Abstract
The aim of the present study was to investigate the role of the adhesion pathway α4 integrins/vascular cell adhesion molecule type 1 (VCAM-1) in rapid eosinophil accumulation induced by the chemoattractants PAF and LTB4. For this purpose we have used an in vivo model of local 111In-eosinophil accumulation to quantify eosinophil accumulation induced by intradermal administration of platelet-activating factor (PAF) and leukotriene B4 (LTB4) in rats. Initial experiments carried out over 4 hr demonstrated that intravenous administration of an anti-VCAM-1 monoclonal antibody (mAb; 5F10) or an anti-α4 integrin mAb (TA2) caused a significant reduction in PAF- or LTB4-induced 111In-labelled eosinophil accumulation. Time–course experiments demonstrated that the anti-VCAM-1 mAb was effective at suppressing early phases of the 111In-labelled eosinophil accumulation induced by PAF and LTB4 (e.g. within the first 60 min). In contrast, 111In-labelled eosinophil accumulation induced by these chemoattractants was unaffected by the local administration of the transcriptional inhibitor actinomycin D, suggesting a role for basally expressed VCAM-1. Indeed, basal expression of VCAM-1 in rat skin sites was demonstrated by the localization of intravenously administered radiolabelled mAb. The localization of the radiolabelled antibody was not altered in skin sites injected with PAF or LTB4. Finally, the inhibitory effects seen with the anti-VCAM-1 mAb were enhanced when the antibody was co-injected into rats with an anti-intercellular adhesion molecule-1 (ICAM-1) mAb (1A29). The combination of these two mAb also caused a significant inhibition of PAF-induced oedema, as quantified by the local accumulation of 125I-labelled human serum albumin. The results indicate a role for α4 integrins/VCAM-1 and ICAM-1, in PAF- and LTB4-induced eosinophil accumulation in vivo and suggest that basally expressed VCAM-1 may have a functional role in rapid accumulation of eosinophils induced by chemoattractants.
INTRODUCTION
Infiltration of eosinophils into inflamed tissues is a characteristic feature of allergic inflammation and contributes to the pathogenesis of disease states such as asthma. This accumulation, and to some extent the activation of the eosinophils at sites of inflammation, are mediated by the interaction of cell surface adhesion molecules expressed on the eosinophils with their counter ligands expressed on vascular endothelial cells and extracellular matrix components.1 In this context, there is much evidence for the involvement of the α4 integrins in eosinophil accumulation and activation.2 The α4 integrins, α4β1 (very late activation antigen-4; VLA-4) and α4β7 are both expressed on eosinophils and share the endothelial cell ligand vascular cell adhesion molecule-1 (VCAM-1) and an alternatively spliced form of the extracellular matrix protein fibronectin. α4β7 also interacts with the mucosal addressin MadCAM-1. Neutralizing anti-α4 integrin monoclonal antibodies (mAb) have been shown to inhibit eosinophil accumulation in a number of allergic and non-allergic animal models of inflammation.2–6
VCAM-1 and intercellular adhesion molecule-1 (ICAM-1), two members of the immunoglobulin-like family of cell surface proteins, are expressed on vascular endothelial cells and interact with leucocyte α4 (as described above) and β2 integrins. In vitro, anti-ICAM-1 mAb block neutrophil, eosinophil and basophil adherence to cytokine-activated vascular endothelium7 whilst anti-VCAM-1 antibodies are more selective in their action in that they effectively inhibit eosinophil but not neutrophil adherence.7,8 In vivo, anti-ICAM-1 antibodies suppress neutrophil accumulation and neutrophil-dependent tissue injury in a number of animal models, such as in rat models of ischaemia/reperfusion injury9 and complement-induced lung injury.10 Anti-ICAM-1 mAb have also been shown to inhibit eosinophil accumulation and airway hyperresponsiveness in a primate model of asthma11 and have been tested in several rat and mouse models of asthma (for review see ref. 12). In the majority of the latter studies, anti-ICAM-1 mAb, when administered alone, had little or no effect on eosinophil recruitment whilst exerting some inhibitory effect on airway hyperreactivity. In contrast to the increasing number of studies with anti-ICAM-1 mAb, there have been very few in vivo studies with anti-VCAM-1 reagents. In this context, in an in vivo model of eosinophil recruitment to mouse trachea, an anti-VCAM-1 but not an anti-ICAM-1 mAb prevented antigen-induced eosinophil infiltration.13 Further, we have previously shown that IL-4-induced eosinophil accumulation into rat skin is inhibited by an anti-VCAM-1 but not an anti-ICAM-1 mAb.6 In these studies, both of which involved 24 hr in vivo test periods, it was considered that anti-VCAM-1 mAb were blocking the function of induced VCAM-1 on vascular endothelial cells. Indeed, based on in vitro and some in vivo studies it is generally considered that there is little or no functionally active basally expressed VCAM-1 on endothelial cells.14–16 To address this point in the rat dermal vasculature, we have investigated the roles of α4 integrins and VCAM-1 in eosinophil accumulation induced by the chemoattractants platelet-activating factor (PAF) and leukotriene B4 (LTB4) in rat skin and have determined the role of VCAM-1 in early phases of these responses. Both PAF and LTB4 have previously been shown to induce chemotaxis of rat eosinophils,17 thus demonstrating their ability to stimulate migration of rat leucocytes directly. The findings reported in the present study show that whilst the eosinophil accumulation elicited by PAF or LTB4 is not susceptible to the RNA synthesis inhibitor actinomycin D, it is significantly inhibited by an anti-VCAM-1 mAb, suggesting a role for basally expressed VCAM-1 in chemoattractant-induced eosinophil accumulation in rat skin. In support of this hypothesis, we provide direct evidence for the presence of a basal level of VCAM-1 in rat skin sites as quantified by the localization of intravenously administered radiolabelled anti-VCAM-1 antibody.
MATERIALS AND METHODS
Animals
Male Sprague-Dawley cell donor rats (400–500 g) and male Sprague-Dawley test rats (200–300 g) were purchased from Harlan-Olac, Oxon, UK.
