Abstract
We previously reported that exogenous interleukin-15 (IL-15) induces proliferation and activation of intestinal intraepithelial lymphocytes (i-IEL) in naive mice. To investigate the ability of endogenous IL-15 to stimulate i-IEL in vivo, we monitored i-IEL and intestinal epithelial cells (i-EC) in mice after an oral infection with Listeria monocytogenes. Although the populations of αβ and γδ i-IEL were not significantly changed after the oral infection, the expression level of interferon-γ (IFN-γ) was increased both at transcriptional and protein levels, and a conversely marked decrease in interleukin-4 (IL-4) was detected in the i-IEL on day 1 after infection as compared with before infection. The T helper 1 (Th1)-biased response of i-IEL coincided with a peak response of IL-15 production in the i-EC after oral infection. These results suggested that IL-15 produced from i-EC may be at least partly involved in the stimulation of i-IEL to produce IFN-γ after oral infection with L. monocytogenes.
INTRODUCTION
Interleukin (IL)-15 is a novel cytokine that uses β (IL-2 receptor-β, IL-2Rβ) and common γ (cγ) chains of IL-2R for signal transduction and shares many of the properties of IL-2 although it has no sequence homology with IL-2.1–4 IL-15 can bind to natural killer (NK) cells, activated T cells or B cells that express unique IL-15Rα, besides IL-2Rβ and cγ chains, and can stimulate these cells to proliferate and exhibit cytotoxic activity, and to produce cytokines or antibody (Ab).5,6 We have previously shown that the γδ T cells that appear at an early stage during the course of infection with Listeria monocytogenes produce interferon-γ (IFN-γ), but not IL-2, after antigen stimulation.7 Furthermore, we have recently found that the γδ T cells that appear early during murine salmonellosis can proliferate and produce various cytokines in response to exogenous IL-15 or IL-15 from infected macrophages (Mφ).8 Our results suggest that IL-15 derived from infected Mφ may contribute to the early activation of γδ T cells well before αβ T cells are activated during the course of salmonellosis.8 Besides activated Mφ, IL-15 is thought to be produced by intestinal epithelial cells (i-EC) but not by activated T cells, whereas IL-2 is exclusively produced by activated T cells.9 Therefore, it can be speculated that IL-15 may play different roles from IL-2 during the course of microbial infection.
Intestinal intraepithelial lymphocytes (i-IEL) are located at the basolateral surfaces of i-EC, which are continuously exposed to numerous environmental antigens via the intestinal epithelium.10 Murine i-IEL comprises an approximately equal number of T-cell receptor (TCR)αβ and γδ i-IEL.10–14 Cytokine requirements for development of γδ i-IEL have been shown to be different from those of αβ i-IEL. γδ T cells are absent in mice lacking IL-7R, IL-2Rβ or the cγ subunit of the receptor for IL-2, IL-4, IL-7, IL-9 and IL-15.15–18 We recently reported that IL-15 preferentially stimulated γδ i-IEL to proliferate and produce IFN-γ.19 Collectively, these results suggest that cytokines such as IL-7 and IL-15 may be important for the development and maintenance of a significant population of i-IEL. i-IEL are known to produce various cytokines upon TCR triggering and play important roles in local immunoglobulin A (IgA) response.20,21 i-IEL also exhibit non-major histocompatibility complex (MHC)-restricted cytotoxicity, via serine esterase-dependent and Fas/Fas-ligand (Fas-L)-dependent mechanisms, against infected cells, premalignant cells and effete cells.22–24 A dominant TCRγδ T-cell response to infections with various microbial pathogens suggests that at least a significant population of γδ T cells represents a first line of host defence against infections with diverse pathogens in nature.25–35 Yamamoto et al. reported that γδ i-IEL produce IFN-γ by per os (p.o.) infection with Listeria monocytogenes, suggesting a function for γδ i-IEL in the host defence against oral bacterial infection.36 We have also shown that γδ i-IEL produced IFN-γ in response to IL-15, of which mRNA are expressed abundantly by i-EC.19 Taken together, it can be speculated that IL-15 derived from i-EC may serve as one of factors for the activation of i-IEL that are responsible for the first line of host defence against microbial invasion.
In this report, we found that IL-15 was produced by i-EC at an early stage after p.o. infection with L. monocytogenes, and concurrently the i-IEL were activated to produce IFN-γ. The implications of these findings for the involvement of IL-15 in the early activation of i-IEL in intestinal infection were discussed.
MATERIALS AND METHODS
Mice
Male C57BL/6 mice were purchased at the age of 7 weeks from Japan SLC (Hamamatsu, Japan). All mice were studied at 8 weeks of age.
Micro-organisms
L. monocytogenes strain EGD was maintained by a previously published method.37 Bacterial virulence was maintained by serial passages in mice. Fresh isolates were obtained from infected spleens, grown in trypto-soya broth (Nissui Pharmaceutical, Tokyo, Japan), washed repeatedly, resuspended in phosphate-buffered saline (PBS) and stored at −70° in small aliquots.
Ab and reagents
Phycoerythrin (PE)-conjugated anti-TCRγδ, anti-CD4 and anti-CD8α; fluorescein isothiocyanate (FITC)-conjugated anti-TCRαβ, anti-CD3, anti-I-Ab, anti-CD45, anti-Vβ2, Vβ3, Vβ5.1,5.2, Vβ7, Vβ8.1,8.2, Vβ12 and Vβ14 TCR; and biotin-conjugated anti-TCRγδ, anti-CD8α, anti-CD8β, anti-NK1.1, anti-Vβ6 and Vβ11 TCR monoclonal Abs (mAbs) were purchased from PharMingen (San Diego, CA). PE-conjugated anti-Thy1.2 was purchased from CALTAG (San Francisco, CA). Red-613-conjugated streptavidin was obtained from Life Technologies (Gaithersburg, MD).
Bacterial growth in the liver, spleen, mesenteric lymph nodes and Peyer’s patch
Mice were starved for 18 hr before infection and then infected p.o. with 1 × 109 viable L. monocytogenes in 0·1 ml of PBS. After infection, the mice were kept without food and water for 4 hr.36 On days 1, 3 and 5 after inoculation, the mice were killed by dislocation of the cervical vertebrae. The control mice were starved for 18 hr and studied after 24 hr. The livers, spleens, mesenteric lymph nodes (MLN) and Peyer’s patch (PP) were removed and placed separately in homogenizers containing 5 ml of cold PBS (for liver tissue) or 2 ml of cold PBS (for spleen tissue and MLNs, PP). The organs were completely homogenized and the homogenates were serially diluted with PBS. Samples were spread on trypto-soya agar (Nissui Pharmaceutical) plates, and colonies were counted after incubation for 24 hr at 37°.
