Abstract
Although the involvement of complement in hyperacute rejection of xenotransplants is well recognized, its role in rejection of devascularized xenografts, such as pancreatic islets, is not completely understood. In this study, we investigated whether complement participates in the immunopathology of xeno-islet transplantation in a concordant rat to mouse model. Rat pancreatic islets were implanted under the kidney capsule of normal and cobra venom factor (CVF)-decomplementized diabetic C57BL/6 mice. Graft survival was monitored by blood glucose levels. Deposition of IgM and C3 on grafted islets in vivo or on isolated islets in vitro (after incubation with normal and decomplementized mouse serum), as well as CD4- and CD8-positive leucocyte infiltration of grafts, was checked by immunohistochemistry. In addition, complement-mediated cytotoxicity on rat islet cells was evaluated by a 3-(4,5-dimethythiazolyl)-2.5-diphenyl-2H-tetrazolium-bromide (MTT) assay. A significant C3 deposition was found on grafted islets from the first day after transplantation in vivo, as well as on isolated islets after incubation with mouse serum in vitro. By MTT assay, complement-mediated cytotoxicity for islet cells was found. Decomplementation by CVF decreased C3 deposition on either isolated or grafted islets, delayed CD4- and CD8-positive leucocyte infiltration, led to significant inhibition of complement-mediated cytotoxicity for islet cells, and prolonged graft survival (mean survival time 21·3 versus 8·5 days; P <0·01). Our results indicate that decomplementation can prolong the survival time of devascularized xenografts across concordant species. The deposition of complement on transplanted islets may contribute to xenograft rejection by direct cytotoxicity and by promoting leucocyte infiltration.
Introduction
Allotransplantation of pancreatic islets is making the step from an experimental procedure to an accepted alternative to pancreas transplantation. While recent results are encouraging,1,2 it is evident that this therapeutic approach will be limited by organ shortage. Therefore, xeno-islet transplantation (XIT) has been considered as a possible solution.3
In discordant species combinations, whole organ xenografts trigger a hyperacute rejection (HAR). It has been recognized that the humoral immune response plays a crucial role in HAR. Xenoreactive natural antibodies and complement pre-existing in recipients induce donor endothelial cell activation and damage, which recruit other effector mechanisms, leading ultimately to graft destruction.4 However, in devascularized xenografts, such as pancreatic islets, HAR does not occur and the term concordant and discordant can not be applied with the same meaning. Although the T-cell response has been recognized as playing a major role in xeno-islet rejection,5 XIT in B-cell-deficient mice exhibits a significantly prolonged survival time, suggesting that some humoral factors may also participate in the rejection.6
In previous studies, a significant deposition of IgM and C3 was found on grafted islets in a rat to mouse model, while this deposition was not seen in islet allotransplantation.7 Although a direct complement-mediated cytotoxicity on xeno-islet cells has been shown in vitro,8 the effect of complement on grafted islets in vivo remains to be investigated.
In order to evaluate the impact of complement on xeno-islet rejection in a concordant model (as related to organ xenotransplantation), rat islets of Langerhans’ were transplanted into mice decomplementized by cobra venom factor (CVF) and the survival of grafted islets, as well as the relevant immunohistological changes, were investigated.
Our results indicate that effective decomplementation by CVF significantly prolongs xeno-islet survival in a rat to mouse model. Complement deposition may participate in the immunopathology of xeno-islet rejection by direct cytotoxicity and by promoting T-cell infiltration.
MATERIALS AND METHODS
Animals
Sprague–Dawley male rats (400–500 g) and C57BL/6 male mice (20–25 g, 6–8 weeks old) were purchased from BRL (Basel, Switzerland) and bred in the animal facilities at the University Hospital of Geneva. All animal studies were approved by the local ethic commission.
