Abstract
CD69 is a differentiation antigen expressed shortly after activation on T lymphocytes and other cells of haematopoietic origin, including natural killer (NK) cells. The function of CD69 on T lymphocytes acting as a costimulatory molecule in proliferation and lymphokine secretion is well established. NK cells express CD69 after activation by different stimuli such as phorbol 12-myristate 13-acetate (PMA), interleukin (IL)-2, IL-12, interferon-α (IFN-α) or anti-CD16 monoclonal antibodies (mAbs). However, although it has been shown that CD69 triggers NK-cell-mediated cytolytic activity, its effect on other NK-cell functions has not been studied. Furthermore, the possible interaction of CD69 triggering with other C-lectin type inhibitory receptors is not known. Thus, the objective of this work is to determine whether CD69-mediated NK cytotoxicity can be regulated by CD94 inhibitory receptor and the role of CD69 on other NK-cell functions different of cytotoxicity. The results show that CD69-mediated NK cytotoxicity can be abrogated by CD94 stimulation in NK cells expressing the CD94 inhibitory form of the receptor, indicating that CD94 regulates the cytotoxic events initiated by a wide variety of NK activatory receptors. We also show that anti-CD69 mAbs, not only triggered NK cytotoxicity, but also induce NK-cell proliferation, CD25 and intracellular adhesion molecule-1 (ICAM-1) expression, TNF-α production and Ca2+ mobilization in preactivated NK cells. These results suggest that CD69 plays a crucial role in NK-cell function contributing to sustain NK-cell activation, as it has been previously demonstrated in T cells.
INTRODUCTION
The human CD69 differentiation antigen is one of the earliest cell surface molecules expressed after activation of T and B lymphocytes and other cells of haematopoietic origin (for review see refs 1 and 2). CD69 is a disulphide-linked homodimer with two chains constitutively phosphorylated belonging to the type II integral protein with an extracellular C-type lectin superfamily domain.3–5 In humans, the CD69 gene is located in chromosome 12 at bands p13–p12 in a region known as natural killer (NK) complex,4,6,7 associated with other C-type lectin genes that control NK-cell activity, such as NKG2, CD94 or hNKRP-1 genes,8 suggesting that CD69 may have a particular significance in NK-cell function. Once expressed on T cells, CD69 acts as a costimulatory molecule leading to cell proliferation, secretion and/or cytotoxicity. CD69 is rapidly induced in NK cells shortly after activation9–11 and its role in NK cytotoxicity has been demonstrated both in human and mice.12,13 However, its possible implications on NK-cell functions other than cytotoxicity, has so far not been analysed. In this work, we study the potential role of CD69 on other NK biological functions and the regulation of CD69 mediated cytotoxicity by the CD94 inhibitory receptor.
MATERIALS AND METHODS
Reagents and monoclonal antibodies (mAbs)
The following murine mAbs were used: T3 (CD3, immunoglobulin G1; IgG1), interleukin (IL)-2R1 (CD25, IgG2a), NKH1 (CD56, IgG1), I2 (HLA-D/DR, IgG2a) and isotype-matched control mAbs purchased from Coulter (Hialeah, FL), fluoroscein isothiocyanate (FITC)-conjugated anti-ICAM-1 (CD54) purchased from AMAC, Inc. (Westbrook, ME), T1/24T6G12 (CD5, IgG2a), B1/H299 (CD20, IgG2a), MY4/322A (CD14, IgG2b), T112/101D2-4C1 (CD2, IgG1), T113/1mono-2A6 (CD2R, IgG3), 3G8 (CD16, IgG1), 3B8 (CD56, IgM) and Leu-23 (CD69, IgG1) from Becton-Dickinson (San Jose, CA), (CD94, IgG2a, provided by Dr M. López-Botet) and TP1/8 (CD69, IgG3, provided Dr F. Sánchez-Madrid). They were used as fluorochrome conjugate, purified antibody, or dilutions of ascites. phorbol 12-nyristate 13-acetate (PMA) was purchased from Sigma Chemical Co. (St. Louis, MO). IL-2 and IL-12 were kindly provided by Dr Gately (Hoffman-La Roche, Nutley, NJ).