Preparation of i-IEL and i-EC
Mice were killed on days 1, 2, 3 and 5 after p.o. infection with L. monocytogenes. Uninfected controls were used in parallel. i-IEL and i-EC were isolated by a modified, previously published method.19,38 Briefly, the small intestine from the mice was cut into 5-mm pieces and stirred at room temperature for 30 min in 199 medium (Gibco, Grand Island, NY) containing 10% inactivated fetal calf serum (FCS) and 1 mm dithiothreitol (DTT). After shaking, the cells were passed through a gauze to remove debris. The passed cells were centrifuged through a 25–40–75% discontinuous Percoll® (Pharmacia, Uppsala, Sweden) gradient, at 600 g, at 20° for 20 min. The i-EC and i-IEL were obtained at the 25–40% and 40–75% interfaces, respectively. The purity of i-EC was assessed by flow cytometry with FITC-conjugated anti-I-Ab or anti-CD45 mAbs,23 and the contamination of i-IEL was less than 2%. In some experiments, αβ and γδ i-IEL were negatively separated by magnetic cell sorting (MACS; Miltenyi Biotec, Bergisch Gladbach, Germany). Briefly, i-IEL were incubated with biotin-conjugated anti-TCRγδ mAb (GL3; PharMingen) or anti-TCRαβ mAb (H57-579, PharMingen) for 15 min at 4°. After washing, cells were incubated with immunomagnetic beads coated with streptavidin (Streptavidin Microbeads; Miltenyi Biotec) for 15 min at 4°. Cells unbound to the magnetic beads were gently separated from the bound cells and used as αβ i-IEL and γδ i-IEL. The contamination of γδ or αβ i-IEL was less than 0·5%, as assessed by flow cytometry.
Flow cytometry
i-EC were stained with FITC-conjugated mAbs. i-IEL were stained with PE-, FITC-, and biotin-conjugated mAbs. All incubation steps were performed at 4° for 30 min. To detect biotin-conjugated mAb, cells were stained with Red-613-conjugated streptavidin after incubation with a primary mAb. The stained cells were analysed by a FACScan™ flow cytometer (Becton-Dickinson, Mountain View, CA). Small lymphocytes were gated by forward and side scatter.
Cell culture
i-IEL were cultured in 200 μl of a complete culture RPMI medium in 96-well flat-bottom plates (Falcon; Becton-Dickinson Ltd, Oxford, UK) at a density of 1×105 cells/well with anti-CD3ε(145 2C11) (10 μg/ml) that had been immobilized on the plates by prior incubation at 4° overnight. The cells were cultured for 72 hr at 37° under 5% CO2 in air and pulsed with 1 μCi [3H]thymidine deoxyribose (TdR) 6 hr before harvest, then [3H]TdR incorporation was determined by liquid scintillation counting. The supernatant was collected to estimate cytokine production after 72 hr of culture. i-EC were cultured in 1.5 ml of complete culture medium in 24-well flat-bottom plates (Falcon) at a density of 2 × 105 cells/well. The cells were cultured for 24 hr at 37° under 5% CO2 in air and the supernatant was collected for estimation of cytokine production.
Cytokine enzyme-linked immunosorbent assay (ELISA)
The cell-free culture supernatants were collected from the 72 hr-culture of i-IEL or 24 hr-culture of i-EC. The cytokine activity in the culture supernatant was assayed by an ELISA using Duo SeT™ mouse IFN-γ, IL-4, IL-2 and IL-12 (Genzyme Diagnostics, Cambridge, MA).
CTLL-2 bioassay
For analysis in the CTLL-2 assay, i-EC supernatants were collected after 24 hr of culture. CTLL cells (2 × 104 cells/well) were cultured in 200 μl of RPMI complete culture medium in 96-well flat-bottom plates (Falcon) containing 50 μl of medium titrated from the supernatants. Triplicate cultures were assayed in the presence of saturating levels of anti-IL-2 or anti-IL-15 mAbs to ensure that the effect was caused by IL-15. Cultures were incubated for 48 hr at 37° under 5% CO2 in air and pulsed with 1 μCi [3H]TdR 24 hr before harvest. [3H]TdR incorporation was determined by liquid scintillation counting.