Materials
Pentobarbitone sodium (Sagatal, 60 mg/ml) was purchased from May and Baker, Dagenham, Essex, UK. Hypnorm (0·315 mg/ml fentanyl citrate and 10 mg/ml fluanisone) was purchased from Janssen Pharmaceutical Ltd, Grove, Oxford, UK. Hypnovel (5 mg/ml midazolam hydrochloride) was purchased from Roche Products Ltd, Welwyn Garden City, UK. 111indium chloride (111InCl3; 10 mCi/ml in pyrogen-free 0·04 m hydrochloric acid), 99mtechnetium sodium pertechnetate (99mTc; 90 mCi/ml in pyrogen-free saline), 125iodine (125I; 101·8 mCi/ml in dilute sodium hydroxide solution, pH 7–11) and 125I-labelled human serum albumin (125I-HSA; 20 mg albumin per ml of sterile isotonic saline, 50 μCi/ml) were purchased from Amersham International, Amersham, Bucks, UK. Actinomycin D, bovine serum albumin (BSA) and 2-mercaptopyridine-N-oxide were purchased from Sigma Chemical Company, Dorset, UK. Horse serum, sterile Hanks’ balanced salt solution (HBSS 10×), HEPES (1 m) and Tyrode’s salt solution were purchased from Gibco Ltd, Paisley, Renfrewshire, UK. Percoll was purchased from Pharmacia Fine Chemicals, Uppsala, Sweden. Pyrogen- and preservative-free heparin sodium (5000 U/ml) was purchased from Pabyrn Laboratories, Greenford, Middlesex, UK. LTB4 was purchased from Cascade Biochemical Ltd, Reading, Berkshire, UK. PAF was purchased from Bachem, Bubendorf, Switzerland. Vectorstain ABC kit and fast red substrate were purchased from Vector, Peterborough, UK. Haematoxylin and OCT compound were purchased from BDH, Essex, UK.
The monoclonal antibodies
The anti-rat ICAM-1 mAb [1A29; immunoglobulin G1 (IgG1)] was produced by immunization of mice with endothelial cells from the high venules of rat lymph nodes as previously described.18 Anti-rat VCAM-1 mAb (5F10, IgG2a) was produced by immunization of mice with COS transfectants expressing rat VCAM-1 as previously described.5 Anti-rat α4 integrins mAb (TA2, IgG1k) was purchased from Endogen Inc., Cambridge, MA. Monoclonal antibodies MOPC-21 (mouse myeloma IgG from Sigma Chemical Company) and P1.17 (IgG2a from Biogen Inc., Cambridge, MA) were used as control mAb.
Purification and 111In-labelling of rat peritoneal eosinophils
Rats were injected intraperitoneally with 5 ml of horse serum and killed 36 hr later by CO2-induced asphyxia. Peritoneal cells were collected by lavage with 30 ml of heparinized saline (10 U/ml) and purified by centrifugation over a three-layer discontinuous Percoll (HBSS gradient (60%/65%/75%) as previously described.19 The fractionated cell population was used only when the eosinophil purity, as determined by Kimura staining, was above 90%. The predominant contaminating cell types were mononuclear leucocytes and a major exclusion criterion was the presence of neutrophils. The cells were then incubated with 111InCl3 (≈100 μCi in 10 μl), chelated with 2-mercaptopyridine-N-oxide [40 μg in 0·1 ml of 50 mm phosphate-buffered saline (PBS), pH 7·4], for 15 min at room temperature. The labelled eosinophils were washed three times and finally resuspended (6×106/ml) in HBSS (pH 7·4) containing cell-free citrated rat plasma to a final concentration of 10%.
Measurement of 111In-eosinophil accumulation and oedema formation in rat skin
Accumulation of 111In-eosinophils over a 4-hr period
Eosinophil infiltration and oedema formation in rat dorsal skin were simultaneously measured using the local accumulation of intravenously (i.v.) injected 111In-labelled eosinophils (111In-eosinophils) and 125I-HSA, as previously described.19 Rats were sedated with Hypnorm (0·1 ml/rat) and their dorsal skin was shaved. Antibodies, freshly diluted in sterile saline to give the final concentration in 0·5 ml, were injected i.v. via a tail vein. Each animal treated with TA2 (5 mg/kg), 5F10 (2 or 5 mg/kg), or 1A29 (5 mg/kg) was paired with an animal treated with MOPC-21 at the same dose. Fifteen minutes after the administration of the antibody, 111In-eosinophils (5×106 eosinophils in 0·5 ml HBSS), mixed with 125I-HSA (2·5 μCi/animal), were injected i.v. via a tail vein. Five minutes later, PAF and LTB4 (both at 10−12−10−10 mol/site), freshly prepared from stock solution in Tyrode with low endotoxin BSA (0·1%), were injected intradermally (100 μl/site) in duplicate, into the back skin, according to a balanced site injection plan. A 4-hr accumulation period was used for this series of experiments. At the end of the 4 hr-test period, the animals were re-anaesthetized and a cardiac blood sample was collected. The animals were then killed with an overdose of sodium pentobarbitone, the dorsal skin was removed, and the injection sites were punched out with a 17-mm diameter punch. Skin, blood and plasma samples were counted in an automatic gamma-counter (Canberra Packard, Berks, UK) and counts were cross-channel corrected for the two isotopes. The 111In count per eosinophil was determined and used to express eosinophil accumulation in each skin site in terms of the number of labelled leucocytes. Oedema formation at each site was expressed as μl of plasma by dividing skin sample 125I counts by counts in 1 μl of plasma.
Time–course of 111 In-eosinophil accumulation induced by PAF and LTB4
In time–course experiments, PAF or LTB4 (both at 10−10 mol/site), were injected at −3, −1, −0·5 and 0 hr before the administration of the labelled cells. The 111In-eosinophils were injected i.v. at t = 0 hr and a 1-hr accumulation period was then allowed. Thus, using this protocol, the rate of 111In-eosinophil accumulation during the measurement periods of 0–1, 0·5–1·5, 1–2 and 3–4 hr was determined. The mAb 5F10 or MOPC-21 (both at 2 mg/kg) were administered 15 min before the cells, as previously described. In a second series of experiments, to elucidate the role of protein synthesis in PAF- and LTB4-induced responses, the DNA/RNA polymerase inhibitor actinomycin D (Act D, 5 × 10−9 molosite) was coinjected intradermally with the chemoattractants or Tyrodes solution following the same time–course protocol described above.
Immunohistochemical localization of VCAM-1
Intradermal injections of PAF, LTB4, or Tyrodes were administered into the dorsal skin of rats as described previously. After 1 or 4 hr, the animals were killed, the skins were removed and the skin sites were punched out. The skin sites were trimmed, snap-frozen in liquid nitrogen-cooled isopentane and embedded in OCT. Cryostat sections (6 μm) were air-dried overnight and acetone-fixed. Following treatment with normal horse serum, the sections were incubated with the anti-VCAM-1 antibody 5F10 (10 μg/ml) or control antibody (mouse IgG; 10 μg/ml) for 60 min. After washing in PBS the sections were incubated for 30 min with biotinylated horse anti-mouse antibody followed by a 30-min incubation with the Vectorstain ABC avidin/alkaline phosphatase complex. The sections were developed in fast red substrate and counterstained with haematoxylin.