NK cell enrichment and purification
Briefly, peripheral blood mononuclear cells (PBMC) were isolated by Ficoll–Hypaque density gradient centrifugation from heparinized venous blood or from cytopheresis buffy coats obtained from normal volunteer donors. Adherent mononuclear cells were depleted by incubation on plastic Petri dishes for 1 hr at 37°. Enriched NK cells were obtained by negative selection using T1/24T6G12 (CD5), MY4/322A (CD14) and B1/H299 (CD20) mAbs and immunomagnetic beads, as previously described.14,15 Purified CD56+ NK cells used in the proliferation assays were isolated by flow cytometry, as previously described.14 Cells from the NK leucocytosis 221707, previously defined as expressing the inhibitory form of CD94 (CD94+ NKG2A+),16 were used in the redirected lysis assays.
Proliferation assay
Purified NK cells were plated at 30 000 cells/well in 96-well U-bottomed microtitre plates (Flow Laboratories, McLean, VA) and [3H]TdR incorporation in response to various stimuli was measured using a 1205 Betaplate liquid scintillation counter (Pharmacia, Turku, Finland) after collecting samples with a 96 Mach II harvester (Tomtec, Orange, CT).
Immunofluorescence studies
Enriched NK cells were cultured for 48 hr in the presence of TP1/8 or 3B8 and/or PMA (10 ng/ml). After washing, the cells were stained with fluorescein-conjugated anti-CD25, anti-CD54 and anti-HLA-DR or control mAb and analysed by flow cytometry as previously described.17
Determination of tumour necrosis factor-α (TNF-α) production
Enriched NK cells were activated previously with PMA (10 ng/ml) during 24 hr. After washing, the cells were incubated in 96-well U-bottomed microtitre plates with the following anti-CD69 mAbs: TP1/8, 3G1 or BL-Ac/p26 alone or in combination with control anti-CD56 mAb 3B8. After incubation for 4 or 24 hr, TNF-α was measured in cell culture supernatants with an enzyme immunoassay for the quantitative determination of human TNF-α levels (Innotest hTNF-α, Innogenetics, Zwindrecht, Belgium).
Calcium flux measurements
Enriched NK cells were preactivated overnight with IL-2 (200 U/ml) or PMA (10 ng/ml) to induce CD69 expression. After washing, the cells (107/ml) were loaded with 20 μg/ml of Indo-1 (Molecular Probes, Junction City, OR) for 30–60 min at 37°. The Indo-1-loaded cells were washed twice and labelled with NKH1–phcoerythrin (PE) and incubated on ice for 30 min. The cells were washed and resuspended in RPMI-1640 with 5% AB human serum. The analysis was performed in the gated NKH1+ population by using an EPICS Elite cytofluorimeter (Coulter, Hialeah, FL). For each sample the 405 nm/485 nm ratio of unstimulated cells was set at 1 and analysis was performed on cells alone for 30–40 s. to obtain a baseline unstimulated measurement. The different mAbs and goat anti-mouse, immunoglobulin (GAM) were added and the analysis was then resumed for a total of 5 min. An increase in [Ca2+]i was revealed by an increase of the ratio of light emitted at 405 nm/485 nm.
NK-redirected lysis assay
NK-redirected lysis assays were performed as previously described. Briefly, P815 cells were incubated for 60 min at 37° with Na51Cr (100 μCi per 106 cells; 1 Ci=37 Gbq) (CIS, Cedex, France). Labelled cells were washed and 50 μl aliquots (5×103 cells per well) were shed into 96 U-well plates. Cells from the NK leucocytosis 221707 expressing the inhibitory form of CD94 (CD94+ NKG2A+)16 were used as effector cells at different effector-:target-cell ratios. This cell line does not express functionally activatory CD94/NKG2C or E receptors.16 To induce redirected lysis anti-CD69 and anti-CD16 mAbs were added to the wells. Anti-CD94 mAb (HP3B1) and the isotype-matched control (U7·27, Immunotech) were also added to analyse the effect of this receptor on NK-redirected lysis. All mAbs were used at a final concentration of 10 μg/ml. After 4 hr at 37° 100 μl of supernatant were collected from each well and the 51Cr released into the supernatant was measured by gamma counting. Results are presented as the mean of triplicate samples. Spontaneous and total 51Cr release were calculated by incubating target cells with culture medium or Triton-X-100, respectively. Specific lysis was calculated as:
