Abstract
We have previously shown that engagement of the T-cell receptor (TCR)/CD3 complex with anti-CD3 antibody induces tyrosine phosphorylation of p105CasL (CasL), a member of the p130Cas docking protein family. In the present work, we attempted to determine which protein tyrosine kinases (PTKs) regulate TCR-mediated phosphorylation of CasL. We show here that an association between CasL and two types of Src family PTKs, Fyn and Lck, is induced by anti-CD3 cross-linking of human H9 T cells. In contrast, ZAP-70, another PTK that also plays a critical role in the TCR signalling, failed to bind CasL, even after anti-CD3 stimulation. In vitro kinase assays revealed that Fyn and Lck, but not ZAP-70, were capable of phosphorylating CasL. Moreover, we found that CasL was constitutively hyperphosphorylated in vivo in splenocytes of MRL-MP-lpr/lpr mice, in which overproduction and excessive activation of Fyn and Lck have previously been shown to occur. Constitutive in vivo binding of CasL to both kinases was also demonstrated in lpr splenocytes. These results strongly suggest that CasL is a substrate for Fyn and Lck PTKs in TCR signal transduction.
INTRODUCTION
p105CasL (CasL; also known as human enhancer of filamentation-1, HEF1) is a recently described cytoplasmic protein that is related to p130Cas (Crk-associated substrate; Cas) and Eft/Sin.1–5 All members in the Cas-related protein family have a single N-terminal Src homology (SH) 3 domain, between eight and 15 potential Crk-SH2-binding motifs and a putative binding site for Src family kinases in their C-terminal portion. Thus, Cas-family proteins are thought to act as ‘docking proteins’, which link one signalling pathway to another. Despite their structural similarities, tissue distribution of each Cas family member is differentially regulated and hence they appear to exert distinctive biological functions.1–5 CasL was originally identified as a protein whose tyrosine phosphorylation is significantly enhanced in response to T-cell adhesion to the extracellular matrix.1,6,7 Like the prototype p130Cas, the SH3 domain of CasL specifically binds to the focal adhesion kinase p125FAK (FAK), which plays an essential role in integrin-mediated signal transduction.1,8 In addition to integrin signals, we and others have recently shown that CasL also participates in T-cell antigen receptor (TCR) signalling pathways.9,10 Engagement of the TCR complex with anti-CD3 monoclonal antibody (mAb) induces a significant increase in tyrosine phosphorylation of CasL and its subsequent binding to the Crk adaptor protein.9,10 Thus, CasL functions at a site where signalling pathways triggered by two distinct receptor systems converge. It has recently been shown that integrin-mediated CasL phosphorylation is primarily regulated by FAK.8 However, the mechanism by which TCR stimulation induces tyrosine phosphorylation of CasL is not yet known.
It has been well established that at least three protein tyrosine kinases (PTK) – Fyn, Lck and ZAP-70 – are involved in the initiation of TCR signal transduction.11,12 Fyn and Lck belong to the Src family, while ZAP-70, along with Syk, makes up the Syk PTK family. Ligation of the TCR induces enzymatic activation of Fyn, Lck and ZAP-70, which subsequently phosphorylate their specific substrates. However, their target proteins have not been fully elucidated. In the present report, we demonstrate that CasL is a potential substrate for Fyn and Lck kinases but not for ZAP-70. Given the previous observations that tyrosine-phosphorylated CasL binds to Crk and C3G,8–10 CasL acts as a docking protein that may link TCR-coupled PTKs to small GTPase pathways.