Quantification of vascular VCAM-1 and ICAM-1 expression by the binding of radiolabelled mAb
The in vivo expression of endothelial cell VCAM-1 and ICAM-1 was quantified by the localization of intravenously administered radiolabelled antibodies as previously described.20–22 Briefly, the anti-VCAM-1 mAb 5F10, the anti-ICAM-1 mAb 1A29 and a control mAb (P1.17) were radiolabelled with 125I, 99mTc and 111In (40 MBq/mg of antibody), respectively, as previously described.22 Radiolabelled antibodies were separated from free isotope by gel filtration on a Sephadex G50 or a PD10 column eluted with PBS. The percentage of total isotope bound to each antibody was determined using instant thin-layer chromatography (Gelman Sciences Inc., Ann Arbor, MI) which in all cases was found to be greater than 95%. Intradermal injections of PAF, LTB4 and Tyrode solution were administered into the dorsal skin of rats as described above. After, 1 or 4 hr, the animals were injected intravenously with a mixture (25 μg each) of 125I-labelled 5F10, 99mTc-labelled 1A29 and 111In-labelled P1.17. Five minutes later, the animals were heavily anaesthetized, the aorta was cannulated and the inferior vena cava was opened to allow the circulation to be perfused with 50 ml of PBS. This step in the procedure minimizes the contribution of blood pool activity to tissue-specific isotope counts. The skin sites were then punched out as described above, weighed and counted in an automated gamma-counter. An aliquot of the injected solution was also counted to allow calculation of the injected dose. After corrections for background, spill over between isotopes and decay, the antibody uptake for each sample was expressed as percentage injected dose (ID)/g of tissue. The uptake of the radiolabelled anti-VCAM-1 mAb and the radiolabelled anti-ICAM-1 mAb were corrected for the non-specific uptake of the control mAb before presenting the results graphically. In order to assess the specific binding of the radiolabelled anti-VCAM-1 mAb 5F10, some animals were injected with a mixture of the radiolabelled antibodies (as detailed above) and a 50-fold excess of unlabelled 5F10.
Statistical analysis
Results are expressed as the mean±SEM for n animals where each datum unit is the average of responses in duplicate sites. Both eosinophil accumulation and oedema formation were corrected for vehicle values before statistical analysis was carried out except where values for Tyrodes are shown. Data were analysed by repeated measures two-way analysis of variance (anova) followed by Tukey’s for comparisons test or Students t-test. With both statistical tests, P < 0·05 was considered statistically significant.
RESULTS
Effect of the anti-VCAM-1 mAb 5F10 on 111In-eosinophil accumulation and oedema formation induced by PAF and LTB4: comparison with the effects of an anti-α4 integrins mAb
To investigate the role of VCAM-1 in PAF- and LTB4-induced eosinophil accumulation and oedema formation in rat skin, using a 4-hr in vivo test period, the effect of the anti-rat VCAM-1 mAb 5F10 was tested. Figure 1 shows that mAb 5F10 (2 mg/kg i.v.) significantly suppressed the 111In-eosinophil accumulation elicited by intradermal PAF and LTB4, at 10−10 mol/site the responses were both inhibited by 39%. A larger dose of mAb 5F10 (5 mg/kg i.v.) did not exert a greater level of inhibition (data not shown). Similarly, the anti-α4 integrins mAb TA2 suppressed the eosinophil accumulation induced by the two chemoattractants (Fig. 2). The responses induced by 10−10 mol/site PAF and LTB4 were inhibited by 47% and 52%, respectively. Monoclonal antibodies 5F10 and TA2 had no significant effects on the circulating leucocyte numbers (results not shown).
Figure 1.
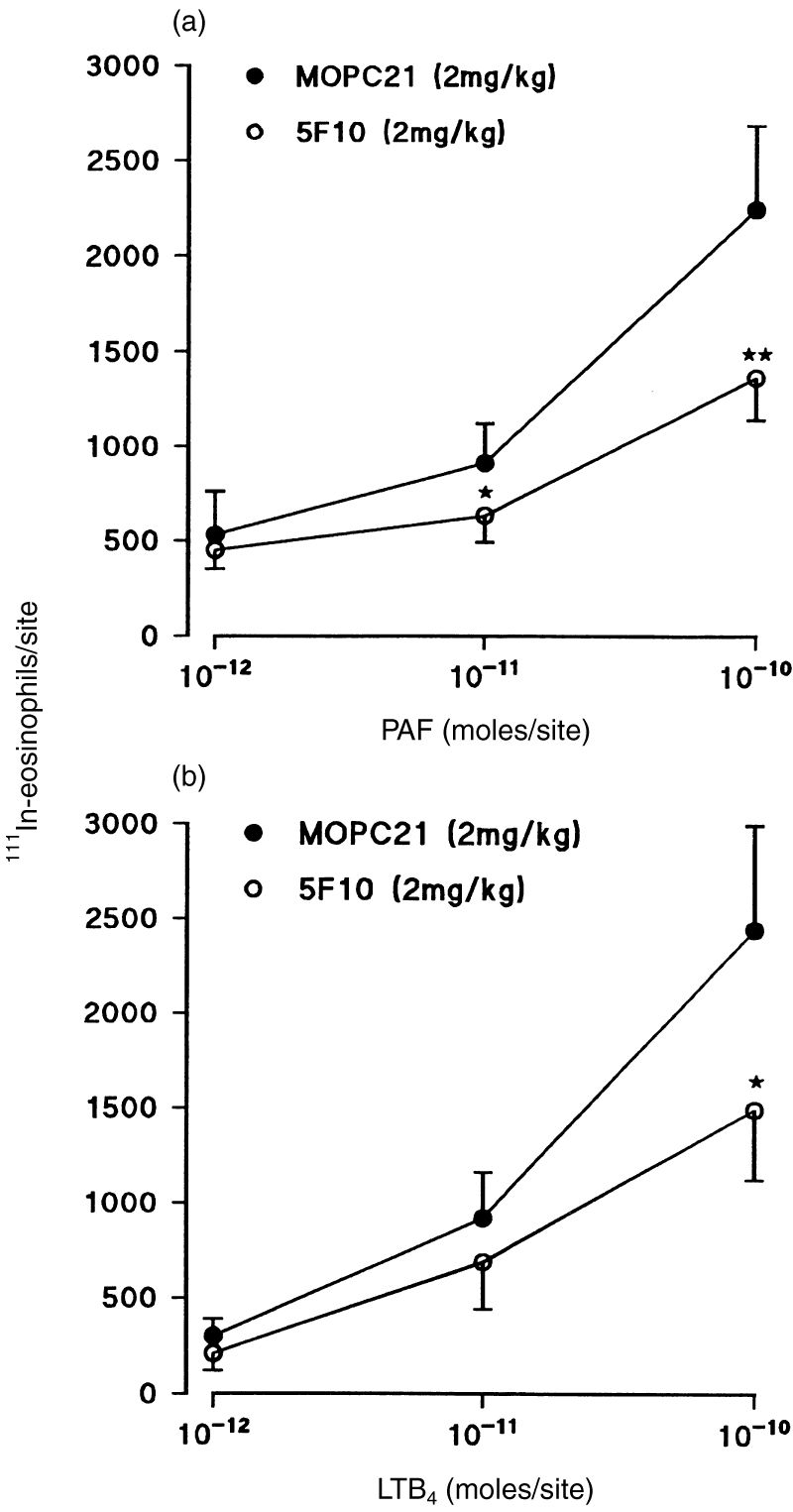
Effect of the anti-VCAM-1 mAb 5F10 on PAF- and LTB4-induced 111In-eosinophil accumulation in rat skin. Animals were pretreated with 5F10 (2 mg/kg i.v.) or MOPC-21 (2 mg/kg i.v.) 15 min prior to the i.v. administration of 111In-eosinophils. (a) PAF or (b) LTB4 were administered intradermally and the responses were quantified 4 hr later as described in the Materials and Methods. The results, which have been corrected for the small level of counts in Tyrode-injected skin sites, are expressed as mean±SEM for at least seven pairs of rats. Significant differences in responses detected between MOPC-21-treated and 5F10-treated rats are shown by asterisks, *P < 0·05 and **P < 0·01.
