Abstract
Interleukin-12 (IL-12) strongly favours the development of T-helper 1 (Th1)-type cells through its ability to induce interferon-γ (IFN-γ) production by natural killer cells and T cells. In the present work we analysed the effects of IL-12 on the synthesis and secretion of IFN-γ and IL-4 by human T-cell clones. Several previously described human T-cell clones exhibiting Th1, Th2 or Th0 phenotypes were used for these analyses. We demonstrated, by enzyme-linked immunosorbent assay (ELISA) and intracytoplasmic staining, that, in Th0 clones, IL-12 up-regulated the production of both IFN-γ and IL-4 and was unable to modulate these cells to Th1-type. The up-regulation of cytokine gene expression was transcriptionally regulated and was not due to differences in mRNA stability. In Th1 cells, IL-12 up-regulated only IFN-γ and not IL-4. However, in Th2 cells, both IFN-γ and IL-4 were up-regulated by IL-12. This suggests that Th2 cells may be less stable than Th1 cells. We also observed that human Th2 cells expressed the IL-12β2 receptor, in contrast to murine Th2, which lacks this receptor. The observed differences in the effects of IL-12 on the three T-cell subsets may have important ramifications for IL-12-based therapies.
INTRODUCTION
Mature CD4+ T cells segregate into two major functional phenotypes, T-helper 1 (Th1) and Th2.1,2 Th1-type cells predominantly secrete interferon-γ (IFN-γ) and interleukin-2 (IL-2), and play an important role in generating protective cell-mediated immune responses against intracellular pathogens. In contrast, Th2-type cells make IL-4, IL-5 and IL-10, and are associated with humoral immunity and allergic responses.3,4 Th0 cells are a third subset of T cells that produce both Th1-and Th2-type cytokines,5 and may represent either a distinct subset of differentiated T cells or precursor T cells in the process of differentiating into Th1- or Th2-type cells.
Multiple factors have been reported that influence the decision of precursor T cells to differentiate into Th1- or Th2-type cells, including cytokines,6,7 antigen dose,8 ligand density,9 intracellular secondary signalling pathways,10 and antigen-presenting cells11 with their associated costimulatory signals.12 Among the factors that control T-cell subset differentiation, the best characterized are the cytokines, which are present during antigen stimulation. Clearly, IL-12 is one of the most powerful cytokines that promotes differentiation of uncommitted T cells into a Th1 phenotype.13,14 IL-4 has the reciprocal activity, inducing differentiation of uncommitted T cells into Th2 cells.7,15,16
The requirement for IL-12 in promoting a Th1 response has been demonstrated both in vitro and in vivo. Using naive T cells from transgenic mice expressing a class II-restricted, ovalbumin-specific T-cell receptor, Hsieh et al.13 showed that priming of naive T cells in the presence of IL-12 skewed the effector cells to a Th1 phenotype. However, addition of exogenous IL-4 resulted in polarization to a Th2 phenotype, even in the presence of IL-12.13 The ability of IL-12 to bias immune responses in vivo to the Th1 type has been well studied in the murine model of leishmania. In vivo, IL-12 protects susceptible BALB/c mice from disseminated Leishmania major infection17,18 by promoting a Th1 response. Conversely, injection of anti-IL-12 antibodies to resistant C57BL/6 mice renders them susceptible, and disease is associated with Th2 development.18 The protective effects of IL-12 are observed only when administered concurrently with pathogens, and not when given after infection.18
We have generated a panel of antigen-specific human T-cell clones that have an unrestricted cytokine profile and are designated as Th0. It is not clear whether these Th0 clones can be further manipulated to differentiate into Th1 or Th2 subsets. In this study we therefore examined the direct effects of IL-12 on IFN-γ and IL-4 mRNA gene expression and protein expression in a panel of antigen-specific human Th0 clones, and compared them with the effects of IL-12 on established human Th1 and Th2 clones. We utilized an accessory cell-independent system in which T-cell clones were pretreated with IL-12 for 48 hr. Cells were then washed and stimulated in 24-well tissue culture plates coated with anti-CD3 antibodies in the absence of IL-12 to assess for their cytokine-producing capacity. We deliberately used polyclonal stimulation in order to avoid any additional biasing that may be induced by antigen-presenting cells. We demonstrate that incubation of resting T-cell clones with IL-12 in the absence of antigen-specific activation results in the up-regulation of both IFN-γ and IL-4 from differentiated T-cell clones. These results are important because they suggest that exogenous administration of IL-12 might up-regulate not only IFN-γ but also IL-4 from primary resting T cells.
MATERIALS AND METHODS
T-cell clones
Mycobacterium leprae, tetanus-toxoid and purified protein derivative (PPD)-reactive clones were established with a limiting dilution technique as described previously.19 The Th1 and Th2 clones were maintained in IL-2, with bi-weekly stimulation with antigen and antigen-presenting cells. To maintain their cytokine profile and provide the least amount of biasing in vitro, the Th0 clones were maintained in IL-2, with bi-weekly stimulation with antigen-presenting cells and phytohaemagglutinin (PHA). Non-specific stimulation with PHA activates both Th1-and Th2-type cytokine gene expression and allows for maintenance of the Th0 phenotype.
Pretreatment of clones with IL-12 and ELISA for IFN-γ and IL-4
T-cell clones, 1×106, were pretreated with 20 U/ml IL-12 for 48 hr. Cells were washed and 0·5×105 cells were stimulated in 24-well tissue culture plates pre-coated with 1 ml of 2·5 μg/ml antibody to CD3 (Biosource International, Camarillo, CA). Supernatants from the stimulated cells were harvested 18–20 hr later, and IFN-γ and IL-4 levels were measured by sandwich enzyme immunoassay using appropriate pairs of capture antibodies and biotinylated detecting antibodies (Pharmingen, San Diego, CA). The sensitivity of the IFN-γ enzyme-linked immunosorbent assay (ELISA) was 0·5 U/ml and the sensitivity of the IL-4 ELISA was 15 picograms/ml.
