Abstract
Both the nitrite and prostaglandin E2 (PGE2) release caused by lipopolysaccharide (LPS) in J774 macrophages are inhibited by SB 203580, a specific p38 mitogen-activated protein kinase (MAPK) inhibitor, in a concentration-dependent manner. The 50% inhibitory concentration (IC50) for nitrite and PGE2 responses was 1 μm and 0·5 μm, respectively. Inhibition was marked following simultaneous treatment with SB 203580 and LPS, and was much reduced when SB 203580 was added 6 hr after LPS treatment. In parallel, LPS induction of inducible NO synthase (iNOS) and cyclo-oxygenase-2 (COX-2) proteins and their steady-state levels of mRNA were reduced by SB 203580. LPS activation of nuclear factor-kappa B (NF-κB), activator protein-1 (AP-1) and p38 MAPK was also inhibited by SB 203580. These results suggest a crucial role of p38 MAPK in regulation of the transcriptional level of endotoxin LPS-induced iNOS and COX-2 protein expression.
INTRODUCTION
p38 mitogen-activated protein kinase (MAPK) is a member of a family of enzymes activated by the dual phosphorylation of threonyl and tyrosyl residues separated by a single amino acid.1–3 The gene for p38 MAPK was originally cloned from Saccharomyces cerevisiae (HOG1)4 but homologues were subsequently found in Xenopus,5 murine (p38)1 and human (CSBP1 and CSBP2) tissues.2 In view of its conservation and wide distribution, it would appear that p38 serves an important function in cellular responses. This kinase is activated by stressful stimuli (e.g. high osmolarity, H2O2, ultraviolet light and shock),4 endotoxin,6,7 inflammatory cytokines, interleukin-1 (IL-1),8,9 tumour necrosis factor-α (TNF-α)3,10 and thrombin.11 p38 MAPK may help cells resist thermal stress12 and may be involved in the synthesis of IL-1, TNF-α,2,13 interleukin-8 (IL-8),14,15 interleukin-6 (IL-6),9,16 cyclo-oxygenase-2 (COX-2),8,9 collagenase-19 and stromelysin-19 in different cell types. Moreover, it provides a crucial signal for platelet aggregation,17 neutrophil chemotaxis,7,18, cytosolic phospholipase A2 (cPLA2) activation,19 E-selectin expression,10 cytosolic alkalinization20 and cell apoptosis.21,22 More recently, this kinase has been identified in macrophages,2 but its regulation and physiological role in these cells have not been clearly explored.
Different regulatory actions of p38 MAPK on protein synthesis, both transcription and translation, have been proposed. For example, in human fibroblasts and umbilical vein endothelial cells stimulated by IL-1, p38 MAPK plays an important role in the regulation of the transcription of COX-2, collagenase-1 and stromelysin-1 genes, while it regulates IL-6 production at the translational level.9 In contrast, in L929 cells, it modulates TNF-induced IL-6 synthesis at the transcriptional level.16 In monocytes, p38 MAPK is also required for the lipopolysaccharide (LPS)-induced synthesis of IL-1 and TNF-α and acts at the translational level.1,2,23 In contrast to its general up-regulatory effect for several proteins in a variety of cells, as mentioned above, p38 MAPK has also been suggested to down-regulate IL-1-induced inducible NO synthase (iNOS) mRNA expression and nitric oxide (NO) biosynthesis in glomerular mesangial cells.8
To date, the roles of p38 MAPK in endotoxin-elicited iNOS and COX-2 induction have not been clearly identified, particularly in macrophages, the major source of NO and eicosanoids during inflammation. Although LPS can stimulate p38 MAPK and induce iNOS and COX-2 expression in a variety of cell types, including macrophages,1,6 the causal relationship between these two events is still unclear. Thus, the aim of this study was to determine the role of p38 MAPK activation, either at the transcriptional or translational level, in LPS-induced iNOS and COX-2 expression and NO and prostaglandin E2 (PGE2) production.