Figure 2.

Effect of the anti-rat α4 integrin mAb TA2 on PAF- and LTB4-induced 111In-eosinophil accumulation in rat skin. Animals were pretreated with TA2 (5 mg/kg i.v.) or MOPC-21 (control, 5 mg/kg i.v.) 15 min prior to the i.v. administration of 111In-eosinophils. (a) PAF or (b) LTB4 were administered intradermally and the responses were quantified 4 hr later as detailed in the Materials and Methods. The results, which have been corrected for the small level of counts in Tyrode-injected sites, are expressed as mean±SEM (n = at least seven pairs of rats). Significant differences in responses detected between MOPC-21-treated and TA2-treated rats are shown by asterisks; *P < 0·05 and **P < 0·01.
As well as inducing eosinophil accumulation, intradermal administration of PAF (but not LTB4) caused a significant level of oedema formation as quantified by the local accumulation of 125I-labelled HSA. The responses induced by 10−11 mol/site and 10−10 mol/site PAF were 45·8±10·3 μl and 76·6±10·8 μl, respectively, as compared to Tyrode-injected skin sites, 9·0±1·1 μl (n = 7; P < 0·05). However, in contrast to the inhibitory effects of 5F10 and TA2 on PAF-induced eosinophil accumulation, these mAb had no effect on PAF-induced oedema (data not shown).
Effect of the anti-VCAM-1 mAb 5F10 on the early phases of 111In-eosinophil accumulation and oedema formation induced by PAF and LTB4
To investigate the role of VCAM-1 in the early phases of chemoattractant-induced responses, we studied the effect of 5F10 on the time–course of PAF- and LTB4-induced 111In-eosinophil accumulation and oedema formation over 4 hr. With both mediators, the maximum rate of eosinophil accumulation occurred in the first 60 min postinjection of the mediator, the rate of accumulation decreasing with increasing time-intervals post-injection of PAF or LTB4 (Fig. 3a,b). Very small levels of accumulation were detected within the time-period of 3–4 hr. With respect to PAF-induced oedema, almost all of the response was detected within the first 60 min after intradermal injection of PAF (Fig. 3c). Interestingly, treatment of animals with mAb 5F10 induced a greater level of inhibition on the eosinophil accumulation detected during the early phases of responses elicited by intradermal PAF and LTB4 (Fig. 3). As found before,mAb 5F10 had no effect on the PAF-induced oedema (Fig. 3c).
Figure 3.
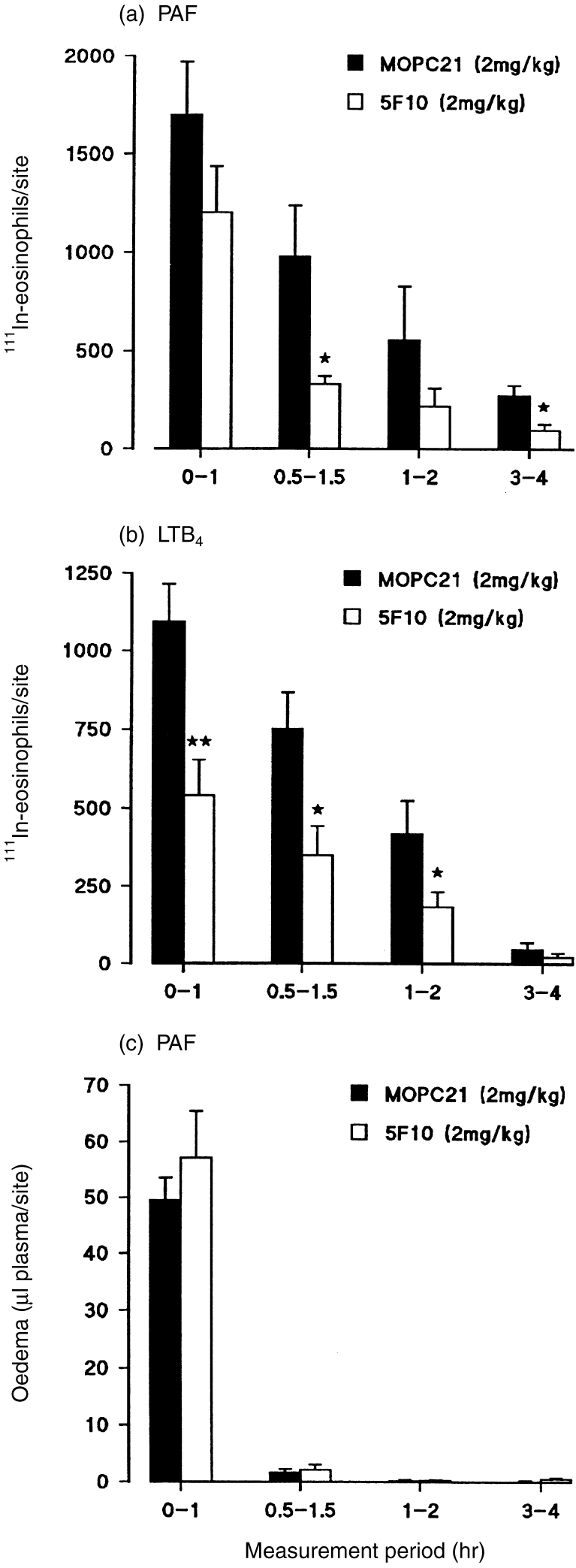
Effect of the anti-VCAM-1 mAb 5F10 on time–course of PAF-and LTB4-induced responses. PAF or LTB4 (both at 10−10 mol/site) were injected intradermally at different time-points, −3 hr, −1 hr, −0·5 hr and 0 hr, prior to the i.v. administration of the antibodies (5F10 and MOPC-21, both at 2 mg/kg), the 111In-eosinophils and 125I-HSA. After a 1-hr accumulation period, the animals were killed and the 111In-eosinophil accumulation/skin site and oedema formation were quantified as described in the Materials and Methods. Panels (a) and (b) show the time–course profiles of 111In-eosnophil accumulation induced by PAF and LTB4, respectively, and panel (c) shows the time–course of PAF-induced oedema. The results, which have been corrected for the small level of counts detected in corresponding Tyrode-injected skin sites, are mean±SEM for (n = at least five pairs of rats). Significant differences in responses detected between MOPC-21-treated and 5F10-treated rats are shown by asterisks, *P < 0·05 and **P < 0·01.