Reverse transcription–polymerase chain reaction (RT–PCR) and Southern blot analysis
mRNA was extracted from isolated cells by using a QuickPrep® Micro mRNA Purification kit (Pharmacia). Serial dilutions of mRNA were primed with 20 pmol of random primer in 20-μl reaction mixtures for reverse transcription. Aliquots were taken for each sample after different numbers of PCR cycles to ensure that reactions were not saturated, thus allowing quantitative estimation to be performed. For quantification, the synthesized cDNA samples were adjusted to equal input concentrations based on their β-actin cDNA content by competitive PCR. For each PCR amplification, cDNA was co-amplified with a known amount of specific-competitor ‘mimic’ fragment. For each sample, cDNA was co-amplified in a series of three reactions with a twofold dilution series of MIMIC concentrations. After electrophoresis of the PCR products on an 1·8% agarose gel, the proportions were estimated by measuring the intensity of ethidium bromide fluorescence, and the cDNA samples were adjusted to equal β-actin cDNA content. The adjusted cDNA was amplified by PCR using primers specific for murine IL-2, IL-4, IL-7, IL-15, IFN-γ, transforming growth factor-β1 (TGF-β1), Fas-L, IL-12 (p40) or IL-18 cDNA sequence. With the exception of TGF-β1, the primers spanned one or more introns (e.g. three introns for IL-2) to distinguish amplicons of cDNA from those of genomic DNA. In the case of TGF-β1, we confirmed that there was no contamination with genomic DNA by PCR amplication without reverse transcriptase. Before the first cycle, a denaturation step of 5 min at 94° and an extension step of 4 min at 72° was performed. After amplification, PCR products were separated by electrophoresis on 1·8% agarose gels containing ethidium bromide and visualized by UV illumination. The specific primers were as follows: IL-4 sense, 5′-CGAAGAACACCACAGAGAGTGAGCT-3′; antisense, 5′-GACTCATTCATGGTGCAGCTTATCG-3′; IL-7 sense, 5′-AAATGCAGCTGACTGCTGCC-3′; antisense, 5′-TCTCCAGTCTAAAACAGGAC-3′; IL-15 exon 7 sense, 5′-GTGATGTTCACCCCAGTTGC-3′; exon 8 antisense, 5′-TCACATTCTTTGCATCCAGA-3′; IL-15 exon 4 sense, 5′-TCCATCTCGTGCTACTTGTGTTTCC-3′; exon 5 antisense, 5′-TCTTACATCTATCCAGTTGGCCTCT-3′; Fas-L sense, 5′-AAAAAGAGCCGAGGAGTGTG-3′; antisense, 5′-GCTGACCTGTTGGACCTTGC-3′; IL-12 (p40) sense, 5′-GGAGACCCTGCCCATTGAACT-3′; antisense, 5′-CAACGTTGCATCCTAGGATCG-3′. The primer sequences of IL-2, IFN-γ, TGF-β1, IL-18 and β-actin were as described in previously published reports.38,39 Southern blot analysis was performed with the specific oligonucleotide probes, which were labelled with [γ-32P]ATP using a MEGA-LABEL labelling kit (Takara Shuzo Co. Ltd, Kyoto, Japan), in accordance with the manufacturer’s instructions. After hybridization for 18 hr at 65° in 1% sodium dodecyl sulphate (SDS), 1 m NaCl, 10% dextran sulphate, and 10 mg/ml heat-denatured salmon sperm DNA, the membrane was washed in 2×standard saline citrate (0·3 m NaCl/0·03 m sodium citrate) and 1% SDS, then exposed to a phosphorimaging plate for visualization on a Fuji BAS-2000 phosphorimaging system (Fuji Photo Film Co., Tokyo, Japan). The specific oligonucleotide probes were as follows: IL-4, 5′-GAGTCTCTGCAGCTCCATGA-3′; IL-7, 5′-ACTCTGAATCTTCATAGCCT-3′; IL-15, 5′-GCAATGAACTGCTTTCTCCT-3′; IL-15φ, 5′-GCTGTCAGATGCACAGAGAC-3′; Fas-L, 5′-AATGGAGGAGAAGAGGTTGAA-3′; and IL-12 (p40), 5′-TGTCTGCGTGCAAGCTCAGGA-3′. Oligonucleotide probe sequences of IL-2, IFN-γ and TGF-β1 were as described in a previous report.38 For determination of the Vγ/δ repertoire, serial dilutions of mRNA were primed with 20 pmol of Cδ (5′-CTTATGGAGATTTGTTTCAGC-3′) or Cγ (5′-CTTGGTCAGTATGGAGATTC-3′) in 20-μl reverse transcription reaction mixtures. Synthesized cDNA was amplified by PCR using specific 3′ primers for Cγ or Cδ and specific 5′ primers for Vγ1/2, Vγ2, Vγ4, Vγ5, Vγ6, Vγ7, Vδ1, Vδ2, Vδ3, Vδ4, Vδ5, Vδ6, Vδ7 or Vδ8, which were as described in previous reports.8,19,34,38 The PCR was performed by a method reported previously.40 Southern blots of the Vγ- or Vδ-PCR products were hybridized with 32P-labelled Cγ2 (MNG6)41 or oligo probe Jδ1 (5′-TTGGTTCCACAGTCACTTGG-3′).34
Statistical analysis
The significance of the data was determined by the Student’s t-test. P <0·05 was taken as significant.
RESULTS
Kinetics of bacterial growth in the organs after p.o. infection with L. monocytogenes
After the mice were inoculated p.o. with 1×109 viable L. monocytogenes, the bacteria in the liver, spleen, MLN and PP were counted on days 1, 3 and 5. In agreement with a previous report,42 a significant number of bacteria were first detected in the PP on day 1 and then in the MLN, liver and spleen on day 3 after oral infection. The number of bacteria reached a peak on day 3 in the PP and MLN and on day 5 in the liver and spleen. No bacteria were detected in the lungs after oral infection (data not shown).
Kinetics of i-IEL during oral infection with L. monocytogenes
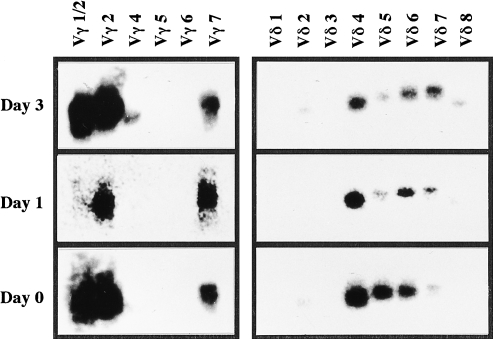
i-IEL consist of unique T-cell subpopulations bearing CD8αα and CD8αβ, or TCRαβ and γδ. The changes in i-IEL subpopulations following listerial infection were analysed by FACScan on days 1, 3 and 5 after oral infection. The mean data from five mice are summarized in Table 1. There were no significant differences in the absolute number and the ratio of i-IEL subpopulations bearing CD8αα and CD8αβ or TCRαβ and γδ on days 1, 3 and 5 after oral infection. We next examined (by RT–PCR) expression of the TCRγ/δ repertoire between i-IEL before infection and on days 1 and 3 after infection. As shown in Fig. 1, the purified γδ i-IEL preferentially used Vγ 2 and 7 on days 1 and 3 after infection, and Vδ 4, 6 and 7 on day 1 after infection, which is comparable with the usage before infection. Similarly, there were no differences in the Vβ repertoire of αβ i-IEL before and after oral infection, as assessed by FACS analysis using anti-Vβs mAb (data not shown). Thus, these results suggest that oral infection with L. monocytogenes may not affect the subpopulations of i-IEL.
Table 1.
Kinetics of T-cell subsets after per os (p.o.) infection with Listeria monocytogenes*
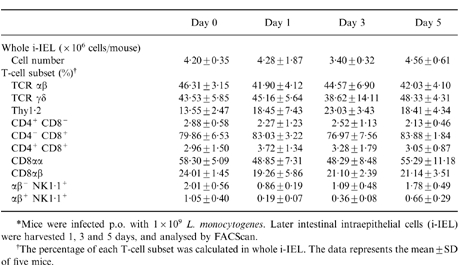
Figure 1.