Intracellular staining
Cells, 2×106, were stimulated with 50 ng/ml phorbol myristate acetate (PMA) and 1 μm ionomycin for 4 hr. Stimulation was performed in the presence of 2 μm monensin, added to inhibit protein secretion and allow for intracytoplasmic accumulation of cytokines. Cells were harvested and fixed in 4% paraformaldehyde in phosphate-buffered saline (PBS) for 20 min. Following two washes in permeablization buffer (PBS containing 1% fetal calf serum, 0·1% NaN3, 0·2% saponin), cells were reacted with fluorescein isothiocyanate (FITC)-conjugated anti-IFN-γ antibodies and phycoerythrin (PE)-conjugated anti-IL-4 antibodies for 30 min (both antibodies were purchased from Pharmingen). Immunofluorescence was detected by flow cytometry (Epics Elite, Coulter Corp., Miami, FL). Unstimulated cells served as controls.
Northern hybridization
Cells were lysed in buffer containing Nonidet P-40 (NP-40), sodium dodecyl sulphate (SDS) and urea. Total RNA was extracted in phenol-chloroform and ethanol precipitated.20 Approximately 10 μg of the total RNA obtained was fractionated by electrophoresis in 1% agarose gels containing 0·66 m formaldehyde and MOPS buffer (1×MOPS: 40 mm morpholinopropanesal fonic acid, 10 mm sodium acetate, 10 mm EDTA, pH 7·2), and blotted onto nylon membrane (MagnaGraph, MSI, Westborough, MA). RNA blots were prehybridized for 2 hr at 65° in 5×SSC, 0·5% SDS, 5×Denhardt's solution and denatured salmon sperm DNA (50 μg/ml). Hybridization was performed for 18 hr at 42° in 5×SSC, 0·5% SDS, 5×Denhardt's solution, denatured salmon sperm DNA (50 μg/ml), 50% formamide and a P32-radiolabelled probe (1×106 c.p.m./ml). Hybridized membranes were washed twice for 15 min at room temperature in 2×SSC, 0·1% SDS, and twice for 15 min at 65° in 0·2×SSC, 0·1% SDS. The blots were probed sequentially for IFN-γ, IL-4 and β-actin. The autoradiograms were analysed and quantified using a Sigma Gel Program (Jandel Corp., San Rafael, CA). The percentage increase in IFN-γ and IL-4 message in IL-12-pretreated cells was determined after normalization with a β-actin message.
Determination of mRNA stability
T cells were cultured in the presence and absence of IL-12 for 48 hr, washed and stimulated with PMA (50 ng/ml) and ionomycin (1 μm). Two hours later actinomycin D was added to the medium at a concentration of 10 μg/ml. Total RNA was isolated at 75-min intervals and analysed by Northern hybridization by probing sequentially for IFN-γ, IL-4 and 18S rRNA. Levels of IFN-γ and IL-4 mRNA were adjusted with 18S rRNA level and quantified using a Fuji phospho-imager (Fuji Photo Film Co Ltd, Tokyo, Japan).
Transcription assay
Transcription of IFN-γ and IL-4 was determined by the 4-thiouridine method.21 This method utilizes the sensitivity of affinity chromatography and Northern hybridization to detect and select newly transcribed RNA following a defined pulse with 4-thiouridine. Thirty minutes after PMA and ionomycin stimulation, 4-thiouridine was added to the medium to a final concentration of 100 μm. Total RNA was isolated 30 min later. Approximately 40 μg of total RNA was resuspended in buffer A (5 mm NaAc, pH 5·5, 0·1% SDS, 0·15 m NaCl and 4 mm EDTA), denatured at 65° for 5 min, cooled on ice and batch adsorbed to phenyl mercury agarose (Affi-gel 501; Bio-Rad Laboratories, Hercules, CA) for 2 hr. The column was washed with five times the column volume of buffer A. The newly synthesized 4-thiouridine containing RNA was eluted with buffer A containing 2-mercaptoethanol. Two micrograms of RNA from both the flow-through and eluted fraction was analysed by Northern hybridization, and levels of IFN-γ and IL-4 mRNA were quantified.
RNase protection assay
A multiprobe template set (RiboQuantTM; Pharmingen) was used to evaluate the mRNA expression levels of IL-12β1 and β2 receptors. A multiprobe was synthesized from the hCR-3 template set that included probes for IL-12β1 and β2 receptors. Total RNA prepared from T-cell clones was hybridized overnight to the probe, followed by RNase treatment and purification of protected probes. Protected fragments were electrophoresed on denaturing acrylamide gels and subjected to autoradiography. A standard curve was plotted of migration distance versus log nucleotide length using the undigested probes as markers. This curve was then used to confirm the identity of ‘RNase protected bands’ in the sample RNA preparations.
Statistical analysis
Statistical differences were determined by the paired Student's t-test.
RESULTS
IL-12 primes Th0 cells for both IFN-γ and IL-4 production
Six separate human Th0 clones, with or without prior exposure to IL-12, were tested by ELISA for their ability to secrete IFN-γ and IL-4. IL-12 stimulated the secretion of both IFN-γ and IL-4 (Fig. 1a,b). IL-12 treatment significantly increased the IFN-γ secretion of Th0 clones (P < 0·01). The increase in IL-4 secretion was also significant (P < 0·05).
Figure 1.