MATERIALS AND METHODS
Reagents
Rabbit and mouse antibodies specific for iNOS and COX-2 were purchased from Transduction Laboratories (Lexington, KY). Rabbit antibody specific for p38 MAPK was purchased from Santa Cruz Biotechnology (Santa Cruz, CA). iNOS and COX-2 cDNA and the enzyme immunoassay (EIA) kit for PGE2 assay were obtained from Cayman Chemical (Ann Arbor, MI). The sequences of the double-stranded oligonucleotides (the binding sites are underlined) used to detect the DNA-binding activities of nuclear factor-kappa B (NF-κB) and activator protein-1 (AP-1) were, respectively, 5′GAT-CAG-TTG-AGG-GGA-CTT-TCC-CAG-GC3′ and 5′GAT-CCG-CTT-GAT-GAC-TCA-GCC-GGA-A3′. RNAzol was obtained from Tel-Test Inc. (Friendswood, TX). Dulbecco’s modified Eagle’s medium (DMEM), fetal bovine serum, penicillin and streptomycin were obtained from Gibco BRL (Grand Island, NY). [α-32P]ATP (3000 Ci/mmol), [γ-32P]ATP (5000 Ci/mmol), horseradish peroxidase-coupled second antibody, and the enhanced chemiluminescence (ECL) detection agent were purchased from Amersham International (Arlington Heights, IL). SB 203580 was obtained from Calbiochem (La Jolla, CA). All other chemicals were obtained from Sigma (St Louis, MO). Protein A–Sepharose beads were purchased from Transduction Laboratories (Lexington, KY).
Activation of J774 cells
The mouse macrophage cell line, J774, obtained from ATCC (Rockville, MD), was cultured, as described previously,24 in DMEM containing 10% fetal bovine serum and antibiotics (100 U/ml of penicillin and 100 μg/ml of streptomycin). Cells in 0·5 ml of DMEM were seeded into 24-well plates and, at confluence, 0·5 ml of fresh culture medium, with or without drugs, was added to each well to activate the cells. After 24 hr (unless otherwise indicated) the medium was collected for nitrite and PGE2 assays. As described below, for the electrophoretic mobility shift assay (EMSA), immunoblot and RNA-blot analysis, cells were grown in 10-cm Petri dishes.
Nitrite production and PGE2 assay
Nitrite was measured by adding 100 μl of Griess reagent (1% sulphanilamide and 0·1% naphthylethylenediamide in 5% phosphoric acid) to 100-μl samples of culture medium. The optical density at 550 nm (OD550) was measured using a microplate reader, and the nitrite concentration calculated by comparison with the OD550 produced using standard solutions of sodium nitrite in culture medium. PGE2 was measured using an EIA kit from Cayman, following the manufacturer’s instructions.
Immunoblot analysis of iNOS and COX-2
After drug treatment, cells were washed twice in ice-cold phosphate-buffered saline (PBS), then solubilized in 20 mm Tris–HCl, 0·5 mm EGTA, 2 mm EDTA, 2 mm DDT, 0·5 mm p-methylsulphonyl fluoride and 10 μg/ml of leupeptin, pH 7·5. Samples of equal amounts of protein (50–100 μg) were applied to 7·5% (for iNOS) and 9% (for COX-2) sodium dodecyl sulphate–polyarcrylamide gel electrophoresis (SDS–PAGE) gels, and electrophoresed under reducing conditions. The separated proteins were transferred onto a nitrocellulose membrane, incubated in TBST buffer (150 mm NaCl, 20 mm Tris, 0·02% Tween-20, pH 7·4) containing 5% milk, and then probed with antibodies specific for iNOS or COX-2, followed by a second antibody. Immunoreactivity was detected by ECL, following the manufacturer’s instructions.
RNA blotting
Confluent cells, grown in 10-cm Petri dishes, were treated with pharmacological agents for different periods and harvested. Equal amounts (about 50 μg) of total RNA, purified using RNAzol reagent, were applied to each lane of 1·2% (w/v) formaldehyde-agarose gels, electrophoresed, and transferred to Immobilon-N membranes (Amersham Pharmacia Biotech, Uppsala, Sweden). After ultraviolet cross-linking and prehybridization for 2 hr at 42°, the membranes were probed for 16–24 hr with iNOS or COX-2 cDNA probes labelled with [α-32P]dATP by random primer (c. 2×108 c.p.m./μg). Hybridization reactions were performed in 50% formamide, 5×SSPE, 10×Denhardt’s solution, 0·5% SDS and 0·1 mg/ml of salmon sperm DNA. The membranes were washed twice in 2×SSC, 0·1% SDS at room temperature for 20 min, then twice in 0·1×SSC, 0·1% SDS at 65° for 30 min before exposure to film, using intensifying screens. Densitometric analyses were performed on a Molecular Dynamics (Sunnyvale, CA) densitometer.