 |
RESULTS AND DISCUSSION
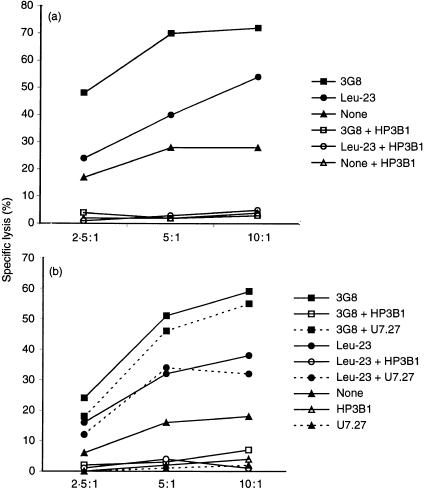
Our results show that the 221707 NK cells mediate both CD69-and CD16-redirected lysis of P815 target cells when incubated in the presence of the appropriate mAb, confirming previous data showing that anti-CD69 and anti-CD16 mAbs triggered the cytolytic activity of NK cells. Redirected lysis has been extensively used to define and analyse NK receptors able to trigger NK cytotoxicity. Our results indicate that both, CD16 and CD69 receptors have the capacity to activate the NK cytolytic machinery in the absence of other NK–target cell adhesion molecule interactions. In order to analyse whether this effect could be neutralized by the action of the inhibitory receptor CD94, redirected lysis by anti-CD69 was measured after anti CD94 treatment of NK cells. Interestingly, our results (Fig. 1) demonstrate that the addition of anti-CD94 mAb to the assay blocks NK-mediated redirected lysis induced either by CD16 or CD69 mAbs. This effect, mediated by CD94/NKG2A, is similar to the inhibitory effect of this molecule observed after its interaction with HLA-E, suggesting that, in this experimental model, crosslinking of CD94 also delivers an inhibitory signal to the NK effector cells. CD94 is a C-type lectin glycoprotein that associates to NKG2 gene products, constituting a family of NK receptors, CD94/NKG2, which recognize HLA-E antigens.16,18,19 The CD94/NKG2A heterodimer, expressed in the 221707 NK cell leucocytosis used in our experiments is functionally inhibitory.16 This inhibitory receptor blocks, not only natural cytotoxicity, but also CD16-redirected cytotoxicity in different experimental models.20–22 Our results indicate that the CD69 triggering of NK cells can also be blocked by the CD94/NKG2A inhibitory receptor at least in the experimental model used. Lanier et al.23 have also shown that the immunoglobulin superfamily inhibitory receptor NKB1 (p70 KIR) inhibits CD69 mediated cytotoxicity. These data further support the hypothesis that NK cytotoxicity is the result of a delicate balance between positive and negative signals delivered by activatory and inhibitory receptors23 and that either immunoglobulin or C-type lectin inhibitory receptors may independently regulate NK cytotoxicity triggered by different receptors.
Figure 1.
CD69-mediated redirected cytotoxicity is inhibited by anti-CD94 mAb (HP3B1). Leu-23 (anti-CD69) and 3G8 (anti-CD16) mAbs induce redirected lysis of P815 target cells when the NK leucocytosis 221707 is used as effector cells (filled symbols). The addition of HP-3B1 (anti-CD94) to the assay blocks NK-mediated redirected lysis induced by both CD16 and CD69 mAbs (empty symbols) (a,b). The addition of U7·27, an isotype-matched control (b), did not significantly affect redirected lysis induced by both CD16 and CD69 mAbs (filled symbols, dashed lines)
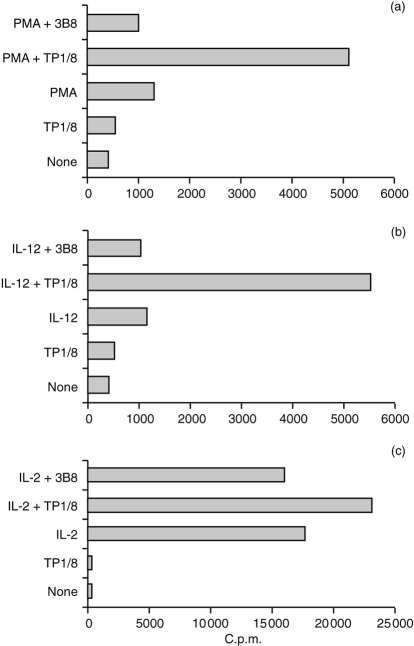
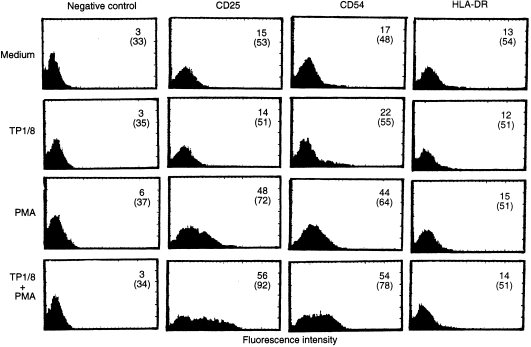