Effect of actinomycin D on 111In-eosinophil accumulation induced by PAF and LTB4
To investigate the role of local protein synthesis induction in PAF-and LTB4-induced 111In-eosinophil accumulation, the mediators were coadministered intradermally with actinomycin D (5×10−9 mol/site). This dose of actinomycin D has previously been shown to suppress cytokine- or LPS-induced leucocyte accumulation into rabbit and guinea-pig skin sites.23–25 Figure 4 shows that actinomycin D had no significant effect on the 111In-eosinophil accumulation induced by LTB4 or PAF at any of the time periods measured. PAF-induced oedema formation was also unaffected by the coadministration of actinomycin D (data not shown). In parallel experiments, coadministration of actinomycin D (5×10−9 mol/site) with recombinant rat interleukin-1β (IL-1β; 5×10−13 mol/site) significantly suppressed the cytokine-induced 111In-eosinophil accumulation over 4 hr in rat skin (rRIL-1β 378±64; rRIL-1β+ actinomycin D 141±66, 111In-eosinophils/site above the small level of counts detected in Tyrode-injected sites, n = 7 rats, P < 0·01).
Figure 4.
Effect of locally administered actinomycin D on (a) PAF- or (b) LTB4-induced 111In-eosinophil accumulation in rat skin. PAF and LTB4 (both at 10−10 mol/site) were injected intradermally with or without actinomycin D (5×10−9 m/site) at −3 hr, −1 hr, −0·5 hr and 0 hr prior to the i.v. administration of 111In-eosinophils. After a 1-hr accumulation period, 111In-eosinophil accumulation/site was quantified as detailed in the Materials and Methods. The results, which have been corrected for the small level of counts detected in corresponding Tyrode/actinomycin D-injected skin sites, are mean±SEM for n = at least five pairs of rats.
Localization of VCAM-1 in rat skin
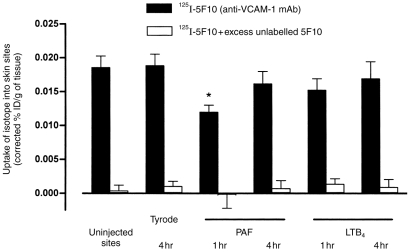
Two experimental approaches were used to investigate the expression of VCAM-1 in uninjected or Tyrode-, PAF-, or LTB4-injected rat skin sites. In initial experiments, using mAb 5F10 to localize VCAM-1 by standard immunohistochemical staining, no detectable levels of VCAM-1 were observed in uninjected skin sites or skin sites injected with Tyrode, PAF, or LTB4(both at 10−10 mol/site) 1 or 4 hr post injection (data not shown). Using the same staining protocol, we have previously found VCAM-1 expression in rat skin sites injected with tumour necrosis factor-α (TNF-α).5 However, using a more sensitive isotopic technique in which the expression of endothelial cell-associated molecules is quantified by the localization of intravenously administered radiolabelled antibodies,21,22 VCAM-1 expression was detected in uninjected rat skin sites. Figure 5 shows the localization of 125I-labelled 5F10 (anti-VCAM-1 mAb) to uninjected rat skin sites. This uptake of 125I-labelled 5F10 was shown to be specific binding to VCAM-1 as demonstrated by the total blockade of binding in the presence of excess unlabelled 5F10. Following intradermal injection of Tyrode, PAF, or LTB4 there was no significant increase in the binding of 125I-5F10 to skin sites above the level detected in uninjected sites. In fact, at 1 hr postinjection of PAF there was a small but significant reduction in the uptake of radiolabelled 5F10. The reason for this drop in localization is unclear but may be associated with the large PAF-induced oedema that occurs within the first 60 min post injection of the mediator (Fig. 3c). PAF-induced contraction of endothelial cells may reduce the surface area available for the binding of the radiolabelled anti-VCAM-1 mAb thus resulting in an apparent reduction in expression of VCAM-1. In parallel experiments, intradermal injection of recombinant rat IL-4 induced a significant increase in the localization of radiolabelled 5F10 (data not shown).
Figure 5.
Uptake of radiolabelled anti-VCAM-1 mAb into rat skin sites. Skin sites were un-injected, or injected intradermally with Tyrode solution, PAF, or LTB4(both at 10−10 mol/site). At 1 or 4 hr post injections, the animals received an intravenous dose of radiolabelled antibodies including 125I-labelled 5F10 (anti-VCAM-1) and 111In-labelled control mAb. Five minutes later, the circulation was perfused and the skin sites removed and localisation of antibodies was quantified as described in the Materials and Methods. The graph shows the percentage uptake of the injected dose (ID) of radiolabelled 5F10 per gram of tissue, corrected for the non-specific uptake of radiolabelled control mAb (filled bars, n = 4 rats). Some animals received both the radiolabelled mAb and a 50-fold excess of unlabelled 5F10 (open bars, n = 3 rats). The results are mean±SEM for n number of rats. Asterisk indicates a significant difference from the uptake in uninjected sites, P < 0·05.
In the above experiments radiolabelled anti-ICAM-1 mAb (1A29) was also used to quantify the expression of endothelial cell-associated ICAM-1 in parallel with the expression of VCAM-1. As found with VCAM-1, there was an uptake of radiolabelled anti-ICAM-1 mAb in uninjected rat skin sites which was not altered in skin sites injected with Tyrode, PAF, or LTB4 (uninjected sites 0·056±0·007; Tyrode 0·050±0·009; PAF at 4 hr 0·041±0·009; LTB4 at 4 hr 0·049±0·007, corrected percentage binding of 99mTc-labelled 1A29/g tissue, n = 6–8 rats).