Vγ or Vδ usages of γδ intestinal intraepithelial lymphocytes (i-IEL) after per os (p.o.) infection with 1 × 109 Listeria monocytogenes. mRNA of γδ i-IEL on days 0, 1 and 3 after infection was extracted, reverse transcribed into cDNA and amplified by the polymerase chain reaction (PCR) using primers for Cγ or Cδ and various Vγ or Vδ segments, respectively. The Southern blot of γ and δ PCR products was hybridized with Cγ2 and Jδ1, respectively.
Activation of i-IEL after oral infection with L. monocytogenes
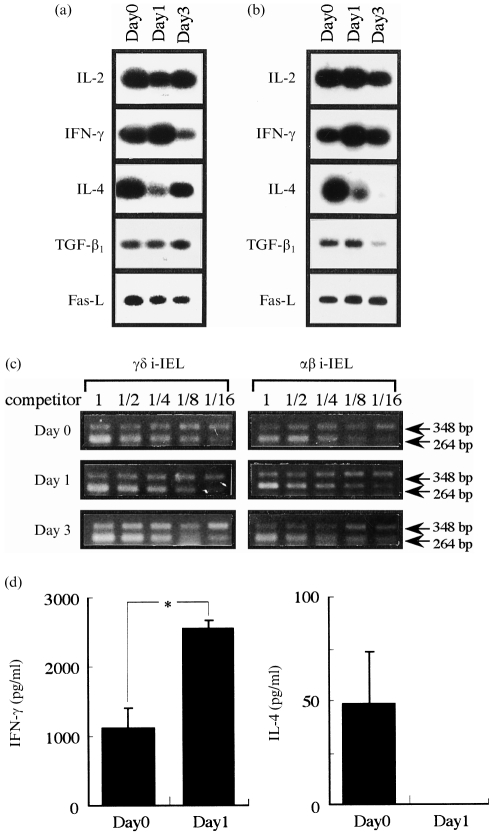
To investigate the qualitative difference of i-IEL before and after p.o. infection with L. monocytogenes, we examined (by RT–PCR) the expression levels of several cytokine genes, including IFN-γ and IL-4, in purified γδ i-IEL (Fig. 2a) and αβ i-IEL (Fig. 2b). The expression levels of IFN-γ mRNA in γδ i-IEL on day 1 after infection were higher than on day 0, and conversely the expression levels of IL-4 mRNA after infection were markedly lower on day 1. Similarly, the expression level of IFN-γ mRNA was increased in αβ i-IEL on day 1, while IL-4 expression was markedly decreased on day 1 after oral infection. The mRNA expression of levels of other genes, such as TGF-β1 and Fas-L, did not vary after infection. These results suggest that oral infection with L. monocytogenes induces a T helper (Th)-biased response by i-IEL at a very early stage after infection.
Figure 2.
Expression of cytokine mRNA in γδ intestinal intraepithelial lymphocytes (i-IEL) (a) or αβ i-IEL (b) and interferon-γ (IFN-γ) or interleukin (IL)-4 production (d) of i-IEL after per os (p.o.) infection with 1 × 109 Listeria monocytogenes. mRNA was extracted from i-IEL on days 1 and 3 after p.o. infection with L. monocytogenes and reverse transcription–polymerase chain reaction (RT–PCR) was carried out with cytokine-specific primers. (a) and (b) PCR products were hybridized with an internal probe specific for each cytokine. PCR products of IL-2, IFN-γ, IL-4, transforming growth factor-β1 (TGF-β1) and Fas-ligand (Fas-L) were: 167 bp, 213 bp, 180 bp, 257 bp and 537 bp, respectively. The typical profiles shown are representative of two independent experiments. (c) Expression of β-actin mRNA in γδ i-IEL (left panel) or αβ i-IEL (right panel). Each sample was assayed for β-actin mRNA by competitive PCR (see the Materials and methods). The target band is the upper band. For each sample, cDNA was co-amplified in a series of three reactions with a twofold dilution series of mimic concentrations. The sizes of PCR products of target and control DNAs are 348 bp and 264 bp, respectively. The expression of each cytokine was determined by PCR-assisted mRNA amplification using an adjusted concentration of cDNA. (d) Whole i-IEL (1 × 105) were cultured for 72 hr with CD3ε monoclonal antibody (mAb) and the culture supernatants were collected. The concentrations of IFN-γ and IL-4 in the culture supernatants were determined by enzyme-linked immunosorbent assay (ELISA). The data are representative of three separate examinations using pooled cells from three C57BL/6 mice and are shown as the mean of triplicate determinations ±SD. Significant differences compared with the value for normal mice are shown: *P <0·001.
We next examined IFN-γ and IL-4 production in the supernatant of the i-IEL cultured on immobilized anti-CD3ε mAb and assessed by ELISA specific for murine IFN-γ or IL-4 (Fig. 2d). IFN-γ production in the supernatant of i-IEL on day 1 after infection was significantly higher than on day 0 (P <0·001), while IL-4 was not detected in the culture supernatants of i-IEL on day 1. Thus, these results suggest that both αβ and γδ i-IEL are activated to produce IFN-γ after oral infection with L. monocytogenes.