Given the significant role of CD69 as a T-cell costimulatory receptor, we have also studied its possible comitogenic effect on preactivated NK cells. Interestingly, the addition of anti-CD69 mAb TP1/8 strongly enhanced NK-cell proliferation of PMA or IL-12 preactivated highly purified NK cells (Fig. 2) while it had a weak synergistic effect on the NK response to IL-2. The addition of an anti-CD56 control mAb (3B8) did not affect PMA-, IL-12- or IL-2-induced proliferation, indicating that the comitogenic effect of TP1/8 is not caused by the activation of CD16 mediated by the Fc fragment of the mAb. Furthermore, neither IL-2 or IL-12 are comitogenic with CD1624,25 and PMA-treated NK cells downmodulate CD16 expression.26 TP1/8 is an IgG3 anti-CD69 mAb that is strongly comitogenic for T lymphocytes. This effect does not seem to be related to the mAb isotype but, on the contrary, to the epitope recognized, E1, which was defined as immunodominant and directly involved in the CD69 stimulatory function.27 Our results showing that anti-CD69 mAb TP1/8 is comitogenic for NK cells in combination with PMA, are similar to the observations on T-cell stimulation, where it was demonstrated that PMA required other costimulatory signals provided either by Ca2+ ionophores or by anti-CD69 mAbs to activate proliferation.28,29 Anti-CD69 mAbs are also comitogenic for T cells activated with mAbs against CD2, CD3, CD5 and CD28.30,31 In NK cells our results show that the addition of the anti-CD69 mAbs moderately enhanced IL-2-induced proliferation, but there was a strong synergistic enhancement of IL-12-induced proliferation. IL-2 and IL-12 are NK activatory lymphokines that enhance NK cytotoxicity, adhesion and cytokine production. However, whereas IL-2 induces strong NK proliferation, IL-12 only induces a low proliferative response in highly purified NK cells.14,32–34 Our results, showing that the addition of anti-CD69 mAb enhanced NK cell proliferation, support the hypothesis that CD69 costimulation plays a physiological role, not only in NK cell cytotoxicity, but also in other aspects of NK-cell activation. Effectively, anti-CD69 mAb used in combination with PMA, increased not only the number of NK cells expressing CD25 and ICAM-1 but also the density of these molecules on NK cells (Fig. 3). CD25 and ICAM-1 are expressed on NK cells when activated by different interleukins such as IL-2 and IL-12.14,17 It has been suggested that the expression of these markers will profoundly alter NK-cell function by promoting NK response to IL-2 and NK-cell adhesion to target cells.17,33 The finding that ICAM-1 expression can be induced in a low percentage of NK cells after treatment with TP1/8 alone, suggests that the low levels of CD69 expressed on resting NK cells obtained from healthy donors are also functionally active.
Figure 2.
Effect of anti-CD69 mAb TP1/8 on proliferation of CD56+ sorted NK cells induced by (a) PMA (10 ng/ml) (b) IL-12 (10 U/ml) and (c) IL-2 (100 pm). TP1/8 and 3B8 (anti-CD56) were ascites used at a final dilution of 1:500. [3H] Thymidine incorporation was measured on day 5. SD is no more than 10%.
Figure 3.
Effect of anti-CD69 mAb TP1/8 on CD25, ICAM-1 and HLA-DR antigen expression on enriched NK cells activated with PMA (10 ng/ml). These results are representative of three different experiments. TP1/8 was used at a final dilution of 1:500. The addition of 3 B8 mAb (anti-CD56) did not affect the expression of CD25, ICAM-1 and HLA-DR induced by PMA (data not shown). The mean fluorescence channel (MFC) is indicated in parentheses.
NK cells are also able to produce and release a range of different cytokines: i.e. TNF-α, interferon-γ (IFN-γ), granulocyte–macrophage colony-stimulating factor (GM-CSF).35 We have found that CD69 induced a strong release of TNF-α by PMA-preactivated NK cells after stimulation with anti-CD69 mAb for 24 hr (Fig. 4), whereas incubation with anti CD56 control mAb did not affect TNF-α production by NK cells. These results support the possible role of CD69 in the control of TNF-α production, as previously shown in T lymphocytes.29,36
Figure 4.