Rat islet isolation
Rat islets were isolated using the intraductal collagenase digestion technique as described previously.9 Briefly, anaesthesia was induced by 5% isofluran (Forene®; Abbott, Cham, Switzerland). After midline incision and exposure of the pancreas, 10 ml Hanks’ balanced saline solution (HBSS) with 2 mg/ml collagenase type XI (Sigma, St Louis, MO) was injected into the pancreatic duct. After pancreatectomy, the pancreas was digested in a 37° water bath with gentle shaking for 19 min. The isolated islets were purified further on a Euro-Ficoll (Sigma) gradient centrifugation. The purified islets were washed three times and resuspended in HBSS solution for islet transplantation.
Decomplementation
C57BL/6 male mice were divided into four groups (control, CVF1, CVF2 and CVF3). In the CVF1 group, 500 μg/kg CVF (Imutran, Cambridge, UK) was injected intraperitoneally (i.p.) into mice 24 hr prior to islet transplantation. The mice of the CVF2 group received 500 μg/kg CVF i.p. 24 hr prior to and 3 days after islet transplantation. For the CVF3 group a single injection of CVF (500 μg/kg) was given to mice on day 4 after transplantation. The mice of the control group were injected with the matching volumes of phosphate-buffered saline (PBS).
In order to evaluate the efficiency of decomplementation by CVF, serum was collected daily and pooled from four non-transplanted mice of the control group, CVF1 and CVF2.
The C3 levels in serum were measured by radial immunodiffusion using a method as described previously.10 Briefly, 1% agar was stabilized at 45° in a water bath, and goat anti-mouse C3 (29·5 mg/ml; courtesy of Dr I. Shozo, Geneva, Switzerland) added at 1 : 150. Gel was poured on a glass plate and circular wells were punched out in the gel. Approximately 7 μl of diluted samples and standards, respectively, was added to the wells. The plates were incubated at room temperature in a moist box for 48 hr, washed in 0·9% NaCl overnight, and coloured with 1% tannic acid (Sigma, Steinheim, Germany).11 Ring-shaped precipitates of samples were measured and compared to a standard curve obtained by plotting the square of diameters of standards against dilutions.
The bioactivity of complement in the serum was analysed using a cytotoxicity test with human brain microvascular endothelial cells (HB-MVEC) by a 3-(4,5-dimethythiazolyl)-2.5-diphenyl-2H-tetrazolium-bromide (MTT) assay as described previously.12 Briefly, fresh normal or decomplementized mouse serum was diluted in 100 μl Dulbecco’s modified Eagle’s medium (DMEM; Gibco BRL, Paisley, UK) in a 96-well plate. Heat-inactivated fetal calf serum (FCS; Gibco) was used as a negative control. HB-MVEC suspension in 100 μl DMEM, containing 2 μg/ml actinomycin D (Merck, Darmstadt, Germany) was added to each well (5±104 cells/well). The final serum concentration was 25%. The cells were incubated with serum at 37° for 20 hr and then 50 μl of 0·5% MTT (Merck) was added to each well. After a 4-hr incubation, the plate was centrifuged at 500 g for 10 min and the supernatant discarded. The cells in each well were lysed with 100 μl/well isopropanol–HCl in a VARI shaker (Dynatech, Zurich, Switzerland) for 5 min. The optical density was measured at 570 nm with an enzyme-linked immunosorbent assay (ELISA) reader. The percentage of cytotoxicity was calculated as follows:
Rat islet transplantation
Diabetes was induced by a single injection of streptozotocin i.p. (200 mg/kg; Sigma, St Louis, MO). Diabetes was defined as plasma glucose levels above 20 mmol/l on 3 consecutive days. Approximately 250–350 hand-picked, freshly isolated rat islets were injected beneath the kidney capsule in mice of each group under 1·5% isofluran (Forene®) anaesthesia. The function of grafted islets was evaluated by measurement of non-fasting plasma glucose levels. Blood samples were taken on the tail vein and glucose was measured in the plasma by an automatic analyser (Cobas Mira; Roche Diagnostic, Basel, Switzerland). Values above 11 mmol/l on 3 consecutive days were considered as rejection.