IL-15 production during L. monocytogenes infection
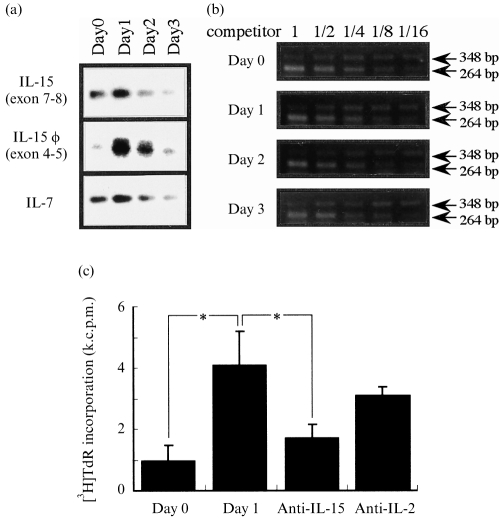
We previously reported that γδ i-IEL are stimulated to produce IFN-γ by IL-15, which are thought to be produced by epithelial cells.19 To investigate IL-15 production by i-EC during L. monocytogenes infection, we first extracted mRNA from i-EC on days 1, 2 and 3 after infection, and examined the expression level of the IL-15 gene in i-EC by RT–PCR using primers specific for exons 7 and 8 of the IL-15 gene. Consistent with our previous report,18 i-EC before infection expressed an appreciable level of IL-15 mRNA as well as IL-7 mRNA (Fig. 3a). The expression levels of IL-15 and IL-7 genes increased on day 1 after infection followed by a rapid decrease on day 3 after oral infection. We recently reported an alternative exon in murine IL-15 mRNA, which is termed ‘φ’. This alternative exon, which exists between exon 4 and 5 of the murine IL-15 gene, effectively enhances the translation of IL-15 mRNA in protein levels.43 Therefore, we next compared (by RT–PCR) the expression of the ‘φ’ exon in IL-15 mRNA in i-EC after oral infection, using primers specific for exon 4 and 5 of the IL-15 gene and Southern hybridization with a φ-specific probe. i-EC on day 1 after oral infection expressed the highest level of the φ exon. Thus, listerial infection causes an early increase in alternative IL-15 mRNA in i-EC, followed by a rapid down-regulation.
Figure 3.
Expression of interleukin (IL)-15 mRNA in intestinal epitherlial cells (i-EC) after per os (p.o.) infection with 1 × 109 Listeria monocytogenes. (a) mRNA was extracted from i-EC on the day indicated, reverse transcribed and cDNA was amplified using specific primers for IL-15 exon 7–8, IL-15 exon 4–5 or IL-7. The polymerase chain reaction (PCR) products were resolved on 1·8% agarose gels and were Southern blotted using radiolabelled, cytokine-specific internal oligonucleotide probes. PCR products of IL-15 exon 7–8, IL-15 exon 4–5 and IL-7 were 180 bp, 215 bp and 250 bp, respectively. The typical profiles shown are representative of three (IL-15 exon 7–8, IL-7) or two (IL-15 exon 4–5) independent experiments. (b) Each sample was assayed for β-actin mRNA by competitive PCR (see the Materials and methods). The target band is the upper band. For each sample, cDNA was co-amplified in a series of three reactions with a twofold dilution series of mimic concentrations. The sizes of PCR products of target and control DNAs were 348 bp and 264 bp, respectively. The expression of each cytokine was determined by PCR-assisted mRNA amplification using an adjusted concentration of cDNA. (c) Proliferative response of CTLL-2 cells stimulated with culture supernatants of i-EC after p.o. infection with 1 × 109 L. monocytogenes. CTLL-2 cells were stimulated with the 24-hr culture supernatants of i-EC, on day 1 after p.o. infection, with anti-IL-15 monoclonal antibody (mAb) (10 μg/ml) or anti IL-2 mAb (10 μg/ml). The value for the proliferative response of CTLL-2 cells without culture supernatant was 864·6±62·7 counts per minute (c.p.m.). The data are representative of two separate examinations using pooled cells from three C57BL/6 mice and are shown as the mean of triplicate determinations ±SD. *P <0·001. k.c.p.m., × 103 c.p.m.
We then examined the production of IL-15 in protein levels in the culture supernatant of i-EC on day 1 and that on day 0, as assessed by proliferation of the CTLL-2 cell line. As shown in Fig. 3(c), the culture supernatant of i-EC on day 1 after infection showed a higher activity for CTLL-2 proliferation than that on day 0 (P <0·001). The proliferation was significantly inhibited by an anti-IL-15 mAb but not by an anti-IL-2 mAb. In addition, the production of IL-2 or IL-4 was not detected (by ELISAs specific for murine IL-2 or IL-4) in the culture supernatants of i-EC on day 1 or day 0. These results suggest that i-EC on day 1 after infection may produce a larger amount of IL-15 protein. IL-12 and IL-18 are known to exert stimulatory activity for IFN-γ production by NK and T cells.39,44 Therefore, we also examined the IL-12 and IL-18 expression by i-EC before and after oral infection with L. monocytogenes. The expression level of the IL-18 gene did not increase on day 1 after infection, and no IL-12 was detected in the culture supernatants of i-EC on day 1 after infection (data not shown).
DISCUSSION
We show here that oral infection with L. monocytogenes may cause an early increase in IL-15 gene expression and consequently IL-15 production in the intestine of mice. Concurrently, i-IEL, both of αβ and γδ T cells, showed Th1-like responses characterized by IFN-γ production at the early stage after infection with L. monocytogenes. i-IEL are unique lymphoid populations exhibiting non-MHC-restricted cytotoxicity mediated by perforin/serine esterase and the Fas/Fas-L system, and produce a variety of cytokines including Th1-type cytokines, Th2-type cytokines and TGF-β, which is important for IgA synthesis.20–24 Yamamoto et al. reported that i-IEL produce IFN-γ following p.o. infection with L. monocytogenes, suggesting a contribution of i-IEL to the host defence against invasion of bacteria in the intestine.36 Hence, it can be speculated that IL-15 produced by infected i-EC may participate in the early activation of i-IEL in the intestine, which provides the first line of host defence against oral infection with microbes.
IL-15 has been shown to stimulate lymphocytes via the IL-2Rβ/cγ complex, and shares several stimulatory properties with IL-2, including the ability to co-stimulate proliferation of primary T cells and the murine CTLL-2 cell line, and to induce the generation of CTL and lymphokine-activated killer cells.1 Furthermore, IL-15 has been shown to activate NK cells, B cells, Mφ and mast cells.1–3,5,6,8,45–47 We have previously reported that IL-15 plays important roles in the proliferation and maintenance of γδ T cells in the peritoneal cavity.8 Although γδ T cells are present in much smaller numbers in the peripheral lymphoid tissues,25 selected subsets of γδ T cells are increased preferentially in the inflamed sites, such as the peritoneal cavity, liver, lung or intestine, early after infection with various pathogens.25–35 We hypothesize that IL-15 released by infected macrohages induces γδ T-cell proliferation in the peritoneal cavity after an intraperitoneal (i.p.) infection with L. monocytogenes and plays an important role at an early stage of listerial infection, well before Listeria-specific αβ T cells are significantly activated for protection.7,8 Besides activated Mφ, IL-15 is thought to be produced by i-EC.9 We have obtained evidence that i-EC express an abundant level of IL-15 mRNA and produce IL-15 after infection with L. monocytogenes and, concurrently, i-IEL are activated to produce IFN-γ. Therefore, it is possible that IL-15 produced by infected i-EC may have an important function in the early activation of i-IEL in the intestine, and consequently may provide the first line of host defence in the intestine against oral infection with microbes. Our data revealed that not only γδ T cells, but also αβ T cells, showed a Th1-like response at an early stage after oral infection with L. monocytogenes. We have reported that αβ i-IEL expressing CD8αα, which are thought to be of a different lineage from those expressing CD8αβ,48 respond preferentially to exogenous IL-15. Therefore, IL-15 produced by i-EC may activate αβ i-IEL, especially those expressing CD8αα, as well as γδ i-IEL, which exclusively express CD8αα.