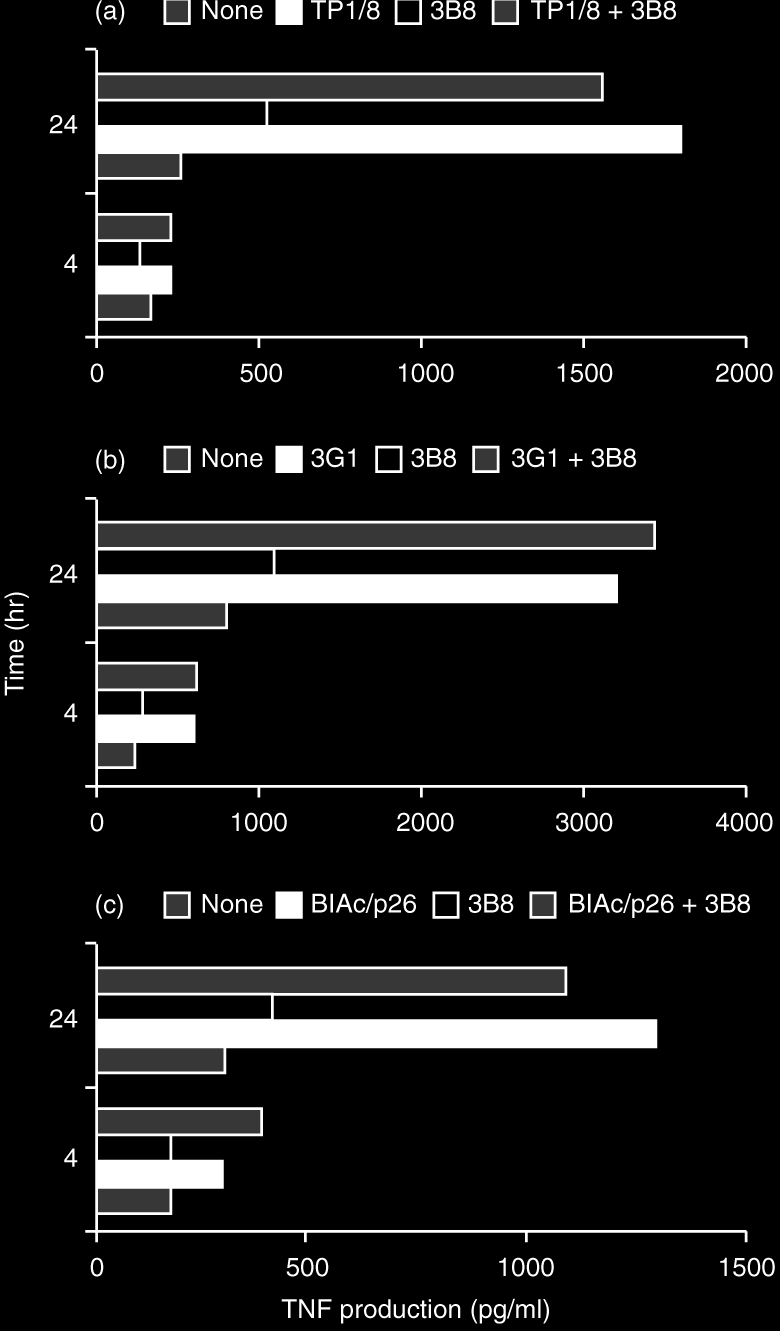
Production of TNF-α by purified NK cells preactivated overnight with PMA (10 ng/ml) and subsequently incubated with anti-CD69 mAbs TP1/8 (a), 3G1 (b) and BL-Ac/p26 (c) alone or in combination with control anti-CD56 mAb 3B8, during 4 and 24 hr. Results from three representative experiments performed independently. The addition of 3B8 (anti-CD56) did not significantly affect TNF-α production by NK cells.
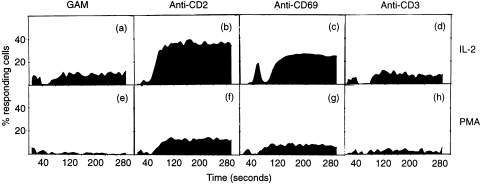
The results presented in Fig. 5 show that crosslinking of CD69 with TP1/8 and GAM induces Ca2+ mobilization in CD69+ NK cells previously activated with IL-2 or PMA. As expected, the addition of T112 + T113 anti-CD2 mAbs (used as positive control) also induced Ca2+ mobilization, while the anti-CD3 mAb T3 plus GAM or GAM alone (used as negative controls) did not affect Ca2+ flux on these cells. The molecular basis of the activation process coupled to CD69 activation has been extensively studied in preactivated T lymphocytes, neutrophils, platelets and monocytes and in all cases CD69 triggering induced Ca2+mobilization.29,37–39 This effect is similar to the observed in T lymphocytes using this mAb (TP1/8), where it has been shown that soluble mAb triggers proliferation although the crosslinking of the receptor is required for calcium mobilization. Our results showing that CD69 also induces Ca2+ mobilization in NK cells support that Ca2+ mobilization acts as a common pathway for CD69 activation in cells from different haematopoietic lineages.
