Synovial fluid macrophages and blood monocytes differ in their responses to IL-4. J Immunol. 1993;151:3370. [PubMed] [Google Scholar]
- 6.Hart PH, Jones CA, Finlay-Jones JJ. Monocytes cultured in cytokine defined environments differ from freshly isolated monocytes in their responses to IL-4 and IL-10. J Leuk Biol. 1995;57:909. doi: 10.1002/jlb.57.6.909. [DOI] [PubMed] [Google Scholar]
- 7.Trinchieri G. Interleukin-12: a cytokine produced by antigen-presenting cells with immunoregulatory functions in the generation of T-helper cell type 1 and cytokine lymphocytes. Blood. 1994;84:4008. [PubMed] [Google Scholar]
- 8.Spitz H, De Waal Malefyt R. Functional characterization of human IL-10. Int Arch Allergy Immunol. 1992;99:8. doi: 10.1159/000236329. [DOI] [PubMed] [Google Scholar]
- 9.Kobayashi M, Fitz L, Ryan M, et al. Identification and purification of natural killer cell stimulatory factor (NKSF), a cytokine with multiple biologic effects on human lymphocytes. J Exp Med. 1989;170:827. doi: 10.1084/jem.170.3.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kubin M, Kamoun M, Trinchieri G. Interleukin 12 synergizes with B7/CD28 interaction in inducing efficient proliferation and cytokine production of human T cells. J Exp Med. 1994;180:211. doi: 10.1084/jem.180.1.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Trinchieri G, Gerosa F. Immunoregulation by interleukin 12. J Leuk Biol. 1996;59:505. doi: 10.1002/jlb.59.4.505. [DOI] [PubMed] [Google Scholar]
- 12.Hashimoto S-I, Yamada M, Motoyoshi K, Akagawa KS. Enhancement of macrophage factor-induced growth and differentiation of human monocytes by IL-10. Blood. 1997;89:315. [PubMed] [Google Scholar]
- 13.Vieira P, De Waal Malefyt R, Dang M-N, et al. Isolation and expression of human cytokine synthesis inhibitory factor cDNA clones: homology to Epstein-Barr virus open reading frame BCRF1. Proc Natl Acad Sci USA. 1991;88:1172. doi: 10.1073/pnas.88.4.1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wanidworanun C, Strober W. Predominant role of tumor necrosis factor-α in human monocyte IL-10 synthesis. J Immunol. 1993;151:6853. [PubMed] [Google Scholar]
- 15.Chomarat P, Rissoan M-C, Banchereau J, Miossec P. Interferon γ inhibits interleukin 10 production by monocytes. J Exp Med. 1993;177:523. doi: 10.1084/jem.177.2.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.De Waal Malefyt R, Abrams J, Bennet B, Figdor CG, De Vries JE. Interleukin 10 (IL-10) inhibits cytokine synthesis by human monocytes: an autoregulatory role of IL-10 produced by monocytes. J Exp Med. 1991;174:1209. doi: 10.1084/jem.174.5.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bonder CS, Dickensheets HL, Finlay-Jones JJ, Donnelly RP, Hart PH. Involvement of the IL-2 receptor γ-chain (γc) in the control by IL-4 of human monocyte and macrophage proinflammatory mediator production. J Immunol. 1998;160:4048. [PubMed] [Google Scholar]
- 18.Ziegler-Heitbrock HWL, Thiel E, Futterer A, Herzog V, Wirtz A, Reitmuller G. Establishment of a human cell line (Mono Mac 6) with characteristics of mature monocytes. Int J Cancer. 1988;41:456. doi: 10.1002/ijc.2910410324. [DOI] [PubMed] [Google Scholar]
- 19.Smith W, Feldmann M, Londei M. Human macrophages induced in vitro by macrophage-colony stimulating factor are deficient in interleukin-12 production. Eur J Immunol. 1998;28:2498. doi: 10.1002/(SICI)1521-4141(199808)28:08<2498::AID-IMMU2498>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 20.D'andrea A, Ma X, Aste-Amezaga M, Paginin C, Trinchieri G. Stimulatory and inhibitory effects of interleukin (IL) -4 and IL-13 on the production of cytokines by peripheral blood mononuclear cells: priming for IL-12 and tumor necrosis factor α production. J Exp Med. 1995;181:537. doi: 10.1084/jem.181.2.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Russell SM, Keegan AD, Harada N, et al. IL-2 receptor γ chain: a functional component of the IL-4 receptor. Science. 1993;262:1880. doi: 10.1126/science.8266078. [DOI] [PubMed] [Google Scholar]
- 22.Kondo M, Takeshita T, Ishii N, et al. Sharing of the interleukin-2 (IL-2) receptor γ chain between receptors for IL-2 and IL-4. Science. 1993;262:1874. doi: 10.1126/science.8266076. [DOI] [PubMed] [Google Scholar]
- 23.Bennett BL, Cruz R, Lacson RG, Manning AM. Interleukin-4 suppression of tumor necrosis factor α-stimulated E-selectin gene transcription is mediated by STAT6 antagonism of NF-κB. J Biol Chem. 1997;272:10212. doi: 10.1074/jbc.272.15.10212. [DOI] [PubMed] [Google Scholar]
- 24.Noguchi M, Yi H, Rosenblatt HM, et al. Interleukin-2 receptor gamma chain mutation results in X-linked severe combined immunodeficiency in humans. Cell. 1993;73:147. doi: 10.1016/0092-8674(93)90167-o. [DOI] [PubMed] [Google Scholar]
- 25.Schnyder B, Lugli S, Feng N, et al. IL-4 and IL-13 bind to a shared heterodimeric complex on endothelial cells mediating vascular cell adhesion molecule-1 induction in the absence of the common γ chain. Blood. 1996;87:4286. [PubMed] [Google Scholar]
- 26.Obiri NI, Debinski W, Leonard WJ, Puri RK. Receptor for IL. Interaction with IL-4 by a mechanism that does not involve the common γ chain shared by receptors for interleukins 2, 4, 7, 9 and 15. J Biol Chem. 1995;270:8797. doi: 10.1074/jbc.270.15.8797. [DOI] [PubMed] [Google Scholar]
- 27.Murata T, Noguchi PD, Puri RK. Receptors for IL-4 do not associate with common γ chain and IL-4 induces the phosphorylation of JAK2 tyrosine kinase in human colon carcinoma cells. J Biol Chem. 1996;270:30829. doi: 10.1074/jbc.270.51.30829. [DOI] [PubMed] [Google Scholar]
- 28.Kammer W, Lischke A, Morigg B, et al. Homodimerisation of interleukin-4 receptor α chain can induce intracellular signalling. J Biol Chem. 1996;271:23634. doi: 10.1074/jbc.271.39.23634. [DOI] [PubMed] [Google Scholar]
- 29.Miloux B, Laurent P, Boonin O, et al. Cloning of the human IL-13RαI chain and reconstruction with the IL-4Rα of a functional IL-4/IL-13 receptor complex. FEBS Lett. 1997;401:163. doi: 10.1016/s0014-5793(96)01462-7. [DOI] [PubMed] [Google Scholar]
- 30.Obiri NI, Leland P, Murata T, Debinski W, Puri RK. The IL-13 receptor structure differs on various cell types and may share more than one component with IL-4 receptor. J Immunol. 1997;158:756. [PubMed] [Google Scholar]