The role of IL-7 in the proliferation and differentiation of T cells, especially γδ T cells, has been noted previously.49–55 The epithelial γδ T cells appear in consecutive waves in the fetal and prenatal thymus, and home to the epidermis and mucosal epithelia of the uterus, vagina and tongue.25 IL-7 is reported to support the growth of γδ T cells from fetal thymocytes and epithelial γδ T cells in the intestine and skin.49–54 IL-7, together with IL-1 and IL-2, can cause expansion of γδ T cells from newborn thymic cells.55 Skeen & Ziegler reported that the peritoneal γδ T cells in Listeria-immune mice are dependent on cytokines produced by activated αβ T cells and Mφ. In particular, a striking synergistic effect of IL-1 and IL-7 on the proliferation of γδ T cells was noted.56 In this study, IL-7 mRNA also increased on day 1 after oral infection with L. monocytogenes. IL-12 and IL-18 are known to exert strong abilities to induce IFN-γ production by both resting and activating NK and T cells.39,44 These cytokines may also play an important role in the early activation of i-IEL during intestinal listeriosis. However, IL-12 was not detected in the culture supernatants of i-EC on day 1 after listerial infection, and IL-18 gene expression was not increased on day 1 after infection, excluding this possibility.
In conclusion, oral infection with L. monocytogenes may cause early IL-15 production by i-EC and, concurrently, Th1-like responses by i-IEL, which are characterized by IFN-γ production at an early stage after infection with L. monocytogenes. Our results suggest that IL-15, which might be produced by infected i-EC, may be one of the cytokines that has an important function in the early activation of i-IEL in intestine, which provides the first line of host defence against oral infection with microbes. However, the possible implication for the roles of IL-15 in host defence awaits the analysis of mice lacking IL-15.
Acknowledgments
This work was supported in part by grants (to Y.Y.) from the Ministry of Education, Science and Culture, and the Ministry of Health and Welfare in Japan, the Ohyama Health Foundation Inc., the Chiba Geigy Foundation (Japan) for the Promotion of Science, and the Ichiro Kanehara Foundation.
Abbreviations
- B6
C57BL/6
- Fas-L
Fas-ligand
- FITC
fluorescein isothiocyanate
- i-EC
intestinal epithelial cells
- IFN-γ
interferon-γ
- i-IEL
intestinal intraepithelial lymphocytes
- IL-15
interleukin-15
- Mφ
macrophage
- MLN
mesenteric lymph node
- PE
phycoerythrin
- PP
Peyer’s patch
- TGF-β1
transforming growth factor-β1.
REFERENCES
- 1.Grabstein KH, Eisenman J, Shanebeck K, et al. Cloning of a T cell growth factor that interacts with the β chain of the interleukin-2 receptor. Science. 1994;264:965. doi: 10.1126/science.8178155. [DOI] [PubMed] [Google Scholar]
- 2.Giri JG, Ahdieh M, Eisenman J, et al. Utilization of the β and γ chains of the IL-2 receptor by the novel cytokine IL-15. EMBO J. 1994;13:2822. doi: 10.1002/j.1460-2075.1994.tb06576.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tagaya Y, Bamford RN, Defilippis AP, Waldmann TA. IL-15: a pleiotropic cytokine with diverse receptor/signaling pathways whose expression is controlled at multiple levels. Immunity. 1996;4:329. doi: 10.1016/s1074-7613(00)80246-0. [DOI] [PubMed] [Google Scholar]
- 4.Bamford RN, Grant AJ, Burton JD, et al. The interleukin (IL) 2 receptor β chain is shared by IL-2 and a cytokine, provisionally designated IL-T, that stimulates T-cell proliferation and the induction of lymphokine-activated killer cells. Proc Natl Acad Sci USA. 1994;91:4940. doi: 10.1073/pnas.91.11.4940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carson WE, Giri JG, Lindemann ML, et al. Interleukin (IL) 15 is a novel cytokine that activates human natural killer cells via components of the IL-2 receptor. J Exp Med. 1994;180:1395. doi: 10.1084/jem.180.4.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Armitage RJ, Macduff BM, Eisenmann J, Paxton R, Grabstein KH. IL-15 has stimulatory activity for the induction of B cell proliferation and differentiation. J Immunol. 1995;154:483. [PubMed] [Google Scholar]
- 7.Hiromatsu K, Yoshikai Y, Matsuzaki G, et al. A protective role of 65-kDa heatshock protein-specific γδ T cells in primary infection with Listeria monocytogenes in mice. J Exp Med. 1992;175:49. doi: 10.1084/jem.175.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nishimura H, Hiromatsu K, Kobayashi N, et al. IL-15 is a novel growth factor for murine γδ T cells induced by Salmonella infection. J Immunol. 1996;156:663. [PubMed] [Google Scholar]
- 9.Reinecker HC, MacDermott RP, Mirau S, Dignass A, Podolsky DK. Intestinal epithelial cells both express and respond to interleukin 15. Gastroenterology. 1996;111:1706. doi: 10.1016/s0016-5085(96)70036-7. [DOI] [PubMed] [Google Scholar]
- 10.Poussier P, Julius M. Thymus independent T cell development and selection in the intestinal epithelium. Annu Rev Immunol. 1994;12:521. doi: 10.1146/annurev.iy.12.040194.002513. [DOI] [PubMed] [Google Scholar]