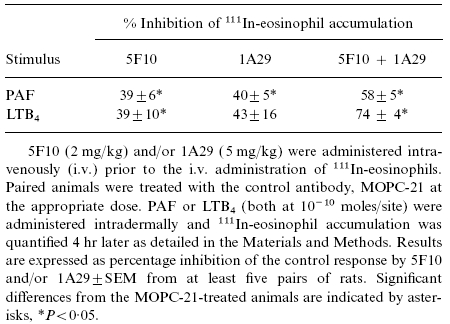
Effect of coadministration of the anti-VCAM-1 mAb 5F10 and the anti-ICAM-1 mAb 1A29 on 111In-eosinophil accumulation and oedema formation induced by PAF and LTB4
As the anti-VCAM-1 mAb, 5F10, was found to exert only a partial inhibitory effect on responses induced by the chemoattractants, and both VCAM-1 and ICAM-1 expression was detected in rat skin sites, the following experiments were carried out to investigate whether combined blockade of VCAM-1 and ICAM-1 would exhibit a greater level of inhibition. Table 1 shows that, when administered alone, the anti-rat ICAM-1 mAb 1A29 (5 mg/kg i.v.) suppressed 111In-eosinophil accumulation induced by PAF or LTB4 (4 hr in vivo test period) though the effect on the LTB4-induced response did not reach statistical significance (P = 0·066). Increasing the dose of 1A29–10 mg/kg did not increase the level of inhibition (data not shown). In accordance with results obtained with both 5F10 and TA2, PAF-induced oedema formation was not affected by intravenous administration of 1A29 (data not shown). Table 1 also shows that coadministration of mAb 5F10 and mAb 1A29 resulted in a greater level of inhibition of the responses elicited by the chemoattractants than that seen with either mAb alone. Further, in contrast to individual antibody pretreatment, combined administration resulted in a small but significant inhibition of oedema induced by 10−10 mol/site PAF (37±12% inhibition, n = 5, P < 0·05).
Table 1.
Effect of combined blockade of VCAM-1 and ICAM-1 on 111In-eosinophil accumulation in response to PAF and LTB4
DISCUSSION
Neutralizing anti-VCAM-1 mAb have been shown to inhibit eosinophil accumulation induced by inflammatory cytokines TNF-α5 and IL-46 in rat skin and eosinophil accumulation into mouse trachea following the inhalation of antigen in sensitized animals.13 In these studies, which involved 4–24 hr in vivo test periods, the inhibitory effects of anti-VCAM-1 mAb were considered to be predominantly due to blockade of induced VCAM-1 on venular endothelial cells. However, as we have previously shown that an anti-α4 integrins mAb blocks the rapid eosinophil accumulation induced by chemoattractants in guinea-pig skin,3 the aim of the present study was to investigate the role of VCAM-1, the principal endothelial cell ligand for leucocyte α4 integrins, in chemoattractant elicited responses. For this purpose, using an in vivo model of 111In-eosinophil accumulation in rat skin we have tested the effect of an anti-rat VCAM-1 mAb on eosinophil accumulation induced by the chemoattractants PAF and LTB4 and have investigated the expression of VCAM-1 in the corresponding skin sites.
In initial studies, using a 4-hr in vivo test period, intravenous administration of the anti-rat VCAM-1 mAb, 5F10, significantly suppressed 111In-eosinophil accumulation induced by intradermal PAF and LTB4. The responses induced by both chemoattractants were suppressed to the same extent. Antibody 5F10 has previously been shown to inhibit both slow developing eosinophil accumulation responses elicited by intradermally administered cytokines TNF-α5 and IL-46 as well as the rapid eosinophil accumulation induced by the chemokine eotaxin.26 Further, in the present study, in agreement with our previous findings in guinea-pig skin,3 an anti-α4 integrins mAb inhibited PAF- and LTB4-induced 111In-eosinophil accumulation. These results, obtained using 2 hr (guinea-pig) or 4 hr (rat)in vivo test periods, suggest a role for the α4 integrins/VCAM-1 adhesion pathways in eosinophil accumulation elicited by PAF and LTB4. However, the studies do not indicate whether VCAM-1 is involved in early or late phases of the responses induced by the chemoattractants. To address this point, a series of experiments was carried out to investigate the effect of the anti-VCAM-1 mAb on the time–course of PAF- and LTB4-induced responses.
Time–course experiments indicated that the rate of eosinophil accumulation into rat skin sites injected with PAF or LTB4 was maximal within the first 60 min post injection of the mediators. With both PAF and LTB4, further influx of 111In-eosinophils was detected up to 2 hr, with very little response being detected within the period of 3–4 hr, post injection. In animals treated with the anti-VCAM-1 mAb there was a significant reduction in the early phases of chemoattractant-induced 111In-eosinophil accumulation, suggesting a role for VCAM-1 in the rapid eosinophil accumulation induced by PAF and LTB4. As these mediators have not been shown to induce expression of VCAM-1 on endothelial cells, the results suggest that basally expressed VCAM-1 on dermal microvascular endothelial cells mediates the rapid accumulation of eosinophils elicited by PAF and LTB4. In support of this suggestion we found that the RNA synthesis inhibitor actinomycin D did not effect responses observed, suggesting that local protein synthesis had no role. Furthermore, whilst using standard immunohistochemical methods we could not detect expression of VCAM-1 in rat skin sites, using a more sensitive assay of in vivo localization of radiolabelled-5F1020–22 basal expression of VCAM-1 was detected in uninjected rat skin sites. This localization of radiolabelled anti-VCAM-1 mAb was shown to be specific as the uptake of the antibody was totally blocked in the presence of excess unlabelled antibody. In parallel experiments, whilst a significant increase in binding of radiolabelled 5F10 was detected in IL-4-injected skin sites (K. Larbi, A. Andrews and S. Nourshargh, manuscript in preparation), there was no significant difference between the specific binding of radiolabelled-5F10 to uninjected skin sites and to skin sites injected with Tyrode, PAF, or LTB4. These in vivo observations support in vitro findings that PAF and LTB4 do not induce VCAM-1 expression on endothelial cells whereas IL-4 is an effective inducer of VCAM-1 expression on cultured endothelial cells.27
Although the in vivo mechanism by which PAF and LTB4 activate α4 integrins/VCAM-1 adhesion pathways remain unknown, our results provide evidence for the presence of a functional level of constitutive expression of VCAM-1 which is below the level of detection of standard immunohistochemical staining procedures. Hence, rapid eosinophil accumulation induced by PAF and LTB4 may be mediated following rapid in vivo activation of α4 integrins on eosinophils by the chemoattractants. Such an activation could render leucocyte molecules capable of interacting with their endothelial cell ligand VCAM-1 which is basally expressed. To date however, there have been no direct in vitro reports on the ability of PAF or LTB4 to activate α4 integrins on eosinophils. In fact, the ability of PAF to induce eosinophil adhesion to VCAM-1 under static conditions is uncertain.28 It is possible that the functional significance of the α4 integrins/VCAM-1 adhesion pathways in chemoattractant-induced eosinophil adhesion is only evident under conditions of flow or in conjunction with the ligation of other eosinophil adhesion molecules, a mechanism proposed for chemoattractant-induced neutrophil adhesion.29 Certain eosinophil chemoattractants such as C5a and the chemokines RANTES and MCP-3 have however, been shown to induce an α4 integrins-dependent rapid and transient adhesion of eosinophils to VCAM-1- or fibronectin-coated plates under static conditions.30 Variations in the role of α4 integrins/VCAM-1 in eosinophil adhesion under static and flow conditions with different inflammatory mediators may very simply be due to different assay conditions employed by different investigators. However, the discrepancies may also indicate the possible existence of complex chemoattractant-specific regulatory mechanisms in eosinophil adhesion.