Preparation of nuclear extracts and EMSA
Nuclear extracts from stimulated or unstimulated cells were prepared by lysing the cells in 100 μl of 10 mm HEPES, pH 7·9, 1·5 mm MgCl2, 10 mm KCl, 0·5 mm DTT, 0·2 mm PMSF, followed by vigorous vortexing for 15 seconds, standing at 4° for 10 min, and centrifugation at 2700 g for 2 min. The pelleted nuclei were resuspended in 30 μl of 20 mm HEPES, pH 7·9, 25% glycerol, 420 mm NaCl, 1·5 mm MgCl2, 0·2 mm EDTA, 0·5 mm DTT, 0·2 mm PMSF for 20 min on ice, then the lysates were centrifuged at 20 000 g for 3 min. For the EMSA, reaction mixtures (15 μl) contained 0·25 μg of poly(dI-dC) (Amersham Pharmacia Biotech, Uppsala, Sweden) and 20 000 d.p.m. of 32P-labelled DNA probe in binding buffer consisting of 10 mm Tris, pH 7·5, 1 mm EDTA, 4% Ficoll, 1 mm DTT and 75 mm KCl; the binding reaction was started by the addition of cell extracts and continued for 30 min. Samples were analysed on native 5% polyacrylamide gels.
p38 MAPK assay
Equal amounts of protein (about 1500 μg) from cytosolic lysates prepared in immunoprecipitation buffer (20 mm Tris, pH 7·5, 1 mm MgCl2, 125 mm NaCl, 1% Triton-X-100, 1 mm PMSF, 10 μg/ml of leupeptin, 10 μg/ml of aprotinin, 50 mm NaF, 25 mm β-glycerophosphate, 100 mm Na3VO4) were incubated with anti-p38 MAPK antibody and protein A–Sepharose beads overnight at 4°, then the beads were washed three times with 1 ml of ice-cold immunoprecipitation buffer and the bound immune complex assayed for p38 kinase activity at 30° for 30 min in 20 μl of kinase reaction buffer [25 mm HEPES, pH 7·4, 20 mm MgCl2, 0·1 mm Na3VO4, 2 mm DTT, 50 μg/ml of myelin basic protein (MBP), 100 μm ATP and 10 μCi [γ-32P]ATP]. The reaction was terminated with 5 μl 5×Laemmli sample buffer, the products resolved by 15% SDS–PAGE gel electrophoresis, and the phosphorylated MBP visualized by autoradiography. A PhosphorImager (Molecular Dynamics) was used to quantify band intensity.
Statistical evaluation
Values are expressed as the mean ± standard error of the mean (SEM) of at least three experiments. The Student’s t-test was used to assess the statistical significance of the differences; P-values less than 0·05 were considered statistically significant.
RESULTS
Inhibition of LPS-induced NO and PGE2 formation by SB 203580
Incubation of cultured J774 macrophages with LPS (1 μg/ml) for 24 hr caused increases in nitrite and PGE2 accumulation in the cell culture medium from 8±2 μm to 40±5 μm (n = 5) and from 250±27 pg/ml to 2250±370 pg/ml (n = 3), respectively. On simultaneous co-incubation with SB 203580, both responses were inhibited by SB 203580 in a concentration-dependent manner over the range of 0·1–10 μm, the 50% inhibitory concentration (IC50) for the nitrite and PGE2 responses being 1·0±0·2 μm and 0·5±0·2 μm, respectively (Fig. 1).
Figure 1.
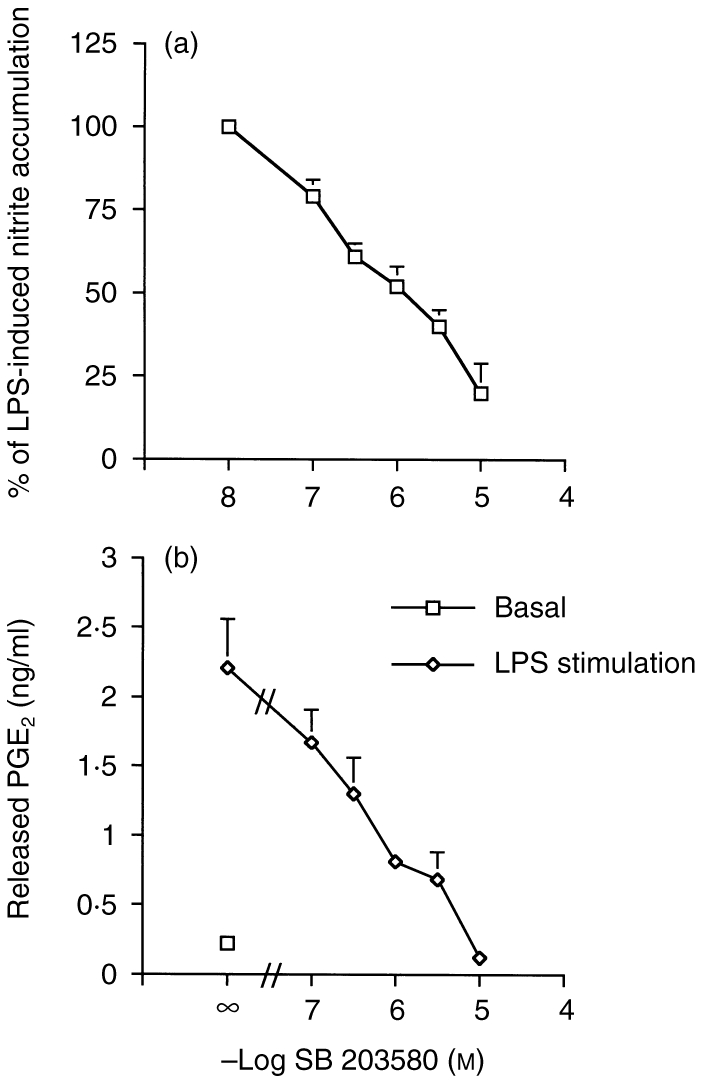
Concentration-dependent inhibition of LPS-induced nitrite and PGE2 formation by SB 203580. Cells were incubated with 1 μg/ml LPS in the presence or absence of SB 203580, at the concentrations indicated, for 24 hr before assaying for nitrite (a) or PGE2 (b). The data represents the mean ± SEM of three experiments performed in duplicate.