Preparation of rat islet cells
In order to investigate the complement-mediated cytotoxicity for rat islet cells, purified rat islets were digested further using an EDTA-dispase technique as described previously.8 Briefly, purified rat islets were incubated in HBSS with 3 μm EDTA for 5 min at room temperature. After centrifugation islets were incubated in dispase (9 mg/ml in HBSS; Dispase II®; Boehringer Mannheim, Mannheim, Germany) for 15 min at 37° with gentle shaking. The digestion was stopped with cold RPMI (Gibco BRL) containing 10% FCS and the cell suspension was passed through a 100-μm mesh screen to discard the aggregated cells and fibrin. Cells were washed three times with RPMI, counted, and then resuspended in DMEM containing 10% FCS for the cytotoxicity assay.
Immunohistochemistry
Kidneys were taken from control and CVF-treated mice on days 1, 4 and 7 after islet transplantation (n = 3 for each group and day) and snap-frozen in Tissue Tek (Miles, Elkhart, Indiana, USA). The samples were soaked in liquid nitrogen and then stored at −80° until immunohistological testing. Tissue sections were incubated with PBS plus 0·5% bovine serum albumen (BSA; Sigma) for 15 min to block non-specific binding, and then stained with guinea-pig anti-porcine insulin (Dako, Zug, Switzerland), goat anti-mouse IgM (Sigma, Buchs, Switzerland), sheep anti-mouse C3 (The Binding Site, Birmingham, UK), rat anti-mouse CD4 and rat anti-mouse CD8 antibody (Serotec, Oxford, UK), respectively. After a 30-min incubation, the sections were washed and further stained with corresponding fluorescein isothiocyanate (FITC)-conjugated second antibodies for 45 min. The results were checked under a fluorescence microscope (Zeiss, Iena, Germany) by three different examiners. Immunostaining was evaluated semi-quantitatively with a scoring system (0, ±, +, ++). Each examiner calculated the mean value of his scoring for each treatment group from 3×2 slides per specific staining and corresponding day after transplantation (days 1, 4 and 7).
The freshly isolated and purified rat islets were fixed with −20° methanol for 10 min, washed twice with PBS, and then incubated with PBS containing 0·1% BSA to block non-specific binding. Islets were incubated with normal or decomplementized mouse serum, respectively, for 1 hr at 4°. After three washings, islets were incubated with a goat anti-mouse IgM or sheep anti-mouse C3 antibody for 1 hr. Islets were washed and further stained by a diluted rabbit anti-goat (Sigma) or rabbit anti-sheep FITC-conjugated antibody (Sigma) for 30 min. The staining was checked under a fluorescence microscope. The experiment was repeated with three different islet isolations.
Cytotoxicity assay
Normal or CVF-decomplementized serum was serially diluted with 100 μl DMEM in a 96-well plate. The isolated islet cell suspension with 2 μg/ml actinomycin D was added to each well (104 cells/well). Rat islet cells were incubated with the serum for 20 hr at 37° and then 50 μl 0·5% MTT was added to each well. After a 4-hr incubation, the plate was centrifuged at 500 g for 10 min and the supernatant was discarded. The cells in each well were lysed with 100 μl/well isopropanol–HCl in a VARI shaker for 5 min. The optical density was measured at 570 nm with an ELISA reader. The results are expressed as a percentage of cell survival. The experiment was repeated with three different islet isolations.
RESULTS
Evaluation of decomplementation by CVF
After the administration of a single dose of CVF (500 μg/kg i.p.), the C3 concentration in the serum had decreased to below 10% of normal levels and persisted for 3 days, as shown in Fig. 1.
Figure 1.
Efficiency of complement depletion by CVF. A single dose of CVF (500 μg/kg i.p.) achieved a drop in C3 levels below 10% of the control for 3 days (measured by radial immunodiffusion). Complement-mediated cytotoxicity, as measured by a MTT-assay on HB-MVEC, was reduced to levels below 20% of the positive control for 4 days. After 1 week C3 levels regained near-normal values. Results are expressed as mean values of triplicates±SD.