As both the anti-α4 integrins mAb and the anti-VCAM-1 mAb exerted only partial inhibitory effects, additional experiments were carried out to investigate whether the simultaneous blockade of both VCAM-1 and ICAM-1 would result in a greater level of inhibition of responses elicited by the two chemoattractants. In this context, combined administration of the anti-VCAM-1 mAb 5F10 and the anti-ICAM-1 mAb 1A29 resulted in a greater suppression of PAF- and LTB4-induced eosinophil accumulation. These results suggest that since both VCAM-1 and ICAM-1 are constitutively expressed on rat cutaneous endothelial cells, both molecules are involved in rapid accumulation ofeosinophils in response to locally generated chemoattractants. Further, we have previously shown that eosinophil accumulation induced by the CC chemokine eotaxin is partially blocked by both an anti-VCAM-1 and an anti-ICAM-1 mAb26 whilst the eosinophil accumulation induced by IL-4 was only inhibited by VCAM-1 blockade.6 These results indicate that the relative contribution of VCAM-1 and ICAM-1 in eosinophil accumulation in vivo is dependent on the type of inflammatory mediator, migration induced by eosinophil chemoattractants being mediated by both adhesion molecules. The roles of VCAM-1 and ICAM-1 have also been investigated in two murine models of allergen-induced eosinophil accumulation. In a model of antigen-induced leucocyte recruitment into the mouse trachea, the blockade of VLA-4/VCAM-1, but not LFA-1/ICAM-1, inhibited the eosinophil accumulation by greater than 70%.13 In contrast, Gonzalo et al. found that antigen-induced pulmonary eosinophilia could not be induced in VCAM-1- or ICAM-1-deficient mice.31 In our study, whilst the combined blockade of ICAM-1 and VCAM-1 resulted in a greater level of inhibition, the eosinophil accumulation induced by PAF and LTB4 was not totally blocked, indicating a role for other adhesion pathways. Of relevance there is now evidence for the involvement of the selectin molecules and their ligands in eosinophil responses in vitro and in vivo.32–35
Intradermal administration of PAF (but not LTB4) in rat skin as well as inducing the accumulation of eosinophils, can also induce oedema formation. However, in contrast to the inhibitory effects seen with the anti-VCAM-1 mAb and the anti-α4 integrins mAb of eosinophil accumulation, PAF-induced oedema was not affected by these mAb. Similarly, the anti-ICAM-1 mAb, when administered on its own, had no effect on PAF-induced plasma protein leakage. The coadministration of anti-VCAM-1 and anti-ICAM-1 mAb did however, cause a small but significant level of inhibition of the oedema induced by intradermal PAF. The reason for this partial inhibitory effect on the PAF-induced oedema is unclear but may be related to the greater inhibition of the eosinophil accumulation seen with dual blockade of VCAM-1 and ICAM-1. In a previous study carried out in guinea-pig skin the results indicated a dissociation between PAF-induced eosinophil accumulation and plasma protein leakage.3 The role of adhesion molecules in chemoattractant-induced oedema formation is still unclear and needs to be further investigated.
In summary, our results demonstrate the presence of a constitutive level of VCAM-1 in rat skin and a functional role for VCAM-1 in the early phases of PAF- and LTB4-induced eosinophil accumulation. The findings suggest that VCAM-1, constitutive or induced, can mediate leucocyte infiltration in both acute and chronic inflammatory reactions, respectively.
Acknowledgments
This work was supported by Biogen Inc. and the Wellcome Trust, UK.
REFERENCES
- 1.Carlos TM, Harlan JM. Leukocyte-endothelial adhesion molecules. Blood. 1994;84:2068. [PubMed] [Google Scholar]
- 2.Lobb RR, Hemler ME. The pathophysiologic role of α4 integrins. In Vivo J Clin Invest. 1994;94:1722. doi: 10.1172/JCI117519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weg VB, Williams TJ, Lobb RR, Nourshargh S. A monoclonal antibody recognising very late activation antigen-4 (VLA-4) inhibits eosinophil accumulation in vivo. J Exp Med. 1993;177:561. doi: 10.1084/jem.177.2.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Das AM, Williams TJ, Lobb RR, Nourshargh S. Lung eosinophilia is dependent on IL-5, and the adhesion molecules CD18 and VLA-4 in a guinea-pig model. Immunology. 1995;84:41. [PMC free article] [PubMed] [Google Scholar]
- 5.Sanz M, Hartnell A, Chisholm P, et al. Tumor necrosis factor α-induced eosinophil accumulation in rat skin is dependent on α4 integrin/vascular cell adhesion molecule-1 adhesion pathways. Blood. 1997;90:4144. [PubMed] [Google Scholar]
- 6.Sanz M, Marinova-Mutafchieva L, Green P, Lobb RR, Feldmann M, Nourshargh S. IL-4-Induced eosinophil accumulation in rat skin is dependent on endogenous TNFα and α4 integrin/VCAM-1 adhesion pathways. J Immunol. 1998;160:5637. [PubMed] [Google Scholar]
- 7.Bochner BS, Luscinskas FW, Gimbrone MA, et al. Adhesion of human basophils, eosinophils, and neutrophils to interleukin 1-activated human vascular endothelial cells: Contributions of endothelial cell adhesion molecules. J Exp Med. 1991;173:1553. doi: 10.1084/jem.173.6.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weller PF, Rand TH, Goelz SE, Chi-Rosso G, Lobb RR. Human eosinophil adherence to vascular endothelium mediated by binding to vascular cell adhesion molecule 1 and endothelial leukocyte adhesion molecule 1. Proc Natl Acad Sci USA. 1991;88:7430. doi: 10.1073/pnas.88.16.7430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Seekamp A, Mulligan MS, Till GO, et al. Role of β2 integrins and ICAM-1 in lung injury following ischemia-reperfusion of rat hind limbs. Am J Pathol. 1993;143:464. [PMC free article] [PubMed] [Google Scholar]
- 10.Mulligan MS, Wilson GP, Todd RF, et al. Role of β1, β2 integrins and ICAM-1 in lung injury after deposition of IgG and IgA immune complexes. J Immunol. 1993;150:2407. [PubMed] [Google Scholar]
- 11.Wegner CD, Gundel RH, Reilly P, Haynes N, Letts LG, Rothlein R. Intercellular adhesion molecule-1 (ICAM-1) in the pathogenesis of asthma. Science. 1990;247:456. doi: 10.1126/science.1967851. [DOI] [PubMed] [Google Scholar]