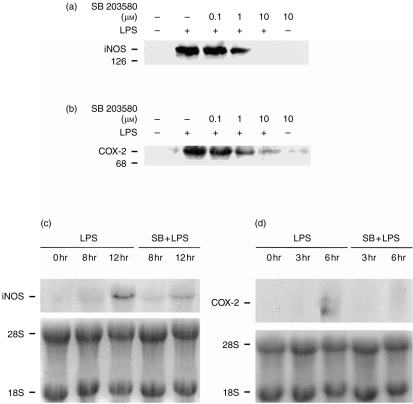
Inhibition of LPS-induced iNOS and COX-2 expression by SB 20358
As shown in Fig. 2a,b, co-incubation of SB 203580 (0·1–10 μm) with 1 μg/ml of LPS inhibited the LPS-mediated induction of iNOS and COX-2 proteins in a concentration-dependent manner. Northern blot analysis of the steady-state levels of iNOS and COX-2 mRNA showed that the induction of iNOS and COX-2 mRNA after LPS treatment for, respectively, 12 and 6 hr was clearly inhibited by the simultaneous addition of SB 203580 (3 μm) (Fig. 2c,d). These results suggest an essential role for p38 MAPK at the transcriptional level of iNOS and COX-2 induction.
Figure 2.
Concentration-dependent inhibition of LPS-induced iNOS and COX-2 expression by SB 203580. iNOS (a) and COX-2 (b) proteins were detected on Western blots by ECL after treatment of the cells with LPS (1 μg/ml) and/or SB 203580 (0·1–10 μm) for 24 hr. Northern blot analysis for iNOS (c) and COX-2 (d) mRNA expression after LPS (1 μg/ml) and SB 203580 (3 μm) co-stimulation is indicated. The example is representative of three independent experiments.
Transcriptional inhibition by SB 203580
To confirm the transcriptional role of p38 MAPK further, we added SB 203580 (3 μm) at different intervals following treatment with LPS. As shown in Fig. 3, the inhibitory effects of SB 203580 on nitrite (Fig. 3a) and PGE2 (Fig. 3b) formation decreased with time after delayed addition of SB 203580.
Figure 3.