A second administration of CVF 4 days after the first could prolong decomplementation for another 3 days (data not shown). The purified CVF, administered at 500 μg/kg i.p., did not have any obvious toxic effect on treated animals.
Normal mouse serum at a concentration of 25% induced 71% cytotoxicity on HB-MVEC, compared with heat-inactivated FCS. This value was used as a positive control (= 100% cytotoxicity), as shown in Fig. 1. With a single administration of CVF (500 μg/kg i.p.) there was a drop in complement-mediated cytotoxicity to below 20% of the positive control. This persisted for 4 days after CVF administration.
In addition, the C3 levels were also checked and were below 10% of the controls in all grafted mice on the day of transplantation (the CVF1 and CVF2 groups) and the day after the second CVF administration (the CVF2 group) or the first CVF administration on day 4 (the CVF3 group) (data not shown).
Prolonged graft survival in complement-depleted recipients
All diabetic mice became normoglycemic within 3 days after islet transplantation. Compared with control mice, the survival time of grafted islets was significantly prolonged in CVF1 and CVF2 mice (19·7 and 21·3 days versus 8·5 days; each P < 0·01). However, no difference was found between the decomplementized groups, CVF1 and CVF2, as shown in Fig. 2. In contrast, a single CVF administration at day 4 after islet transplantation (the CVF3 group) did not prolong the survival time of grafted islets, suggesting an involvement of complement at early, but not late, stages after XIT.
Figure 2.
Effect of decomplementation by CVF on xeno-islet graft survival in a rat to mouse model. Plasma glucose values above 11 mmol/l on 3 consecutive days were considered as a rejection. MST of grafts in the control group (n = 6) was 8·5 (7–11) days, while in prior to transplantation complement-depleted recipients the MST was prolonged significantly to 19·7 (10–26) days for the CVF1 group (n = 6) and 21·3 (18–30) days for the CVF2 group (n = 6) (both P < 0·01). There was no significant difference between CVF1 and CVF2 groups or between control and CVF3 groups.
Immunohistological changes after decomplementation
Immunohistochemistry was performed on kidneys taken on days 1, 4 and 7 after islet transplantation.
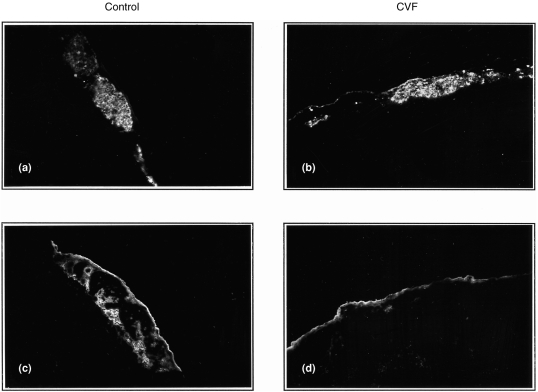
In the control group, IgM deposition was found on grafted islets from day 1 and persisted weakly until day 7, while a more marked C3 deposition was seen on grafted islets at days 1 and 4, and had decreased on day 7 after transplantation. In the CVF1 and CVF2 groups, C3 deposition was significantly reduced (Fig. 3a–d) while the IgM deposition was not affected. Moreover, the difference of C3 deposition between control and CVF1/CVF2 groups became less obvious at day 7 after transplantation, but the insulin staining showed a different pattern. Insulin staining in the control group presented a scattered pattern, while that in the CVF groups was relatively intact (Fig. 4a,b). There was no obvious difference in immunohistology between the CVF1 and CVF2 groups, and between the control and CVF3 groups.
Figure 3.
Effect of decomplementation on C3 deposition on grafted islets. Immunofluorescence staining on xeno-islets at day 1 after transplantation. Insulin and C3 staining in control (a, c) and CVF-treated recipients (b, d) Original magnification×150.
Figure 4.