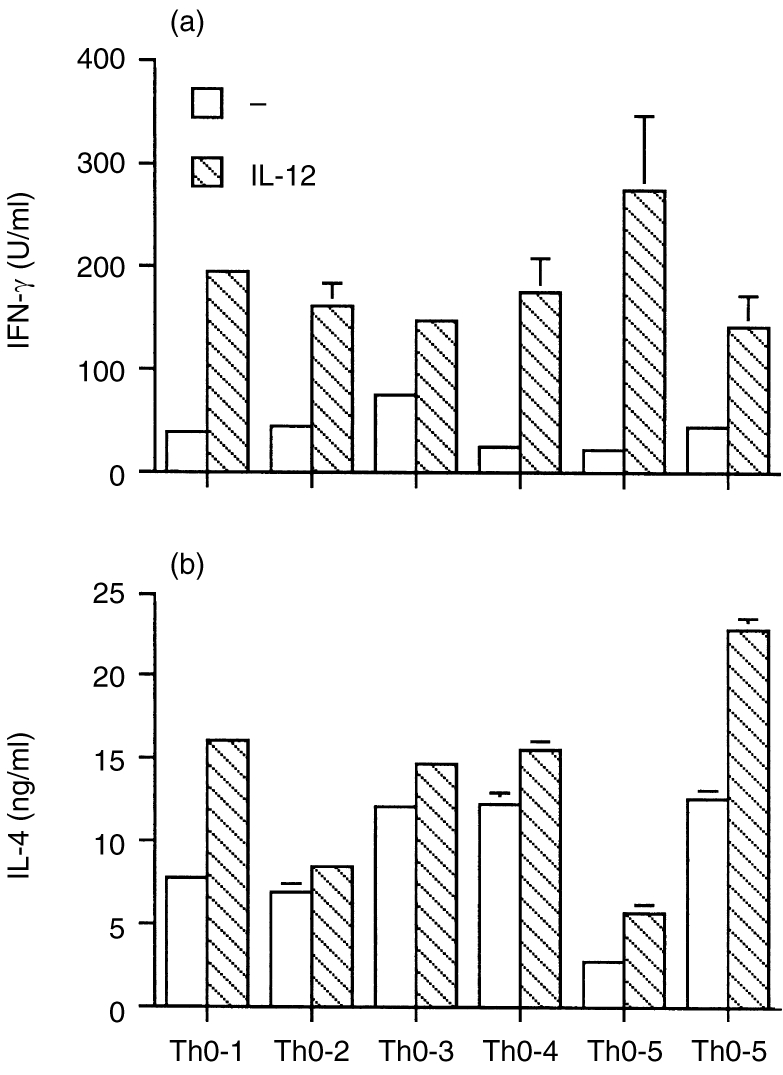
ELISA for IFN-γ (a) and IL-4 (b) in six IL-12 pretreated Th0 clones. Approximately 1×106 T-cell clones were pretreated with 20 U/ml IL-12 for 48 hr. Cells were washed, and 0·5×105 cells were stimulated by immobilized antibodies to CD3. Supernatants were harvested 18–20 hr later, and IFN-γ and IL-4 levels were measured by sandwich enzyme immunoassay using appropriate pairs of capture antibodies and biotinylated detecting antibodies. The mean and SD are shown for each individual clone. Differences in IFN-γ and IL-4 production between IL-12 untreated and treated cells were statistically significant.
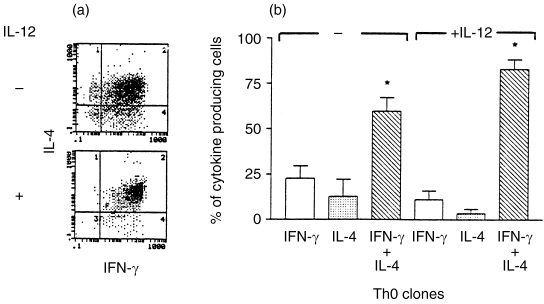
Three of the six Th0 clones (Th0-3, Th0-5 and Th0-6) were analysed further for intracytoplasmic IFN-γ and IL-4 protein by two-colour immunofluorescence staining followed by flow cytometry. A fluorescence-activated cell sorter (FACS) profile of a representative clone (Th0-6), before and after IL-12 pretreatment, is shown in Fig. 2a. A compilation of data from FACS profiles of three clones is shown in Fig. 2b. The majority of the cells in the clonal populations responded to stimulation by producing both IFN-γ and IL-4, thereby confirming their Th0 phenotype. However, there appeared to be a small percentage of IFN-γ and IL-4 single-positive cells in all clonal Th0 populations (Fig. 2a,b). Interestingly, pretreatment with IL-12, prior to stimulation, resulted in a decrease in the frequency of individual cells that were positive for either IFN-γ or IL-4 with a concomitant increase in the frequency of cells that stained positively for both cytokines (Fig. 2a,b). These results indicate that IL-12 pretreatment of clonal populations of Th0 cells increases their cytokine secretion and converts single-positive to double-positive Th0 cells.
Figure 2.
Flow cytometric determination of intracytoplasmic IFN-γ and IL-4 protein in three IL-12-pretreated Th0 clones. Intracytoplasmic cytokine levels were determined by two-colour FACS analysis of paraformaldehyde-fixed and saponin-permeablized T cells using FITC-labelled anti-IFN-γ antibodies and PE-labelled anti-IL-4 antibodies. (a) A FACS profile of one such clone. The upper left-hand quadrant shows IL-4 single-positive cells; the lower right-hand quadrant, IFN-γ single-positive cells; the upper right-hand quadrant, double-positive cells; and the lower left-hand quadrant, double-negative cells. (b) Data obtained from the FACS profile of three individual clones. The bar diagram indicates the percentage of cells that stain for either IFN-γ and IL-4 alone, and the percentage of cells that stain for both cytokines, before and after IL-12. The asterisk above the two columns represents statistically significant differences between them (P < 0·05), as determined by paired Student's t-test.
Effect of IL-12 on IFN-γ and IL-4 mRNA
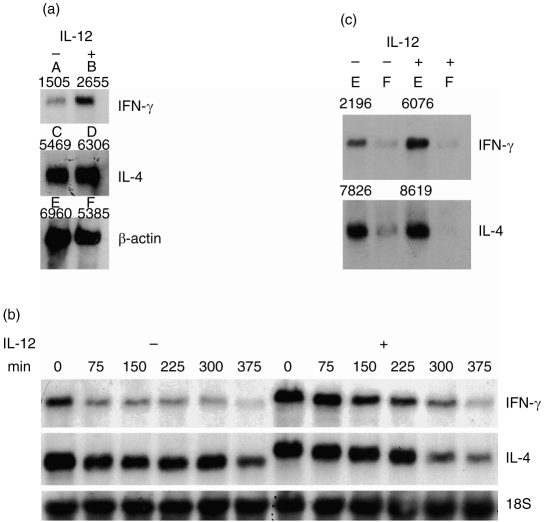
In the experiments described here, the effect of IL-12 on the transcription of IFN-γ and IL-4 by Th0 clones was studied. Th0 clones, with or without prior treatment with IL-12, were stimulated with anti-CD3 antibodies for 4 hr, and analysed by Northern blotting for β-actin, IFN-γ and IL-4 mRNA levels. Figure 3a (a representative blot of data obtained from two different clones) demonstrates that IL-12 up-regulated the mRNA levels for both IFN-γ and IL-4. In IL-12-pretreated cells there was a 128% increase in the IFN-γ message levels, and a 48% increase in the IL-4 message levels. The increase in the transcript levels is reflected in the increases in protein levels observed in Fig. 1a,b.