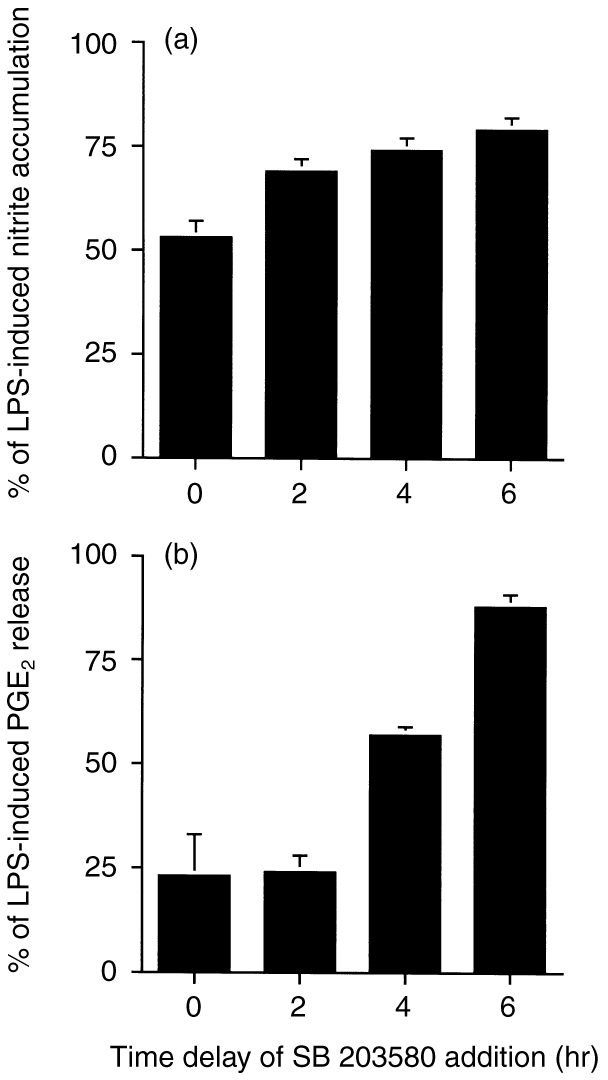
Time-dependent effects of SB 203580 on LPS-induced nitrite and PGE2 production. SB 203580 (3 μm) was added to the cell cultures at the same time as, or 2, 4 or 6 hr after, LPS (1 μg/ml). Twenty-four hours after LPS addition, nitrite (a) and PGE2 (b) production in the medium was determined. The data represents the mean±SEM of three experiments.
Because activation of transcription factors NF-κB and AP-1 has been shown previously to be indispensable for induction of iNOS and COX-2 gene activation by various stimuli,25,26 we explored the possible role of p38 MAPK in the activation of NF-κB and AP-1. Direct EMSA measurement of the activation of transcription factors revealed that the activation of NF-κB and AP-1 by LPS (1 μg/ml) was abolished by co-addition of SB 203580 (10 μm) (Fig. 4a,b).
Figure 4.
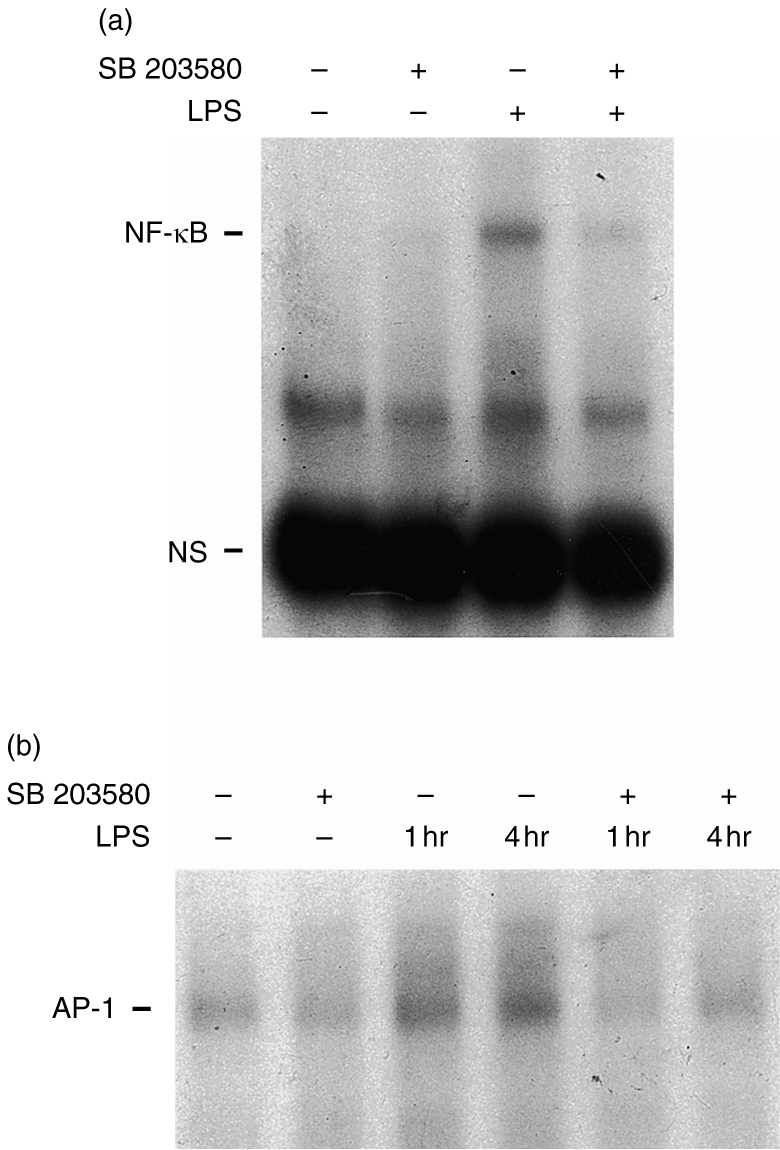
Effects of SB 203580 on LPS-induced transactivation of NF-κB and AP-1. Cells were either untreated or preincubated with SB 203580 (10 μm) for 20 min, then incubated with or without, LPS (1 μg/ml) for 1 or 4 hr. NF-κB activation at 1 hr (a) and AP-1 activation at either 1 or 4 hr (b) were determined by EMSA. Typical traces, representative of three experiments, are shown.