- 11.Guy-Grand D, Cerf-Bensussan N, Malissen B, et al. Two gut intraepithelial CD8+ lymphocyte populations with different T cell receptors: a role for the gut epithelium in T cell differentiation. J Exp Med. 1991;173:471. doi: 10.1084/jem.173.2.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Klein JR. Advances in intestinal T-cell development and function. Immunol Today. 1995;16:322. doi: 10.1016/0167-5699(95)80145-6. [DOI] [PubMed] [Google Scholar]
- 13.Guy-Grand D, Rocha B, Vassalli P. Mucosal immunology. In: Kiyono H, McGhee JR, editors. Intraepithelial Lymphocytes. New York: Raven Press; 1993. p. 21. [Google Scholar]
- 14.Lefrancois L, Puddington L. Extrathymic intestinal T-cell development: virtual reality? Immunol Today. 1995;16:16. doi: 10.1016/0167-5699(95)80065-4. [DOI] [PubMed] [Google Scholar]
- 15.He YW, Malek TR. Interleukin-7 receptor a is essential for the development of γδ+ T cells, but not natural killer cells. J Exp Med. 1996;184:289. doi: 10.1084/jem.184.1.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maki K, Sunaga S, Komagane Y, et al. Interleukin 7 receptor-deficient mice lack γδ T cells. Proc Natl Acad Sci USA. 1996;93:7172. doi: 10.1073/pnas.93.14.7172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cao X, Shores EW, Hu-Li J, et al. Defective lymphoid development in mice lacking expression of the common cytokine receptor γδ chain. Immunity. 1995;2:223. doi: 10.1016/1074-7613(95)90047-0. [DOI] [PubMed] [Google Scholar]
- 18.Suzuki H, Duncan GS, Takimoto H, Mak TW. Abnormal development of intestinal intraepithelial lymphocytes and peripheral natural killer cells in mice lacking the IL-2 receptor β chain. J Exp Med. 1997;185:499. doi: 10.1084/jem.185.3.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Inagaki-Ohara K, Nishimura H, Mitani A, Yoshikai Y. Interleukin-15 preferentially promotes the growth of intestinal intraepithelial lymphocytes bearing γδ T cell receptor in mice. Eur J Immunol. 1997;27:2885. doi: 10.1002/eji.1830271121. [DOI] [PubMed] [Google Scholar]
- 20.Yamamoto M, Fujihashi K, Beagley KW, McGhee JR, Kiyono H. Cytokine synthesis by intestinal intraepithelial lymphocytes. J Immunol. 1993;150:106. [PubMed] [Google Scholar]
- 21.Fujihashi K, Taguchi T, Alcher WK, et al. Immunoregulatory function for murine intraepithelial lymphocytes: γ/δ T cells abrogate oral tolerance, while α/β TCR+ T cells provide B cell help. J Exp Med. 1992;175:695. doi: 10.1084/jem.175.3.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sakai T, Kimura Y, Inagaki-Ohara K, et al. Fas-mediated cytotoxicity by host intestinal intraepithelial lymphocytes is involved in the enteropathy during acute graft-vs.-host disease. Gastroenterology. 1997;113:168. doi: 10.1016/s0016-5085(97)70092-1. [DOI] [PubMed] [Google Scholar]
- 23.Inagaki-Ohara K, Nishimura H, Inagaki H, et al. Potential for involvement of fas antigen/fas ligand interaction in apoptosis of epithelial cells by intraepithelial lymphocytes in murine small intestine. Lab Invest. 1997;77:421. [PubMed] [Google Scholar]
- 24.Guy-Grand D, Malassis-Seris M, Briottet C, Vassalli P. Cytotoxic differentiation of mouse gut thymodependent and independent intraepithelial T lymphocytes is induced locally: correlation between functional assays, presence of perforin and granzyme transcripts, and cytoplasmic granules. J Exp Med. 1991;173:1549. doi: 10.1084/jem.173.6.1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Haas W, Pereira P, Tonegawa S. γ/δ cells. Annu Rev Immunol. 1993;11:637. doi: 10.1146/annurev.iy.11.040193.003225. [DOI] [PubMed] [Google Scholar]
- 26.Kaufmann SHE. Immunity to intracellular bacteria. Annu Rev Immunol. 1993;11:129. doi: 10.1146/annurev.iy.11.040193.001021. [DOI] [PubMed] [Google Scholar]
- 27.Modlin RL, Pirmez C, Hofman FM, et al. Lymphocytes bearing antigen-specific γδ T-cell receptors accumulate in human infectious disease lesions. Nature. 1989;339:544. doi: 10.1038/339544a0. [DOI] [PubMed] [Google Scholar]
- 28.Janis EM, Kaufmann SHE, Schwartz RH, Pardoll DM. Activation of γδ T cells in the primary immune response to Mycobacterium tuberculosis. Science. 1989;244:713. doi: 10.1126/science.2524098. [DOI] [PubMed] [Google Scholar]
- 29.Ho M, Webster HK, Tongtawe P, Pattanapanyasat K, Weidanz WP. Increased γδ T cells in acute Plasmodium falciparum malaria. Immunol Lett. 1990;25:139. doi: 10.1016/0165-2478(90)90105-y. [DOI] [PubMed] [Google Scholar]
- 30.Inoue T, Yoshikai Y, Matsuzaki G, Nomoto K. Early appearing γ/δ-bearing T cells during infection with Calmette–Guerin bacillus. J Immunol. 1991;146:2754. [PubMed] [Google Scholar]
- 31.Ohga S, Yoshikai Y, Takeda Y, Hiromatsu K, Nomoto K. Sequential appearance of γ/δ- and α/β-bearing T cells in the peritoneal cavity during an i.p. infection with Listeria monocytogenes. Eur J Immunol. 1990;20:533. doi: 10.1002/eji.1830200311. [DOI] [PubMed] [Google Scholar]
- 32.Eichelberger M, Allan W, Carding SR, Bottomly K, Doherty PC. Activation status of the CD4−CD8−γδ-T cells recovered from mice with influenza pneumonia. J Immunol. 1991;147:2069. [PubMed] [Google Scholar]
- 33.Hasegawa T, Tanaka T, Yoshikai Y. The appearance of γδ T cells in the peritoneal cavity and liver during primary infection with Listeria monocytogenes in rats. Int Immunol. 1992;4:1129. doi: 10.1093/intimm/4.10.1129. [DOI] [PubMed] [Google Scholar]