Figure 3.
(a) Northern analysis for IFN-γ and IL-4 in IL-12-pretreated Th0 clones. T-cell clones with or without IL-12 pretreatment were stimulated with immobilized anti-CD3 monoclonal antibodies. After 4 hr of stimulation the cells were harvested, total RNA was prepared and Northern analysis performed. The blots were sequentially probed for IFN-γ, IL-4 and β-actin. The autoradiograms were analysed and quantified using a Sigma Gel Program. The densitometric numbers obtained by the above analysis is indicated above each band of the autoradiogram. Lane 1, IL-12 untreated cells; lane-2, cells pretreated with IL-12 for 48 hr. The percentage increase in IFN-γ message was calculated as (B/F−A/E)/A/E×100. The percentage increase in IL-4 message was calculated as (D/F−C/E)/C/E×100. (b) Determination of mRNA stability. Three Th0 clones were pretreated with IL-12 for 48 hr or left untreated. The cells were then stimulated and 2 hr later actinomycin D was added at 10 μg/ml. Total RNA was isolated at 75-min intervals and subjected to Northern analysis. Data obtained from one such clone are depicted in this figure. Lanes 1–6 represent different time-points at which IL-12 untreated cells were harvested, and lanes 7–12 represent different time-points at which IL-12 pretreated cells were harvested. (c) Transcription of IFN-γ and IL-4 message in IL-12 pretreated clone Th0-1. This was determined by the thiouridine method. Lane 1, eluted fraction of IL-12 untreated cells; lane 2, flow-through of IL-12 untreated cells; lane 3, eluted fraction of IL-12 pretreated cells; lane 4, flow-through of IL-12 pretreated cells.
Effect of IL-12 on IFN-γ and IL-4 mRNA stability
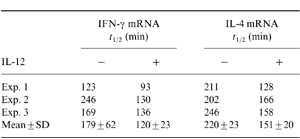
We next tested if the increase in mRNA levels of IFN-γ and IL-4 was due to an increase in message stability. T cells were treated with IL-12 for 48 hr, washed and stimulated with PMA and ionomycin. Actinomycin was added to the cultures 4 hr later, and total RNA was harvested at 75-min intervals. Stability of mRNA was measured by Northern hybridization (Fig. 3b). The t½-values for IFN-γ mRNA in the presence and absence of IL-12 were 179±62 and 120±23 min, respectively (Table 1). These data indicate that in the presence of IL-12 there is clearly no increase in message stability, but there may be a decrease in message stability. Nevertheless, on statistical analysis the differences were not found to be significant (P > 0·2). Similarly, the t½-values for IL-4 mRNA in the presence and absence of IL-12 were 220±23 and 151±20 min, respectively (Table 1). Again, this difference was not statistically significant (P > 0·1). As there were no statistically significant differences in the t½-values between the IL-12-treated and untreated groups, it leads us to conclude that up-regulation of IFN-γ and IL-4 genes by IL-12 is not due to increased message stability.
Table 1.
Degradation of the IFN-γ and IL-4 message was evaluated by Northern blot hybridization and densitometric readings obtained for each of the hybridized bands. Relative percentage differences were calculated as densitometric readings at the various time-points/reading at 0 hr time-point×100. The t½-values were determined from the computer-generated equation of the line formed by plotting the log of the relative differences of the densitometric readings versus time. Data from three individual Th0 clones are presented
Effect of IL-12 on transcription of IFN-γ and IL-4 genes
In order to determine if IFN-γ and IL-4 genes were transcriptionally regulated, we analysed newly synthesized mRNA by in vivo labelling with 4-thiouridine. Total RNA isolated from labelled cells was adsorbed to a phenyl mercury agarose column, and 2 μg of the flow-through and eluted fractions was analysed by Northern hybridization for IFN-γ and IL-4 message levels. Densitometric readings for IFN-γ message in the elute (newly synthesized RNA) of untreated and IL-12-treated samples were 2196 and 6076, respectively, indicating a 177% increase in newly synthesized mRNA. Similarly, readings for IL-4 message in the elute of untreated and treated samples were 7826 and 8619, respectively, with a 10% increase in newly synthesized IL-4 mRNA (Fig. 3c). These data indicate that the increases in protein levels of IFN-γ and IL-4 in IL-12 treated cells are likely to be transcriptionally regulated.
Effect of IL-12 on differentiated Th1 and Th2 clones
Human Th1 and Th2 clones were then tested by ELISA for their ability to up-regulate IFN-γ and IL-4 secretion in response to IL-12 treatment. IL-12 up-regulated IFN-γ secretion in the three Th1 clones tested. There was a 1·3–3·4-fold increase in IFN-γ secretion (Fig. 4a). Because Th1 clones that were not pretreated with IL-12 made large amounts of IFN-γ, the increase after IL-12 pretreatment was moderate. However, pretreatment of the Th1 clones with IL-12 did not induce the cells to produce any IL-4 (Fig. 4b). In contrast, in Th2 clones (Fig. 4c,d) IL-12 pretreatment induced significant up-regulation of both IFN-γ (P < 0·01) and IL-4 (P < 0·05).
Figure 4.