p38 MAPK activation by LPS
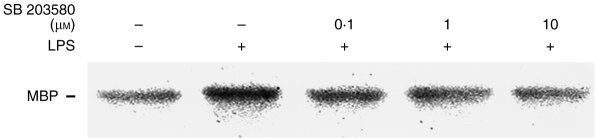
To determine whether p38 MAPK was directly responsive to LPS, J774 cells were treated with LPS (1 μg/ml) for 5 min in the presence or absence of SB 203580 and the cytosolic lysates analysed by the immune complex kinase assay. As shown in Fig. 5, LPS was able to increase p38 MAPK activity by 61±7% (n = 3) at 5 min, and this effect was inhibited in a concentration-dependent manner by SB 203580.
Figure 5.
Inhibition of LPS-induced p38 MAPK activation by SB 203580. J774 cells were pretreated with vehicle or SB 203580 at the concentrations indicated; they were then treated with vehicle or 1 μg/ml LPS for 5 min, extracted and subjected to the immune complex kinase assay using anti-p38 antibody. p38 activity was measured by phosphorylation of MBP as described in the Materials and Methods.
DISCUSSION
Using a class of pyridinyl imidazoles, including SB 203580,13 that are highly specific p38 MAPK inhibitors, evidence has been accumulated that suggests p38 MAPK activation may be essential for some cellular responses associated with acute or chronic inflammation. In the present study, the role of p38 MAPK in LPS-elicited iNOS and COX-2 induction, leading to the generation of NO and PGE2, was investigated in murine J774 macrophages.
From the results, several conclusions can be drawn regarding the involvement of p38 MAPK in the transcriptional regulation of macrophage iNOS and COX-2 induction by LPS. Firstly, SB 203580 over the range of 0·1–10 μm potently attenuated the induction of the iNOS and COX-2 proteins, and this was related to its ability to inhibit p38 MAPK activity. Secondly, SB 203580 almost completely inhibited nitrite and PGE2 formation when given together with LPS, but the inhibition diminished when the inhibitor was added 2–4 hr after stimulus induction, suggesting a role for p38 MAPK in the transcriptional processes of iNOS and COX-2 induction. To assess this possibility further, the effects of SB 203580 on the activation of the transcription factors NF-κB and AP-1, which act, respectively, on the two key promoter regions of iNOS and COX-2 and are responsible for initiation of iNOS and COX-2 mRNA transcription in response to LPS,25–28 were studied; the fact that the LPS-induced DNA binding of both NF-κB and AP-1 was attenuated by SB 203580 indicates a transcriptional role of p38 MAPK in iNOS and COX-2 induction. Thirdly, consistent with the ability of LPS to activate p38 MAPK in other cells, such as human neutrophils,7 B cells,6 murine RAW 264.7 macrophages,6 HeLa, Chinese hamster ovary and transfected COS-1 cell lines,3 LPS increased SB 203580-susceptible p38 MAPK activity in J774 macrophages.
In this study, we show, for the first time, that SB 203580 can inhibit LPS-mediated NF-κB and AP-1 activation, suggesting a crucial role for a p38-dependent signalling mechanism upstream of the activation of both transcription factors. These findings are in contrast to those seen for TNF-α, the action of which, on NF-κB activation in L929 cells, was not modulated by 10 μm SB 203580.6 Thus, it appears that multiple signalling cascades converge to elicit NF-κB activation, depending on the stimuli and/or cell systems studied, and further studies are needed to link the action of p38 MAPK to NF-κB and AP-1 activation.
Taken together, our data suggest that the p38 MAPK– NF-κB/AP-1–iNOS/COX-2 cascade is an important signalling pathway involved in the regulation of nitric oxide and prostaglandin biosynthesis by bacterial endotoxin.
Acknowledgments
This work was supproted by a grant from the National Science Council, Taiwan (NSC 88-2314-B002-28).