Effect of decomplementation on leucocyte infiltration. Immunofluorescence staining on xeno-islets at day 7 after transplantation. Staining for insulin, CD4 and CD8 in control (a, c, e) and CVF1 and CVF2 recipients (b, d, f). Original magnification×150.
Further immunohistological analysis of grafted islets revealed a CD4- and CD8-positive leucocyte infiltration beginning at day 4 and becoming more evident on day 7 after transplantation in the control group (Fig. 4c,e) as well as in the CVF3 group. In contrast, CD4- and CD8-positive leucocyte infiltration was absent in the CVF1 and CVF2 groups on day 4 and very weak on day 7 after transplantation (Fig. 4d,f), suggesting an involvement of complement in leucocyte infiltration. The results of immunohistology are summarized in Table 1.
Table 1.
Summary of the immunohistology of xeno-islet transplantation

Complement and IgM binding on isolated xeno-islets
In order to confirm C3 deposition seen in vivo, isolated rat islets were incubated with normal or decomplementized mouse serum, and complement and IgM binding was investigated further in vitro.
By immunofluorescence staining, IgM binding was found only on microvessel-like structures of rat islets (Fig. 5a). In contrast, C3 binding was homogeneously distributed on the islet surface (Fig. 5c), suggesting that C3 deposition on xeno-islets might be IgM independent. After incubation of rat islets with serum from CVF-treated mice, C3 binding on islets was significantly decreased while IgM deposition was unchanged (Fig. 5b,d).
Figure 5.
Effect of decomplementation on IgM and C3 binding on isolated rat islets in vitro. Immunofluorescence staining for IgM and C3 on freshly isolated rat islets after incubation with intact (a, c) or complement-depleted mouse serum (b, d). Original magnification×400.
Complement-mediated cytotoxicity of mouse serum on rat islet cells
Intact serum of untreated mice exerted a strong complement-mediated cytotoxicity on rat islet cells incubated at higher serum concentrations, as measured by the MTT assay. This cytotoxicity was serum-concentration dependent. In vivo decomplementation by CVF could abrogate completely the cytotoxicity of low concentrations of mouse serum (12·5%) against rat islet cells (97·7±6·8 SD versus 78·7±6·5 SD percentage islet cell survival; P < 0·05). Using higher concentrations of mouse serum (50%), in vivo decomplementation by CVF could strongly reduce, but not completely block, cytotoxicity of mouse serum on rat islet cells (54·9±3·4 SD versus 24·9±1·7 SD percentage islet cell survival; P < 0·01). As a control, Sprague–Dawley rat serum, even at higher concentrations, did not have any cytotoxic effect on rat islet cells (Fig. 6).
Figure 6.
Complement-mediated cytotoxicity on isolated rat islet cells. Isolated rat islet cells were incubated with different concentrations of fresh serum and the cytotoxicity was measured by an MTT assay. Intact homologous rat serum was used as a negative control. Results are expressed as mean values of triplicates±SD. *P < 0·05, **P < 0·01 for statistical difference between normal and complement-depleted mouse serum.
DISCUSSION
It is known that activation of complement by xenoreactive IgM is responsible for HAR of solid organs xenotransplanted across discordant species.4 However, little is known about the role of complement in devascularized tissue xenografts, such as pancreatic islets, where HAR does not usually occur. In this study, we show that complement also participates in the rejection of xenografted islets and that decomplementation by CVF prolongs graft survival of xeno-islets in a concordant rat to mouse model.
In our previous and present studies, complement-deposition was found on concordant and discordant xenografted pancreatic islets early after transplantation.7,13 Complement deposition was demonstrated further on isolated rat islets in vitro, and immunohistological patterns suggested an IgM-independent pathway consistent with previous reports.8 In order to evaluate the role of complement in xeno-islet rejection, rat islets were transplanted in complement-depleted mice. Decomplementation was performed by CVF, a constituent of cobra venom with extensive structural homology to mammalian C3. CVF activates complement and leads to complement consumption.14 Decomplementation by CVF has been used widely to investigate the role of complement in HAR.15
With a single injection of CVF (500 μg/kg i.p.), a reduction of 90% in C3 levels and of 80% in bioactivity was obtained and persisted for 3–4 days. Immunohistology showed that decomplementation with CVF significantly reduced complement deposition on grafted islets in vivo and on isolated islets in vitro. These results indicate that CVF is efficient for decomplementation in mice.