- 34.Emoto M, Danbara H, Yoshikai Y. Induction of γ/δ T cells in murine salmonellosis by an avirulent but not by a virulent strain of Salmonella choleraesuis. J Exp Med. 1992;176:363. doi: 10.1084/jem.176.2.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ogasawara T, Emoto M, Kiyotani K, et al. Sendai virus pneumonia: evidence for the early recruitment of γδ T cells during the disease course. J Virol. 1994;68:4022. doi: 10.1128/jvi.68.6.4022-4027.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yamamoto S, Russ F, Teixeira HC, Conradt P, Kaufmann SHE. Listeria monocytogenes-induced gamma interferon secretion by intestinal intraepithelial γδ T lymphocytes. Infect Immun. 1993;61:2154. doi: 10.1128/iai.61.5.2154-2161.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kimura Y, Tomida S, Matumoto Y, Hiromatsu K, Yoshikai Y. Evidence for the early recruitment of T-cell receptor γδ+ T cells during rat listeriosis. Immunology. 1996;87:21. [PMC free article] [PubMed] [Google Scholar]
- 38.Inagaki-Ohara K, Kobayashi N, Nishimura H, et al. Effects of a nonapeptide thymic hormone on intestinal intraepithelial lymphocytes in mice following administration of 5-fluorouracil. Cell Immunol. 1996;171:30. doi: 10.1006/cimm.1996.0169. [DOI] [PubMed] [Google Scholar]
- 39.Okamura H, Tsutsi H, Komatsu T, et al. Cloning of a new cytokine that induces IFN-gamma production by T cells. Nature. 1995;378:88. doi: 10.1038/378088a0. [DOI] [PubMed] [Google Scholar]
- 40.Atsuta N, Nishimura H, Nakamura N, et al. Diversity of Vγ gene segments rearranged to the Jγ 4 gene in mice. J Immunol. 1995;154:676. [PubMed] [Google Scholar]
- 41.Yoshikai Y, Reis MD, Mak TW. Athymic mice express a high level of functional γ-chain but greatly reduced levels of α- and β-chain T-cell receptor messages. Nature. 1986;324:482. doi: 10.1038/324482a0. [DOI] [PubMed] [Google Scholar]
- 42.MacDonald TT, Carter PB. Cell-mediated immunity to intestinal infection. Infect Immun. 1980;28:516. doi: 10.1128/iai.28.2.516-523.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nishimura H, Washizu J, Nakamura N, Enomoto A, Yoshikai Y. Translational efficiency is up-regulated by an alternative exon in murine interleukin-15 mRNA. J Immunol. 1998;160:936. [PubMed] [Google Scholar]
- 44.Trinchieri G. Interleukin-12: a proinflammatory cytokine with immunoregulatory functions that bridge innate resistance and antigen-specific adaptive immunity. Annu Rev Immunol. 1995;13:251. doi: 10.1146/annurev.iy.13.040195.001343. [DOI] [PubMed] [Google Scholar]
- 45.Alleva DG, Kaser SB, Monroy MA, Fenton MJ, Beller DI. IL-15 functions as a potent autocrine regulator of macrophage proinflammatory cytokine production: evidence for differential receptor subunit utilization associated with stimulation or inhibition. J Immunol. 1997;159:2941. [PubMed] [Google Scholar]
- 46.Doherty TM, Seder RA, Sher A. Induction and regulation of IL-15 expression in murine macrophages. J Immunol. 1996;156:735. [PubMed] [Google Scholar]
- 47.Tagaya Y, Burton JD, Miyamoto Y, Waldmann TA. Identification of a novel receptor/signal transduction pathway for IL-15/T in mast cells. EMBO J. 1996;15:4928. [PMC free article] [PubMed] [Google Scholar]
- 48.Murosaki S, Inagaki-Ohara K, Kusaka H, Ikeda H, Yoshikai Y. Apoptosis of intestinal intraepithelial lymphocytes induced by exogenous and endogenous glucocorticoids. Microbiol Immunol. 1997;41:139. doi: 10.1111/j.1348-0421.1997.tb01179.x. [DOI] [PubMed] [Google Scholar]
- 49.Havran W, Allison JP. Developmentally ordered appearance of thymocyte expression by pre-T cells in murine fetal liver cultures. Nature. 1988;335:443. doi: 10.1038/335443a0. [DOI] [PubMed] [Google Scholar]
- 50.Okazaki H, Ito M, Sudo T, et al. IL-7 promotes thymocyte proliferation and maintains immunocompetent thymocytes bearing α/β or γ/δ T cell receptors in vitro: synergism with IL-2. J Immunol. 1989;143:2917. [PubMed] [Google Scholar]
- 51.Watoson JD, Morrissey PJ, Namen AE, Conlon PJ, Widmer MB. Effect of IL-7 on the growth of fetal thymocytes in culture. J Immunol. 1989;143:1215. [PubMed] [Google Scholar]
- 52.Watanabe Y, Sudo T, Minato N, Ohnishi A, Katsura Y. Interleukin-7 preferentially supports the growth of γδ T cell receptor-bearing T cells from fetal thymocytes in vitro. Int Immunol. 1991;3:1067. doi: 10.1093/intimm/3.11.1067. [DOI] [PubMed] [Google Scholar]
- 53.Appasamy PM. IL-7-induced T cell receptor-γ gene expression by pre-T cells in murine fetal liver cultures. J Immunol. 1992;149:1649. [PubMed] [Google Scholar]
- 54.Uehira M, Matsuda H, Hikita I, et al. The development of dermatitis infiltrated by γδ T cells in IL-7 transgenic mice. Int Immunol. 1993;5:1619. doi: 10.1093/intimm/5.12.1619. [DOI] [PubMed] [Google Scholar]
- 55.Lynch F, Shevach EV. Activation requirements of newborn thymic γδ T cells. J Immunol. 1992;149:2307. [PubMed] [Google Scholar]
- 56.Skeen MJ, Ziegler HK. Intracellular interactions and cytokine responsiveness of peritoneal α/β and γ/δ T cells from Listeria-infected mice: synergistic effects of interleukin 1 and 7 on γ/δ T cells. J Exp Med. 1993;178:985. doi: 10.1084/jem.178.3.985. [DOI] [PMC free article] [PubMed] [Google Scholar]