Abbreviations
- AP-1
activator protein-1
- COX-2
cyclo-oxygenase-2
- cPLA2
cytosolic phospholipase A2
- DMEM
Dulbecco's modified Eagle's medium
- EMSA
electrophoretic mobility shift assay
- IL-1
interleukin-1
- IL-6
interleukin-6
- IL-8
interleukin-8
- iNOS
inducible NO synthase
- LPS
lipopolysaccharide
- MAPK
mitogen-activated protein kinase
- MBP
myelin basic protein
- NF-κB
nuclear factor-kappa B
- NO
nitric oxide
- PGE2
prostaglandin E2
- TNF-α
tumour necrosis factor-α.
REFERENCES
- 1.Han J, Lee JD, Bibb L, Uievitch RJ. A MAP kinase targeted by endotoxin and hyperosmolarity in mammalian cells. Science. 1994;265:808. doi: 10.1126/science.7914033. [DOI] [PubMed] [Google Scholar]
- 2.Lee JC, Layton JT, McDonnell PC, et al. A protein kinase involved in the regulation of inflammatory cytokine biosynthesis. Nature. 1994;372:739. doi: 10.1038/372739a0. [DOI] [PubMed] [Google Scholar]
- 3.Raingeaud J, Gupta S, Rogers J, et al. Pro-inflammatory cytokines and environmental stress cause p38 mitogen-activated protein kinase activation by dual phosphorylation on tyrosine and threonine. J Biol Chem. 1995;270:7420. doi: 10.1074/jbc.270.13.7420. [DOI] [PubMed] [Google Scholar]
- 4.Brewster JL, De Valoir T, Dyer ND, Winter E, Gustin MC. An osmosensing signal transduction pathway in yeast. Science. 1993;259:1760. doi: 10.1126/science.7681220. [DOI] [PubMed] [Google Scholar]
- 5.Rouse J, Cohen P, Trigon S, et al. A novel kinase cascade triggered by stress and heat shock that stimulates MAPKAP kinase-2 and phosphorylation of the small heat shock proteins. Cell. 1994;78:1027. doi: 10.1016/0092-8674(94)90277-1. [DOI] [PubMed] [Google Scholar]
- 6.Han J, Lee JD, Tobias PS, Ulevitch RJ. Endotoxin induced rapid protein tyrosine phosphorylation in 70Z/3 cells expressing CD14. J Biol Chem. 1993;268:25009. [PubMed] [Google Scholar]
- 7.Nick JA, Avdi NJ, Gerwins P, Johnson GL, Worthen GS. Activation of a p38 mitogen-activated protein kinase in human neutrophils by lipopolysaccharide. J Immunol. 1996;156:4867. [PubMed] [Google Scholar]
- 8.Guan Z, Baier LD, Morrison AR. p38 mitogen-activated protein kinase down-regulates nitric oxide and up-regulates prostaglandin E2 biosynthesis stimulated by interleukin-1β. J Biol Chem. 1997;272:8083. doi: 10.1074/jbc.272.12.8083. [DOI] [PubMed] [Google Scholar]
- 9.Ridley SH, Sarsfield SJ, Lee JC, et al. Actions of IL-1 are selectively controlled by p38 mitogen-activated protein kinase. Regulation of prostaglandin H synthase 2, metalloproteinase, and IL-6 at different cells. J Immunol. 1997;158:3165. [PubMed] [Google Scholar]
- 10.Read MA, Whitley MZ, Gupta S, et al. Tumor necrosis factor α-induced E-selectin expression is activated by the nuclear factor-κ B and c-JUN N-terminal kinase/p38 mitogen-activated protein kinase pathways. J Biol Chem. 1997;272:2753. doi: 10.1074/jbc.272.5.2753. [DOI] [PubMed] [Google Scholar]
- 11.Kramer RM, Roberts EF, Um SL, et al. p38 Mitogen-activated protein kinase phosphorylated cytosolic phospholipase A2 (cPLA2) in thrombin-stimulated platelets. Evidence that proline-directed phosphorylation is not required for mobilization of arachidonic acid by cPLA2. J Biol Chem. 1996;271:27723. doi: 10.1074/jbc.271.44.27723. [DOI] [PubMed] [Google Scholar]
- 12.Knauf U, Jakob U, Engel K, Buchner J, Gaestel M. Stress- and mitogen-induced phosphorylation of the small heat shock protein Hsp 25 by MAPKAP kinase 2 is not essential for chaperone properties and cellular thermoresistance. EMBO J. 1995;13:54. doi: 10.1002/j.1460-2075.1994.tb06234.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cuenda A, Rouse J, Doza Y, et al. SB 203580 is a specific inhibitor of a MAP kinase homologue which is stimulated by cellular stress and interleukin-1. FEBS Lett. 1995;364:229. doi: 10.1016/0014-5793(95)00357-f. [DOI] [PubMed] [Google Scholar]