Figure 5.
Ca2+ mobilization on NK cells preactivated overnight with PMA (10 ng/ml) (a–d) or IL-2 (100 pm) (e–h) and subsequently triggered with GAM (a,e), T112 + T113 (b,f), TP1/8 + GAM (c,g) or T3 + GAM (d,h). Results are indicated as (a) the ratio of emitted light of cells at 405 nm/485 nm, and (b) the percentage of responding cells.
In summary, in the present work we provide data supporting that CD69 mediates NK cytotoxicity that can be inhibited by CD94 inhibitory receptor. Furthermore, we also show that, beyond its role in NK cell cytotoxicity, CD69 is involved in regulating other NK-cell functions such as proliferation, TNF-α production and the expression of other, functionally relevant, activation antigens, such as CD25 and ICAM-1 by a mechanism that involves Ca2+ mobilization. This is relevant not only for a better knowledge of CD69 biology, but also for understanding the role of the CD94 inhibitory receptor in the inhibition of the cytotoxicity process triggered by a wide variety of NK receptors.
Acknowledgments
We are very grateful to Dr F. Sánchez-Madrid (Madrid, Spain) for kindly providing TP1/8 mAb and to Dr M. López-Botet (Madrid, Spain) for his HP3BI mAb. IL2 and IL12 were provided by Dr M. Gately (Hoffman-LaRoche). This work was supported by grants FIS95/1242 (RS), FIS98/1052 (RS) and FIS98/1051 (JP) from Ministry of Health and Junta de Andalucia (Spain). MJR was supported by US NIH grant CA-01730. FB is a fellow FPI from the Ministry for Science and Education (Spain).
Abbreviations
- NK
natural killer
- NKC
NK complex;α
- mAb
monoclonal antibody
- TNF-α
tumour necrosis factor-α
- IL
interleukin
REFERENCES
- 1.Testi R, D’Ambrosio D, de Maria R, Santoni A. The CD69 receptor: a multipurpose cell-surface trigger for hematopoietic cells. Immunol Today. 1994;15:479. doi: 10.1016/0167-5699(94)90193-7. [DOI] [PubMed] [Google Scholar]
- 2.Sanchez-Madrid F. Overview of CD69. In: Schlossman L S F, Boumsell W, Gilks J, et al., editors. Leucocyte Typing V. Oxford, New York, Tokio: Oxford University Press; 1995. p. 1123. [Google Scholar]
- 3.Hamann J, Fiebig H, Strauss M. Expression cloning of the early activation antigen CD69, a type II integral membrane protein with a C-type lectin domain. J Immunol. 1993;150:4920. [PubMed] [Google Scholar]
- 4.Lopez-Cabrera M, Santis AG, Fernandez-Ruiz E, et al. Molecular cloning, expression, and chromosomal localization of the human earliest lymphocyte activation antigen AIM/CD69, a new member of the C-type animal lectin superfamily of signal-transmitting receptors. J Exp Med. 1993;178:537. doi: 10.1084/jem.178.2.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ziegler SF, Ramsdell F, Hjerrild KA, et al. Molecular characterization of the early activation antigen CD69: a type II membrane glycoprotein related to a family of natural killer cell activation antigens. Eur J Immunol. 1993;23:1643. doi: 10.1002/eji.1830230737. [DOI] [PubMed] [Google Scholar]
- 6.Ziegler SF, Levin SD, Johnson L, et al. The mouse CD69 gene. Structure, expression, and mapping to the NK gene complex. J Immunol. 1994;152:1228. [PubMed] [Google Scholar]
- 7.Schnittger S, Hamann J, Dannenberg C, Fiebig H, Strauss M, Fonatsch C. Regional sublocalization of the human CD69 gene to chromosome bands 12p12.3–p13.2, the predicted region of the human natural killer cell gene complex. Eur J Immunol. 1993;23:2711. doi: 10.1002/eji.1830231051. [DOI] [PubMed] [Google Scholar]
- 8.Renedo M, Arce I, Rodriguez A, et al. The human natural killer gene complex is located on chromosome 12p12–p13. Immunogenetics. 1997;46:307. doi: 10.1007/s002510050276. [DOI] [PubMed] [Google Scholar]
- 9.Borrego F, Pena J, Solana R. Regulation of CD69 expression on human natural killer cells: differential involvement of protein kinase C and protein tyrosine kinases. Eur J Immunol. 1993;23:1039. doi: 10.1002/eji.1830230509. [DOI] [PubMed] [Google Scholar]