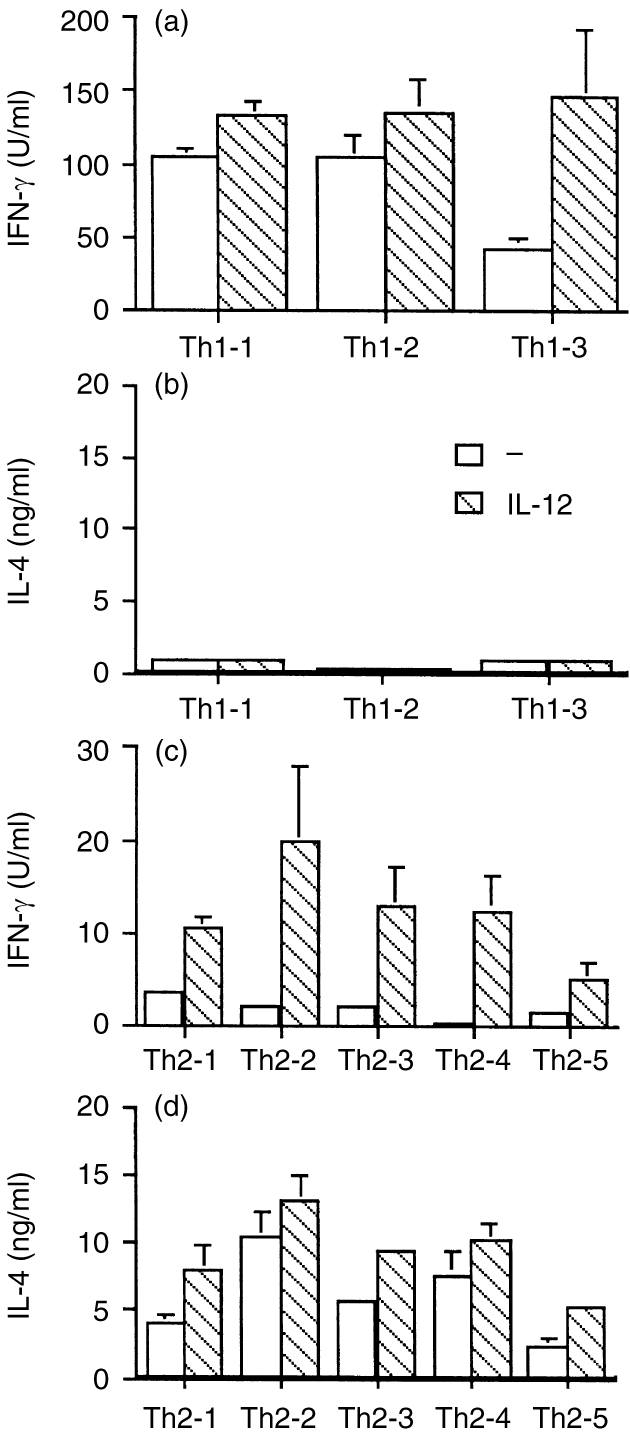
ELISA for IFN-γ (a) and IL-4 (b) in IL-12-pretreated Th1 clones, and ELISA for IFN-γ (c) and IL-4 (d) in IL-12-pretreated Th2 clones. There was a statistically significant increase in IFN-γ (P < 0·01) and IL-4(P < 0·05) production in IL-12 -pretreated Th2 cells compared with the untreated cells.
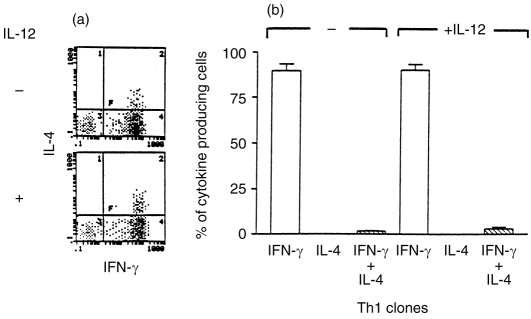
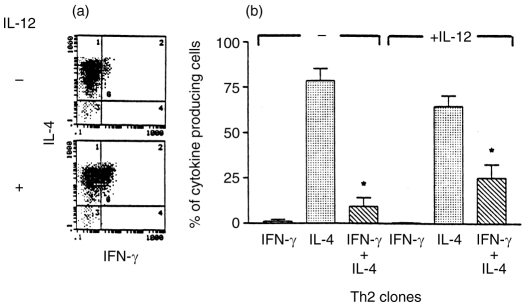
Three Th1 and three Th2 clones were analysed further by two-colour intracytoplasmic immunofluorescence staining followed by flow cytometry. There was no difference in the frequency of IFN-γ-producing cells in the Th1 clones before and after treatment with IL-12 (Fig. 5a,b). On analysis of the mean fluorescence intensity (MFI) of IFN-γ staining (data not shown), it was observed that there was an increase in the MFI in IL-12-pretreated cells. This suggests that the increase in IFN-γ that was observed in the ELISA was as a result of increased IFN-γ production in individual cells. IL-12 treatment did not increase the IL-4 levels in Th1 clones (Fig. 5a,b) and concomitantly no increase in the frequency of Th0-type cells was observed (Fig. 5b). On the other hand, pretreatment of Th2 clones with IL-12 prior to stimulation resulted in a significant increase (P < 0·05) in the percentage of Th0 cells secreting both IFN-γ and IL-4 (Fig. 6a,b). Thus the increase in IFN-γ and IL-4 that was observed in the ELISA is attributable to the increase in Th0-like cells in the Th2 clonal population.
Figure 5.
Flow cytometric determination of intracytoplasmic IFN-γ and IL-4 protein in three IL-12-pretreated Th1 clones. Intracytoplasmic staining was performed as described in Fig. 2. A FACS profile of a representative Th1 clone before and after IL-12 pretreatment is presented in (a). Compilation of FACS data from three individual clones is presented in (b).
Figure 6.
Flow cytometric determination of intracytoplasmic IFN-γ and IL-4 protein in three IL-12-pretreated Th2 clones. Intracytoplasmic staining was performed as described in Fig. 2. A FACS profile of a representative Th2 clone before and after IL-12 pretreatment is presented in (a). Compilation of FACS data from three individual clones is presented in (b). The asterisk above the two columns represents statistically significant differences between them (P < 0·05), as determined by paired Student's t-test.