Complement depletion by CVF the day before transplantation significantly prolonged graft survival, from a mean survival time (MST) of 8·5 days to 20 days. A single dose of CVF on day 4 did not achieve any prolongation of graft survival. Thus, complement seems to have an impact on early but not late stages of xeno-islet-rejection.
By MTT assay, a cytotoxicity of mouse serum on rat islet cells was demonstrated. This cytotoxicity was serum-concentration dependent and complement mediated, as it was reduced after CVF administration. These results conform with a previous report by Schaapherder et al. showing that complement across species is cytotoxic for isolated islet cells.8
Although there was a significant cytotoxic effect of complement on isolated islet cells in vitro, it was not seen on whole islets either in vitro16 or in vivo. In fact, in spite of a marked complement deposition on grafted islets early after transplantation, insulin staining at days 1 and 4 did not show a different pattern between control and CVF groups.
IgM was only found on microvessels within whole islets, while a significant IgM binding was found on all isolated rat islets cells after incubation with mouse serum by flow cytometric analysis (data not shown). These results may provide an explanation for the difference in complement-mediated cytotoxicity on the isolated islet cells in vitro and on the grafted islets in vivo, i.e. the exposure of antigens by the islet cell isolation procedure may induce IgM binding on the recovered cells, thereby activating complement by the classical pathway.
Although it has been shown that decomplementation by CVF can reduce the incidence of primary non-function in a dog to rat XIT model,17 in our model the prolongation of graft survival could not be explained solely by the abrogation of complement-mediated cytotoxicity on xeno-islets.
A significant T-cell infiltration around grafted islets has been shown by immunohistological studies and flow cytometric analysis.18 There is some evidence that T-cell-derived cytokines exert a cytotoxic effect on islets cells.19 Anti-CD4 pretreatment of recipients significantly prolongs islet survival and in some models induces tolerance.5 In T-cell-deficient nude mice, xeno-islets survive indefinitely.20 These results indicate that a T-cell-mediated or -dependent immune response plays a major role in the rejection of xenografted islets.
Complement can influence different stages of the immune response. The effector functions, such as lysis and opsonization for phagocytosis, are well known but complement is also involved in the control of antigen-specific immune responsiveness.21 In vitro, complement enhances the presentation of antigen to T and B lymphocytes22 and interleukin-2-dependent T-lymphocyte proliferation.23 In vivo, complement plays a role in the primary immune response leading to T-cell activation.24
Our immunohistochemical results show that the CD4- and CD8-positive leucocyte infiltration was significantly delayed in the CVF1 and CVF2 groups compared with control and CVF3 groups, suggesting that the prolongation of xeno-islet survival by CVF might be mediated by interference with leucocyte infiltration. Nevertheless, although delayed, rejection also occurred in CVF1 and CVF2 recipients with a similar pattern, including CD4- and CD8-positive leucocyte infiltration (data not shown). As no significant difference in survival time or immunohistological pattern was found between the CVF1 and CVF2 groups, persisting decomplementation could not further delay or finally prevent leucocyte infiltration and rejection. Due to the redundancy of mechanisms that promote chemo-attraction and activation of cellular responses, it is evident that complement is only one of many factors triggering leucocyte infiltration.
The results of the present study demonstrate that complement is also involved in the immunopathology of rejection of non-primary vascularized xenografted tissue. Complement may participate in the rejection of xeno-islets by direct cytotoxicity and by promoting CD4- and CD8-positive leucocyte infiltration. Efficient decomplementation by CVF significantly prolongs the survival of xenotransplanted islets of Langerhans’ in a concordant rat to mouse model.