- 10.Borrego F, Galiani MD, Garcia-Cozar F. CD69 expression and function on NK cells. In: Schlossman S F, Boumsell L, Gilks W, et al., editors. Leucocyte Typing V. Oxford, New York, Tokio: Oxford University Press; 1995. p. 1427. [Google Scholar]
- 11.Gerosa F, Tommasi M, Benati C, et al. Differential effects of tyrosine kinase inhibition in CD69 antigen expression and lytic activity induced by rIL-2, rIL-12, and rIFN-α in human NK cells. Cell Immunol. 1993;150:382. doi: 10.1006/cimm.1993.1206. [DOI] [PubMed] [Google Scholar]
- 12.Moretta A, Poggi A, Pende D, et al. CD69-mediated pathway of lymphocyte activation: anti-CD69 monoclonal antibodies trigger the cytolytic activity of different lymphoid effector cells with the exception of cytolytic T lymphocytes expressing T cell receptor α/β. J Exp Med. 1991;174:1393. doi: 10.1084/jem.174.6.1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Karlhofer FM, Yokoyama WM. Stimulation of murine natural killer (NK) cells by a monoclonal antibody specific for the NK1.1 antigen. IL-2-activated NK cells possess additional specific stimulation pathways. J Immunol. 1991;146:3662. [PubMed] [Google Scholar]
- 14.Robertson MJ, Soiffer RJ, Wolf SF, et al. Response of human natural killer (NK) cells to NK cell stimulatory factor (NKSF): cytolytic activity and proliferation of NK cells are differentially regulated by NKSF. J Exp Med. 1992;175:779. doi: 10.1084/jem.175.3.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Luque I, Solana R, Galiani MD, et al. Threonine 80 on HLA-B27 confers protection against lysis by a group of natural killer clones. Eur J Immunol. 1996;26:1974. doi: 10.1002/eji.1830260845. [DOI] [PubMed] [Google Scholar]
- 16.Borrego F, Ulbrecht M, Weiss EH, Coligan JE, Brooks AG. Recognition of human histocompatibility leukocyte antigen (HLA)-E complexed with HLA class I signal sequence-derived peptides by CD94/NKG2 confers protection from natural killer cell-mediated lysis. J Exp Med. 1998;187:813. doi: 10.1084/jem.187.5.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Robertson MJ, Caligiuri MA, Manley TJ, Levine H, Ritz J. Human natural killer cell adhesion molecules. Differential expression after activation and participation in cytolysis. J Immunol. 1990;145:3194. [PubMed] [Google Scholar]
- 18.Braud VM, Allan DS, O’Callaghan CA, et al. HLA-E binds to natural killer cell receptors CD94/NKG2A, B and C. Nature. 1998;391:795. doi: 10.1038/35869. [DOI] [PubMed] [Google Scholar]
- 19.Lee N, Llano M, Carretero M, et al. HLA-E is a major ligand for the natural killer inhibitory receptor CD94/NKG2A. Proc Natl Acad Sci USA. 1998;95:5199. doi: 10.1073/pnas.95.9.5199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brumbaugh KM, Perez-Villar JJ, Dick CJ, Schoon RA, Lopez-Botet M, Leibson PJ. Clonotypic differences in signaling from CD94 (kp43) on NK cells lead to divergent cellular responses. J Immunol. 1996;157:2804. [PubMed] [Google Scholar]
- 21.Brooks AG, Posch PE, Scorzelli CJ, Borrego F, Coligan JE. NKG2A complexed with CD94 defines a novel inhibitory natural killer cell receptor. J Exp Med. 1997;185:795. doi: 10.1084/jem.185.4.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lopez-Botet M, Perez-Villar JJ, Carretero M, et al. Structure and function of the CD94 C-type lectin receptor complex involved in recognition of HLA class I molecules. Immunol Rev. 1997;155:165. doi: 10.1111/j.1600-065x.1997.tb00949.x. [DOI] [PubMed] [Google Scholar]
- 23.Lanier LL, Corliss B, Phillips JH. Arousal and inhibition of human NK cells. Immunol Rev. 1997;155:145. doi: 10.1111/j.1600-065x.1997.tb00947.x. [DOI] [PubMed] [Google Scholar]