Murine Th2 clones down-regulate their IL-12 β2 receptor expression and therefore are no longer capable of responding to IL-12. Because the human Th2 clones responded to IL-12, we examined the mRNA expression levels of the receptor on four Th2 clones and two Th0 clones. We observed that all four Th2 clones expressed an abundant message for both the IL-12 β1 and β2 receptors, similar to the expression levels on the two Th0 clones (Fig. 7).
Figure 7.
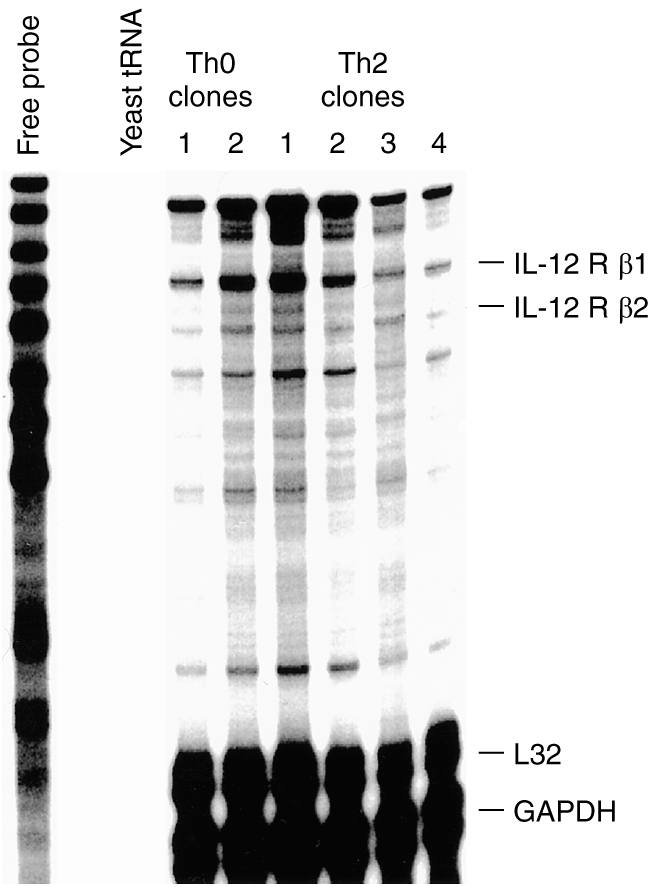
Expression of IL-12β1 and β2 receptors on Th2 and Th0 clones. Total RNA was isolated from four Th2 clones and two Th0 clones and used for RNase a protection assay using the hCR-3 probe kit (Pharmingen) and developed by autoradiography for 24 hr.
DISCUSSION
On activation, naive T cells pass through a Th0 phase when they express both Th1 and Th2 cytokines.22,23 Depending on the cytokine environment, these cells then rapidly down-regulate the expression of either the Th1 or Th2 cytokines, and develop into mature Th1 and Th2 subsets.23 We tested the possibility that antigen-specific, yet uncommitted, human Th0 clones could similarly be modulated to become Th1-type in the presence of IL-12.
Our results show that IL-12 did not bias the human Th0 clones to Th1-type, and the cells retained their Th0 phenotype. As previously observed, IL-12 pretreatment of the clones primed the cells for increased IFN-γ production. Furthermore, we also observed an increase in IL-4 production by the IL-12-treated Th0 clones. By ELISA, it was not possible to determine cytokine secretion by individual cells. Consequently, we examined IL-4 and IFN-γ protein levels further by performing a two-colour immunofluorescence assay that measured intracellular cytokine levels in individual cells. It is interesting to note that analysis of individual cells in the clonal Th0 population revealed intraclonal variation. The majority of the cells in the clonal population were Th0-type; however, single-positive cells were also present. Pretreatment with IL-12 up-regulated cytokine production in all cells and the single positives were converted to Th0. This further confirms that IL-12 does not modulate Th0 cells to become Th1-like, but in fact accentuates their Th0 phenotype. These data therefore suggest that antigen-specific Th0-effectors are possibly a third distinct subset of T cells, and principles that govern naive Th0 differentiation may not be applicable to this subset.
Induction of IL-4 in Th0 clones by IL-12 was unexpected, and prompted us to examine if IL-12 would similarly up-regulate IL-4 production in established Th1 and Th2 clones. IL-12 up-regulated IFN-γ in Th1 clones, but did not induce any IL-4 production, thus not altering their Th1 profile. In contrast, in Th2 clones IL-12 up-regulated not only IFN-γ, as previously reported,24 but also IL-4. With the treatment regimen that we utilized here the conversion of Th2 to Th0-like cells was not a 100%, nevertheless there was a significant increase in Th0 cells after IL-12 pretreatment. This is in contrast to previous studies where IL-12 was shown to have multiple effects on Th1 cells, but none on Th2 cells.25 These data also differ from previous reports where IL-12 inhibited IL-4 production in KLH-primed CD4 T cells26 and in allergen-specific Th2 cells.27 However, in these studies the inhibition observed was indirect through the effects of IL-12 on antigen-presenting cells. In our system the cells were stimulated in an antigen-presenting cell-free system with insoluble anti- CD3 antibodies. Thus the differential ability of IL-12 to regulate IL-4 production may be influenced by the environment in which the T cells are activated. When administered exogenously as an immunotherapeutic agent, IL-12 acts on T cells in the absence of antigen. Therefore our system may more closely mimic the in vivo situation when IL-12 is being used in the absence of antigen priming.
The present data also highlight differences in the ability of murine and human T-cell subsets to be modulated by cytokines. The phenotype of murine Th2 cells cannot be reversed by IL-12, as during commitment to the Th2 lineage they lose their ability to respond to IL-12 by down-regulating the signalling β2 component of the IL-12 receptor.28,29 Nevertheless, this down-regulation is not as tightly controlled in the human Th2 cells, because they do express low levels of β2 receptor.28 This may partly explain why human Th2 clones can up-regulate cytokine expression in response to IL-12. Plasticity of human Th2 cells has been described in other systems as well. IFN-γ production is induced in human Th2 cells upon non-specific stimulation with phorbol ester and calcium ionophore.30 Similarly, transformation with herpes virus saimiri also induces constitutive IFN-γ production in human Th2 cells.