Acknowledgments
Tis work was supported by grant no. 32-50865.97 from the Swiss National Science Foundation (to Ph. Morel J. Philippe and J. Lou). We thank Professor Shoso Izui and Professor J¨rg A. Schifferli for their valuable scientific suggestions. We also thank Tuzi Radelgruber, Liliane Fossati, Corinne Sinigaglia, David Matthey-Doret, as well as Raymond Mage for technical assistance.
Abbreviations
- BSA
bovine serum albumin
- CVF
cobra venom factor
- DMEM
Dulbecco’s modified Eagle’s medium
- FITC
fluorescein isothiocyanate
- HAR
hyperacute rejection
- HB-MVEC
human brain microvascular endothelial cells
- HBSS
Hanks’ buffered salt solution
- MST
mean survival time
- MTT
3-(4,5-dimethythiazolyl)- 2.5-diphenyl-2H-tetrazolium-bromide (= thiazolyl blue)
- PBS
phosphate-buffered saline
- XIT
xeno-islet transplantation.
REFERENCES
- 1.Alejandro R, Lehmann R, Ricordi C, et al. Long-term function (6 years) of islet allografts in type 1 diabetes. Diabetes. 1997;46:1983. doi: 10.2337/diab.46.12.1983. [DOI] [PubMed] [Google Scholar]
- 2.Keymeulen B, Ling Z, Gorus F, et al. Implantation of standardized beta-cell grafts in a liver segment of IDDM patients: graft and recipient characteristics in two cases of insulin-independence under maintenance immunosuppression for prior kidney graft. Diabetologia. 1998;41:452. doi: 10.1007/s001250050929. [DOI] [PubMed] [Google Scholar]
- 3.Thomas F. Isolated pancreatic islet xenografting. In: Cooper D, Kemp E, Platt J, White D, editors. Xenotransplantation. Germany: Springer-Verlag, Heidelberg; 1997. p. 545. [Google Scholar]
- 4.Platt JL. Xenotransplantation: recent progress and current perspectives. Curr Opin Immunol. 1996;8:721. doi: 10.1016/s0952-7915(96)80091-4. [DOI] [PubMed] [Google Scholar]
- 5.Marchetti P, Scharp DW, Finke EH, et al. Prolonged survival of discordant porcine islet xenografts. Transplantation. 1996;61:1100. doi: 10.1097/00007890-199604150-00019. [DOI] [PubMed] [Google Scholar]
- 6.Mirenda V, Sigalla J, Fiche M, et al. Pig pancreatic islet xenografts in a B-cell-deficient mouse model. Transplant Proc. 1997;29:762. doi: 10.1016/s0041-1345(96)00472-1. [DOI] [PubMed] [Google Scholar]
- 7.Deng S, Buhler L, Lou J, et al. Study of concordant xenografted islets of Langerhans rejection: humoral or cellular mechanism? Transplant Proc. 1994;26:1184. [PubMed] [Google Scholar]
- 8.Schaapherder AF, Wolvekamp MC, te Bulte MT, Bouwman E, Gooszen HG, Daha MR. Porcine islet cells of Langerhans are destroyed by human complement and not by antibody-dependent cell-mediated mechanisms. Transplantation. 1996;62:29. doi: 10.1097/00007890-199607150-00006. [DOI] [PubMed] [Google Scholar]
- 9.Shapiro AM, Hao E, Rajotte RV, Kneteman NM. High yield of rodent islets with intraductal collagenase and stationary digestion – a comparison with standard technique. Cell Transplant. 1996;5:631. doi: 10.1177/096368979600500606. [DOI] [PubMed] [Google Scholar]
- 10.Mancini G, Carbonara AO, Heremans JF. Immunochemical quantitation of antigens by single radial immunodiffusion. Immunochemistry. 1965;2:235. doi: 10.1016/0019-2791(65)90004-2. [DOI] [PubMed] [Google Scholar]