- 12.Nourshargh S. Adhesion molecules and asthma. J Pharm Pharmacol. 1997;49:33. [PubMed] [Google Scholar]
- 13.Nakajima H, Sano H, Nishimura T, Yoshida S, Iwamoto I. Role of vascular cell adhesion molecule 1/very late activation antigen 4 and intercellular adhesion molecule 1/lymphocyte function-associated antigen 1 interactions in antigen-induced eosinophil and T cell recruitment into the tissue. J Exp Med. 1994;179:1145. doi: 10.1084/jem.179.4.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Briscoe DM, Cotran RS, Pober JS. Effects of tumor necrosis factor, lipopolysaccharide, and IL-4 on the expression of vascular cell adhesion molecule-1 in vivo. J Immunol. 1992;149:2954. [PubMed] [Google Scholar]
- 15.Osborn L, Hession C, Tizard R, et al. Direct expression cloning of vascular cell adhesion molecule 1, a cytokine-induced endothelial protein that binds to lymphocytes. Cell. 1989;59:1203. doi: 10.1016/0092-8674(89)90775-7. [DOI] [PubMed] [Google Scholar]
- 16.Rice GE, Munro JM, Corless C, Bevilacqua MP. Vascular and nonvascular expression of INCAM-110. A target for mononuclear leukocyte adhesion in normal and inflamed human tissue. Am J Pathol. 1991;138:385. [PMC free article] [PubMed] [Google Scholar]
- 17.Alves AC, Pires AL.A, Cruz HN, et al. Selective inhibition of phosphodiesterase type IV suppresses the chemotactic responsiveness of rat eosinophils in vitro. Eur J Pharmacol. 1998;312:89. doi: 10.1016/0014-2999(96)00357-3. [DOI] [PubMed] [Google Scholar]
- 18.Tamatani T, Miyasaka M. Identification of monoclonal antibodies reactive with the rat homolog of ICAM-1, and evidence for a differential involvement of ICAM-1 in the adherence of resting versus activated lymphocytes to high endothelial cells. Int Immunol. 1990;2:165. doi: 10.1093/intimm/2.2.165. [DOI] [PubMed] [Google Scholar]
- 19.Sanz M, Weg VB, Bolanowski MA, Nourshargh S. IL-1 is a potent inducer of eosinophil accumulation in rat skin: inhibition of response by a PAF antagonist and an anti-human IL-8 antibody. J Immunol. 1995;154:1364. [PubMed] [Google Scholar]
- 20.Binns RM, Licence ST, Harrison AA, Keelan ET.D, Robinson MK, Haskard DO. In vivo E-selectin upregulation correlates with early infiltration of PMN, later with PBL-entry: mAbs block both. Am J Physiol Heart Circ Physiol. 1996;270:H183. doi: 10.1152/ajpheart.1996.270.1.H183. [DOI] [PubMed] [Google Scholar]
- 21.Binns RM, Whyte A, Licence ST, et al. The role of E-selectin in lymphocyte and polymorphonuclear cell recruitment into cutaneous delayed hypersensitivity reactions in sensitized pigs. J Immunol. 1996;157:4094. [PubMed] [Google Scholar]
- 22.Harrison AA, Stocker CJ, Chapman PT, et al. Expression of vascular cell adhesion molecule-1 by vascular endothelial cells in immune and nonimmune inflammatory reactions in the skin. J Immunol. 1997;159:4546. [PubMed] [Google Scholar]
- 23.Rampart M, Williams TJ. Evidence that neutrophil accumulation induced by interleukin-1 requires both local protein biosynthesis and neutrophil CD18 antigen expression in vivo. J Pharmacol. 1988;94:1143. doi: 10.1111/j.1476-5381.1988.tb11632.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cybulsky MI, McComb DJ, Movat HZ. Protein synthesis dependent and independent mechanisms of neutrophil emigration. Am J Pathol. 1989;135:227. [PMC free article] [PubMed] [Google Scholar]
- 25.Weg VB, Walsh DT, Faccioli LH, Williams TJ, Feldmann M, Nourshargh S. LPS-induced 111In-eosinophil accumulation in guinea-pig skin: evidence for a role for TNFα. Immunology. 1995;84:36. [PMC free article] [PubMed] [Google Scholar]
- 26.Sanz M, Ponath PD, Mackay CR, et al. Human eotaxin induces α4 and β2 integrin dependent eosinophil accumulation in rat skin in vivo: Delayed generation of eotaxin in response to IL-4. J Immunol. 1998;160:3569. [PubMed] [Google Scholar]
- 27.Schleimer RP, Sterbinsky SA, Kaiser J, et al. IL-4 induces adherence of human eosinophils and basophils but not neutrophils to endothelium. Association with expression of VCAM-1. J Immunol. 1992;148:1086. [PubMed] [Google Scholar]
- 28.Nagai K, Larkin S, Hartnell A, et al. Human eotaxin induces eosinophil extravasation through rat mesenteric venules: role of α4 integrins and VCAM-1. Immunology. 1999;96:176–183. doi: 10.1046/j.1365-2567.1999.00673.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tsang YT.M, Neelamegham S, Hu Y, et al. Synergy between l-selectin signaling and chemotactic activation during neutrophil adhesion and transmigration. J Immunol. 1997;159:4566. [PubMed] [Google Scholar]
- 30.Weber C, Kitayama J, Springer TA. Differential regulation of β1 and β2 integrin avidity by chemoattractants in eosinophils. Proc Natl Acad Sci USA. 1996;93:10939. doi: 10.1073/pnas.93.20.10939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gonzalo J, Lloyd CM, Kremer L, et al. Eosinophil recruitment to the lung in a murine model of allergic inflammation. The role of T cells, chemokines and adhesion receptors. J Clin Invest. 1996;98:2332. doi: 10.1172/JCI119045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kitayama J, Fuhlbrigge RC, Puri KD, Springer TA. P-selectin, l-selectin, and α4 integrin have distinct roles in eosinophil tethering and arrest on vascular endothelial cells under physiological flow conditions. J Immunol. 1997;159:3929. [PubMed] [Google Scholar]
- 33.Patel KD, McEver RP. Comparison of tethering and rolling of eosinophils and neutrophils through selectins and, p-selectin glycoprotein ligand-1. J Immunol. 1997;159:4555. [PubMed] [Google Scholar]
- 34.Sriramarao P, von Andrian UH, Butcher EC, Bourdon MA, Broide DH. l-selectin and very late antigen-4 integrin promote eosinophil rolling at physiological shear rates in vivo. J Immunol. 1994;153:4238. [PubMed] [Google Scholar]
- 35.Henriques MG.M.O, Miotla JM, Cordeiro RS.B, Wolitsky BA, Wolley ST, Hellewell PG. Selectins mediate eosinophil recruitment in vivo: a comparison with their role in neutrophil influx. Blood. 1996;87:1. [PubMed] [Google Scholar]