One of the most distinguishing effects of IL-12 is its ability to induce transcription of the IFN-γ gene. In Th0 clones IL-12 pretreatment increased IFN-γ production by several fold, and the increase was transcriptionally regulated. However, the molecular mechanism of this up-regulation is not understood. IL-12 signalling in Th1 cells induces the phosphorylation of Stat3 and Stat4.31 The Stat molecules may directly or indirectly regulate IFN-γ gene transcription in Th0 cells. The up-regulation of IL-4 gene expression in Th0 cells was also transcriptionally regulated and was not due to differences in mRNA stability. The molecular mechanism of IL-4 up-regulation by IL-12 in Th0 cells also remains undefined. It has been described that in Th2 cells the IFN-γ gene is not transcribed, due to methylation of a site in the IFN-γ promoter between the CAAAT and the TATA box.32 Whether IL-12 demethylates this region in Th2 cells and allows for IFN-γ gene transcription also needs to be examined.
The presence of IL-12 during initiation of the immune response prevents Th2 development, with concomitant biasing towards Th1 development, both in vivo18 and in vitro.13 In contrast, IL-12 does not inhibit already developed Th2 responses,33 and in IFN-γ knockout mice even exacerbates an established Th2 response.34 Immunization of mice with TNP-KLH and IL-12 as an adjuvant promotes a Th1 response.35 Nevertheless, these mice are still capable of developing a recall Th2 response in the absence of IL-12, suggesting that IL-12 does not induce a long-lasting suppression of Th2 responses.35 Thus IL-12 has differential effects on a primary versus an established secondary immune response. Our in vitro data and those of others36,37 provide an explanation for the inability of IL-12 to inhibit an already established Th2 response.
The present findings therefore have important implications for the clinical use of IL-12 as an immunomodulator of Th1 and Th2 responses in human diseases. Delineation of the molecular mechanisms whereby IL-12 controls cytokine up-regulation in T cells, especially Th2 cells, will provide better understanding for the appropriate clinical use of IL-12.
Acknowledgments
We than the Genetics Institute and National Cancer Institute for their generous gifts of IL-12 and IL-2, respectively.
REFERENCES
- 1.Mosmann TR, Coffman RL. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol. 1989;7:145. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- 2.Janeway CRJ, Carding S, Jones B, et al. CD4+ T cell: specificity and function. Immunol Rev. 1988;101:39. doi: 10.1111/j.1600-065x.1988.tb00732.x. [DOI] [PubMed] [Google Scholar]
- 3.Bloom BR, Salgame P, Diamond B. Revisiting and revising T suppresser cells. Immunol Today. 1992;13:131. doi: 10.1016/0167-5699(92)90110-S. [DOI] [PubMed] [Google Scholar]
- 4.Romagnani S. Lymphokine production by human T cells in disease states. Annu Rev Immunol. 1994;12:227. doi: 10.1146/annurev.iy.12.040194.001303. [DOI] [PubMed] [Google Scholar]
- 5.Paliard X, de Waal Malefyt R, Yssel H, et al. Simultaneous production of IL-2, IL-4, and IFN-γ by human CD4+ and CD8+ T cell clones. J Immunol. 1988;136:2348. [PubMed] [Google Scholar]
- 6.Seder RA, Paul WE. Acquisition of lymphokine-producing phenotype by CD4+ T cells. Annu Rev Immunol. 1994;12:635. doi: 10.1146/annurev.iy.12.040194.003223. [DOI] [PubMed] [Google Scholar]
- 7.Swain SL, Weinberg AD, English M, Huston G. IL-4 directs the synthesis of Th2-like helper effectors. J Immunol. 1990;145:3796. [PubMed] [Google Scholar]
- 8.Bretscher PA, Wei G, Menon JN, Bielefeldt OH. Establishment of stable, cell-mediated immunity that makes ‘susceptible’ mice resistant to Leishmania major. Science. 1992;257:539. doi: 10.1126/science.1636090. [DOI] [PubMed] [Google Scholar]
- 9.Pfeiffer C, Murray J, Madri J, Bottomly K. Selective activation of Th1- and Th2-like cell in vivo-response to human collagen IV. Immunol Rev. 1991;123:65. doi: 10.1111/j.1600-065x.1991.tb00606.x. [DOI] [PubMed] [Google Scholar]
- 10.Novak TJ, Rothenberg EV. cAMP inhibits induction of interleukin 2 but not of interleukin 4 in T cells. Proc Natl Acad Sci USA. 1990;87:9353. doi: 10.1073/pnas.87.23.9353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weaver CT, Hawrylowicz CM, Unanue ER. T helper cell subsets require the expression of distinct costimulatory signals by antigen-presenting cells. Proc Natl Acad Sci USA. 1988;85:8181. doi: 10.1073/pnas.85.21.8181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gajewski TF, Schell SR, Fitch FW. Evidence implicating utilization of different T cell receptor-associated signalling pathways by Th1 and Th2 clones. J Immunol. 1990;144:4100. [PubMed] [Google Scholar]
- 13.Hsieh C-S, Macatonia SE, Tripp CS, Wolf SF, O’Garra A, Murphy KM. Development of TH1, CD4+ T cells through IL-12 produced by Listeria-induced macrophages. Science. 1993;260:547. doi: 10.1126/science.8097338. [DOI] [PubMed] [Google Scholar]
- 14.McKnight AJ, Zimmer GJ, Fogelman I, Wolf SF, Abbas AK. Effects of IL-12 on helper T cell dependent immune responses in vivo. J Immunol. 1994;152:2172. [PubMed] [Google Scholar]