- 24.Azzoni L, Kamoun M, Salcedo TW, Kanakaraj P, Perussia B. Stimulation of Fc gamma RIIIA results in phospholipase C-gamma 1 tyrosine phosphorylation and p56lck activation. J Exp. 1992;176:1745. doi: 10.1084/jem.176.6.1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Robertson MJ, Manley TJ, Donahue C, Levine H, Ritz J. Costimulatory signals are required for optimal proliferation of human natural killer cells. J Immunol. 1993;150:1705. [PubMed] [Google Scholar]
- 26.Borrego F, Lopez-Beltran A, Pena J, Solana R. Downregulation of Fc gamma receptor IIIA α (CD16-II) on natural killer cells induced by anti-CD16 mAb is independent of protein tyrosine kinases and protein kinase C. Cell Immunol. 1994;158:208. doi: 10.1006/cimm.1994.1268. [DOI] [PubMed] [Google Scholar]
- 27.Sanchez-Mateos P, Sanchez-Madrid F. Structure–function relationship and immunochemical mapping of external and intracellular antigenic sites on the lymphocyte activation inducer molecule, AIM/CD69. Eur J Immunol. 1991;21:2317. doi: 10.1002/eji.1830211005. [DOI] [PubMed] [Google Scholar]
- 28.Cebrian M, Yague E, Rincon M, Lopez-Botet M, de Landazuri MO, Sanchez-Madrid F. Triggering of T cell proliferation through AIM, an activation inducer molecule expressed on activated human lymphocytes. J Exp Med. 1988;168:1621. doi: 10.1084/jem.168.5.1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Testi R, Phillips JH, Lanier LL. T cell activation via Leu-23 (CD69) J Immunol. 1989;143:1123. [PubMed] [Google Scholar]
- 30.Risso A, Smilovich D, Capra MC, et al. CD69 in resting and activated T lymphocytes. Its association with a GTP binding protein and biochemical requirements for its expression. J Immunol. 1991;146:4105. [PubMed] [Google Scholar]
- 31.Vandenberghe P, Verwilghen J, Van Vaeck F, Ceuppens JL. Ligation of the CD5 or CD28 molecules on resting human T cells induces expression of the early activation antigen CD69 by a calcium- and tyrosine kinase-dependent mechanism. Immunology. 1993;78:210. [PMC free article] [PubMed] [Google Scholar]
- 32.Rabinowich H, Herberman RB, Whiteside TL. Differential effects of IL12 and IL2 on expression and function of cellular adhesion molecules on purified human natural killer cells. Cell Immunol. 1993;152:481. doi: 10.1006/cimm.1993.1306. [DOI] [PubMed] [Google Scholar]
- 33.Robertson MJ, Cameron C, Lazo S, Cochran KJ, Voss SD, Ritz J. Costimulation of human natural killer cell proliferation: role of accessory cytokines and cell contact-dependent signals. Nat Immun. 1996;15:213. [PubMed] [Google Scholar]
- 34.Yago T, Sukuda M, Fukushima H, et al. IL-12 promotes the adhesion of NK cells to endothelial selectins under flow conditions. J Immunol. 1998;161:1140. [PubMed] [Google Scholar]
- 35.Trinchieri G. Definition and biology of natural killer cells. In: Solana R, Pena J, editors. MHC Antigens and NK Cells. Austin TX: R. G. Landes Co.; 1994. p. 1. [Google Scholar]
- 36.Santis AG, Campanero MR, Alonso JL, et al. Tumor necrosis factor-α production induced in T lymphocytes through the AIM/CD69 activation pathway. Eur J Immunol. 1992;22:1253. doi: 10.1002/eji.1830220521. [DOI] [PubMed] [Google Scholar]
- 37.Testi R, Pulcinelli F, Frati L, Gazzaniga PP, Santoni A. CD69 is expressed on platelets and mediates platelet activation and aggregation. J Exp Med. 1990;172:701. doi: 10.1084/jem.172.3.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.De Maria R, Cifone MG, Trotta R, et al. Triggering of human monocyte activation through CD69, a member of the natural killer cell gene complex family of signal transducing receptors. J Exp Med. 1994;180:1999. doi: 10.1084/jem.180.5.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gavioli R, Risso A, Smilovich D, et al. CD69 molecule in human neutrophils: its expression and role in signal-transducing mechanisms. Cell Immunol. 1992;142:186. doi: 10.1016/0008-8749(92)90279-x. [DOI] [PubMed] [Google Scholar]