- 14.Shapiro L, Dinarello CA. Osmotic regulation of cytokine synthesis in vitro. Proc Natl Acad Sci USA. 1995;92:12230. doi: 10.1073/pnas.92.26.12230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee JC, Badger AM, Grisword DE, et al. Bicyclic imidazoles as a novel class of cytokine biosynthesis inhibitors. Ann NY Acad Sci. 1993;696:149. doi: 10.1111/j.1749-6632.1993.tb17149.x. [DOI] [PubMed] [Google Scholar]
- 16.Beyaert R, Cuenda A, Berghe W, et al. The p38/RK mitogen-activated protein kinase pathway regulates interleukin-6 synthesis in response to tumour necrosis factor. EMBO J. 1996;15:1914. [PMC free article] [PubMed] [Google Scholar]
- 17.Saklatvala J, Rawlinson L, Waller RJ, et al. Role for p38 mitogen-activated protein kinase in platelet aggregation caused by collagen or a thromboxane analogue. J Biol Chem. 1996;271:6586. doi: 10.1074/jbc.271.12.6586. [DOI] [PubMed] [Google Scholar]
- 18.Krump E, Sanghera JS, Pelech SL, Furuya WZ, Grinstein S. Chemotactic peptide N-formyl-Met-Leu-Phe activation of p38 mitogen-activated protein kinase (MAPK) and MAPK-activated protein kinase-2 in human neutrophils. J Biol Chem. 1997;272:937. doi: 10.1074/jbc.272.2.937. [DOI] [PubMed] [Google Scholar]
- 19.Waterman WH, Molski TF.P, Huang CK, Adams JL, Sha'afi RI. Tumour necrosis factor-α induced phosphorylation and activation of cytosolic phospholipase A2 are abrogated by an inhibitor of the p38 mitogen-activated protein kinase cascade in human neutrophils. Biochem J. 1996;319:17. doi: 10.1042/bj3190017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shrode LD, Rubie EA, Woodgett JR, Grinstein S. Cytosolic alkalinization increases stress-activated protein kinase/c-Jun NH2-terminal kinase (SAPK/JNK) activity and p38 mitogen-activated protein kinase activity by a calcium-independent mechanism. J Biol Chem. 1997;272:13653. doi: 10.1074/jbc.272.21.13653. [DOI] [PubMed] [Google Scholar]
- 21.Xia Z, Dickens M, Raingeaud J, Davis RJ, Greenberg ME. Opposing effects of ERK and JNK-p38 MAP kinases on apoptosis. Science. 1995;270:1326. doi: 10.1126/science.270.5240.1326. [DOI] [PubMed] [Google Scholar]
- 22.Polverino AJ, Patterson SD. Selective activation of caspases during apoptotic induction in HL-60 cells. Effect of a tetrapeptide inhibitor. J Biol Chem. 1997;272:7013. doi: 10.1074/jbc.272.11.7013. [DOI] [PubMed] [Google Scholar]
- 23.Prichett W, Hand A, Sheilds J, Dunnington D. Mechanism of activation of bicyclic imidazoles defines a translational regulatory pathway for tumor necrosis factor α. J Inflamm. 1995;45:97. [PubMed] [Google Scholar]
- 24.Lin WW, Chen BC. Involvement of protein kinase C in the UTP-mediated potentiation of cyclic AMP accumulation in mouse J774 macrophages. Br J Pharmacol. 1997;121:1749. doi: 10.1038/sj.bjp.0701300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Appleby SB, Ristimaki A, Neilson K, Narko K, Hla T. Structure of the human cyclo-oxygenase-2 gene. Biochem J. 1994;302:723. doi: 10.1042/bj3020723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xie QW, Whisnant R, Nahtan C. Promoter of the mouse gene encoding calcium-independent nitric oxide synthase confers inducibility by interferon γ and bacterial lipopolysaccharide. J Exp Med. 1993;177:1779. doi: 10.1084/jem.177.6.1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lowenstein CJ, Alley EW, Raval P, et al. Macrophage nitric oxide synthase gene: two upstream regions mediate induction by interferon γ and lipopolysaccharide. Proc Natl Acad Sci USA. 1993;90:9730. doi: 10.1073/pnas.90.20.9730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Newton R, Kuitert LM, Bergmann M, Adcock IM, Barnes PJ. Evidence for involvement of NF-κB in the transcriptional control of COX-2 gene expression by IL-1β. Biochem Biophys Res Commun. 1997;237:28. doi: 10.1006/bbrc.1997.7064. [DOI] [PubMed] [Google Scholar]