- 15.Seder RA, Paul WE, Davis MM, Fazekas DS, Groth B. The presence of interleukin-4 during in vitro priming determines the lymphokine-producing potential of CD4+ T cells from T cell receptor transgenic mice. J Exp Med. 1992;176:1091. doi: 10.1084/jem.176.4.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Le Gros G, Ben-Sasson SZ, Seder R, Finkelman FD, Paul WE. Generation of interleukin-4 (IL-4)-producing cells in vivo and in vitro: IL-2 and IL-4 are required for in vitro generation of IL-4-producing cells. J Exp Med. 1990;172:921. doi: 10.1084/jem.172.3.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heinzel FP, Schoenhaut DS, Rerko RM, Rosser LE, Gately MK. Recombinant interleukin 12 cures mice infected with Leishmania major. J Exp Med. 1993;177:1505. doi: 10.1084/jem.177.5.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sypek JP, Chung CL, Mayor SEH, et al. Resolution of cutaneous leishmaniasis: interleukin 12 initiates a protective T helper type 1 immune response. J Exp Med. 1993;177:1797. doi: 10.1084/jem.177.6.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Salgame PR, Modlin RL, Bloom BR. On the mechanism of human T cell suppression. Int Immunol. 1989;1:121. doi: 10.1093/intimm/1.2.121. [DOI] [PubMed] [Google Scholar]
- 20.Gough NM. Rapid and quantitative preparation of cytoplasmic RNA from small numbers of cells. Anal Chem. 1988;173:93. doi: 10.1016/0003-2697(88)90164-9. [DOI] [PubMed] [Google Scholar]
- 21.Woodford TA, Schlegel R, Pardee AB. Selective isolation of newly synthesized mammalian mRNA after in vivo labelling with 4-thiouridine or 6-thioguanosine. Anal Biochem. 1988;171:166. doi: 10.1016/0003-2697(88)90138-8. [DOI] [PubMed] [Google Scholar]
- 22.Kamogawa Y, Minasi LE, Carding SR, Bottomly K, Flavell RA. The relationship of IL-4 and IFNγ-producing T cells studied by lineage ablation of IL-4-producing cells. Cell. 1993;75:985. doi: 10.1016/0092-8674(93)90542-x. [DOI] [PubMed] [Google Scholar]
- 23.Nakamura T, Kamagawa Y, Bottomly K, Flavell RA. Polarization of IL-4 and IFN-γ-producing CD4+ T cells following activation of naive CD4+ T cells. J Immunol. 1997;158:1085. [PubMed] [Google Scholar]
- 24.Manetti R, Gerosa F, Giudizi MG, et al. Interleukin-12 induces stable priming for interferon-γ production during differentiation of human T helper cells and transient IFN-γ production in established Th2 cell clones. J Exp Med. 1994;179:1273. doi: 10.1084/jem.179.4.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Germann T, Gately MK, Schoenhaut DS, et al. Interleukin-12/T cell stimulating factor, a cytokine with multiple effects on T helper type 1 (Th1) but not on Th2 cells. Eur J Immunol. 1993;23:1762. doi: 10.1002/eji.1830230805. [DOI] [PubMed] [Google Scholar]
- 26.Dekruyff RH, Fang Y, Wolf SF, Umetsu DT. IL-12 inhibits IL-4 synthesis in keyhole limpet hemocyanin-primed CD4+ T cells through an effect on antigen-presenting cells. J Immunol. 1995;154:2578. [PubMed] [Google Scholar]
- 27.Marshall JD, Secrist H, Dekruyff RH, Wolf SF, Umetsu DT. IL-12 inhibits the production of IL-4 and IL-10 in allergen-specific human CD4+ T lymphocytes. J Immunol. 1995;155:111. [PubMed] [Google Scholar]
- 28.Szabo SJ, Dighe AS, Gubler A, Murphy KM. Regulation of the interleukin (IL)-12R, B2 subunit expression in developing T helper 1 (Th1) and Th2 cells. J Exp Med. 1997;185:817. doi: 10.1084/jem.185.5.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rogge BL, Barberis-Maino L, Biffi M, et al. Selective expression of an interleukin-12 receptor component by human T helper 1 cells. J Exp Med. 1997;185:825. doi: 10.1084/jem.185.5.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yssel H, Johnson KE, Schneider PV, et al. T cell activation inducing epitopes of the house dust mite allergen Der pi. Induction of a restricted cytokine production profile of Der pi-specific T cell clones upon antigen-specific activation. J Immunol. 1992;148:738–. [PubMed] [Google Scholar]
- 31.Jacobson NG, Szabo SJ, Weber-Nordt RM, et al. Interleukin 12 signaling in T helper type 1 (Th1) cells involves tyrosine phosphorylation of signal transducer and activator of transcription (Stat) 3 and Stat4. J Exp Med. 1995;181:1755. doi: 10.1084/jem.181.5.1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pang Y, Norihisa Y, Benjamin D, Kantor RR.S, Young HA. Interferon-γ gene expression in human B-cell lines: induction by interleukin-2, protein kinase C activators, and possible effects of hypomethylation on gene regulation. Blood. 1990;80:724. [PubMed] [Google Scholar]
- 33.Finkelman FD, Madden KB, Cheever AW, et al. Effects of interleukin-12 on immune responses and host protection in mice infected with intestinal nematode parasites. J Exp Med. 1994;179:1563. doi: 10.1084/jem.179.5.1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wynn TA, Jankovic D, Hieny S, et al. IL-12 exacerbates rather than suppresses T helper 2-dependent pathology in the absence of endogenous IFN-γ. J Immunol. 1995;154:3999. [PubMed] [Google Scholar]
- 35.Bliss J, Van Cleave V, Murray K, et al. IL-12, as an adjuvant, promotes a T helper 1 cell, but does not suppress a T helper 2 recall response. J Immunol. 1996;156:887. [PubMed] [Google Scholar]
- 36.Yssel H, Fasler S, de Vries JE, de Waal Malefyt R. IL-12 transiently induces IFN-gamma transcription and protein synthesis in human CD4+ allergen-specific Th2 T cell clones. Int Immunol. 1994;6:1091. doi: 10.1093/intimm/6.7.1091. [DOI] [PubMed] [Google Scholar]
- 37.Jeannin P, Deineste Y, Life P, Gauchat J-F, Kaiserlian D, Bonnefoy J-Y. Interleukin-12 increases interleukin-4 production by established human Th0 and Th2-like T cell clones. Eur J Immunol. 1995;25:2247. doi: 10.1002/eji.1830250820. [DOI] [PubMed] [Google Scholar]