- 11.Alpert E, Monroe M, Schur PH. A method for increasing the sensitivity of radial-immunodiffusion assay. Lancet. 1970;i:1120. doi: 10.1016/s0140-6736(70)92796-0. [DOI] [PubMed] [Google Scholar]
- 12.Lou J, Ythier A, Burger D, et al. Modulation of soluble and membrane-bound TNF-induced phenotypic and functional changes of human brain microvascular endothelial cells by recombinant TNF binding protein I. J Neuroimmunol. 1997;77:107. doi: 10.1016/s0165-5728(97)00067-2. [DOI] [PubMed] [Google Scholar]
- 13.Buhler L, Deng S, Andereggen E, Bubloz C, Rohner A, Morel P. Comparison of concordant and discordant xenografted islets of Langerhans rejection. Transplant Proc. 1994;26:3453. [PubMed] [Google Scholar]
- 14.Vogel C. Cobra venom factor. In: Roitt I, Delves P, editors. Encyclopedia of Immunology. London, UK: Academic Press; 1992. p. 363. [Google Scholar]
- 15.Leventhal JR, Dalmasso AP, Cromwell JW, et al. Prolongation of cardiac xenograft survival by depletion of complement. Transplantation. 1993;55:857. doi: 10.1097/00007890-199304000-00033. [DOI] [PubMed] [Google Scholar]
- 16.Kin T, Nakajima Y, Kanehiro H, et al. Effect of complement activation in human serum on isolated porcine islets. Cell Transplant. 1996;5:45. doi: 10.1016/0963-6897(96)00038-3. [DOI] [PubMed] [Google Scholar]
- 17.Deng S, Ketchum RJ, Levy MM, et al. Long-term culture or complement inhibition improves early islet function in dog to rat islet xenotransplantation. Transplant Proc. 1996;28:805. [PubMed] [Google Scholar]
- 18.Karlsson-Parra A, Ridderstad A, Wallgren AC, Moller E, Ljunggren HG, Korsgren O. Xenograft rejection of porcine islet-like cell clusters in normal and natural killer cell-depleted mice. Transplantation. 1996;61:1313. doi: 10.1097/00007890-199605150-00005. [DOI] [PubMed] [Google Scholar]
- 19.Rabinovitch A, Sumoski W, Rajotte RV, Warnock GL. Cytotoxic effects of cytokines on human pancreatic islet cells in monolayer culture. J Clin Endocrinol Metab. 1990;71:152. doi: 10.1210/jcem-71-1-152. [DOI] [PubMed] [Google Scholar]
- 20.Krieger NR, Ito H, Fathman CG. Rat pancreatic islet and skin xenograft survival in CD4 and CD8 knockout mice. J Autoimmun. 1997;10:309. doi: 10.1006/jaut.1997.0126. [DOI] [PubMed] [Google Scholar]
- 21.Erdei A, Fust G, Gergely J. The role of C3 in the immune response. Immunol Today. 1991;12:332. doi: 10.1016/0167-5699(91)90011-H. [DOI] [PubMed] [Google Scholar]
- 22.Arvieux J, Yssel H, Colomb MG. Antigen-bound C3b and C4b enhance antigen-presenting cell function in activation of human T-cell clones. Immunology. 1988;65:229. [PMC free article] [PubMed] [Google Scholar]
- 23.Erdei A, Spaeth E, Alsenz J, et al. Role of C3b receptors in the enhancement of interleukin-2-dependent T-cell proliferation. Mol Immunol. 1984;21:1215. doi: 10.1016/0161-5890(84)90013-0. [DOI] [PubMed] [Google Scholar]
- 24.Pratt JR, Hibbs MJ, Laver AJ, Smith RA, Sacks SH. Allograft immune response with sCR1 intervention. Transpl Immunol. 1996;4:72. doi: 10.1016/s0966-3274(96)80041-4. [DOI] [PubMed] [Google Scholar]