MATERIALS AND METHODS
Antibodies
Mouse mAb against CD3 (OKT3) was used in our study. Mouse mAb against p130Cas (clone 21) and p56Lck were obtained from Transduction Laboratories (Lexington, KY). Rabbit antiserum (Cas2) against p130Cas has been described previously.5 Antiphosphotyrosine mAb 4G10 was purchased from Upstate Biotechnology, Inc. Laboratories (Lake Placid, NY). Rabbit polyclonal antibody against ZAP-70 and mouse mAb against p59Fyn were obtained from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). A rabbit polyclonal antibody (designated as U71), which specifically recognizes CasL, was generated by immunizing rabbits with a synthetic peptide corresponding to amino acid residues 562–583 (GSKHLKNGPESIMNSTEYPHGG) of CasL.
Cells
The human T-cell line H9 was cultured in RPMI-1640 containing 10% heat-inactivated fetal calf seum (FCS) and 2 mm glutamine. Single-cell suspensions of splenocytes were prepared from spleens of MRL-MP-lpr/lpr mice (lpr mice) or its congenic MRL-MP-+/+ strain (+/+ mice) by removing red blood cells in hypotonic NH4Cl lysis buffer.
Stimulation of cells and preparation of cell lysates
Cells were washed three times, resuspended in RPMI serum-free medium and dispensed into 1·5 ml Eppendorf tubes with 10 × 107 cells/ml per sample. The samples were left as controls or incubated with saturating amount of OKT3 for 15 min at 4°, washed once with cold medium, and then incubated with 200 μl of medium containing antimouse immunoglobulin (10 μg/ml) at 37° for 2 min. The reaction was terminated by addition of 1 ml of stop solution (cold phosphate-buffered saline (PBS) containing 5 mm EDTA, 10 mm NaF, 10 mm sodium pyrophosphate and 0·4 mm sodium orthovanadate). Cells were pelleted and then solubilized in lysis buffer (1% Nonidet P-40 (NP-40), 150 mm NaCl, 50 mm Tris HCl, pH 8·0, 5 mm EDTA, 1 mm phenylmethylsulphonyl fluoride (PMSF), 10 mm iodoacetamide, 10 mm NaF, 10 mm sodium pyrophosphate and 0·4 mm sodium orthovanadate) for 15 min on ice. After removing insoluble material by centrifugation at 15 000 g for 10 min, supernatants were stored at −80° until use.
Immunoprecipitation and immunoblotting
Immunoprecipitation was performed as described previously.13 In brief, cell extracts were incubated with various antibodies for 1 hr at 4°, followed by additional incubation with protein G–Sepharose beads for 1 hr at 4°. Beads were then washed five times with 1% NP-40 lysis buffer to remove unbound proteins. Immune complexes were treated with sample buffer, and subjected to sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS–PAGE). Proteins on the gel were electrotransferred to nitrocellulose membranes, and then subjected to immunoblotting using an enhanced chemiluminescence kit (Amersham, Arlington Heights, IL) as a detection system.
In vitro kinase assay
Immunoprecipitated proteins were washed twice with lysis buffer, twice with kinase buffer (10 mm HEPES, pH 7·3, 50 mm NaCl, 5 mm MnCl2, 5 mm MgCl2, 100 mm Na2VO4) and then incubated for 30 min at room temperature in kinase buffer containing 0·1 mm ATP. Proteins were then eluted by boiling with 0·5% SDS in 50 mm Tris-HCl, pH 7·0, 5 mm EDTA, 10 mm dithiothreitol (DTT), and then reimmunoprecipitated.
RESULTS
Ligation of the TCR/CD3 complex with anti-CD3 mAb induces tyrosine phosphorylation of CasL
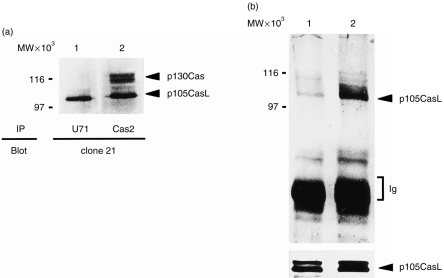
As an initial step in the present work, we generated a rabbit polyclonal antibody (designated as U71), which specifically recognizes CasL, by immunizing rabbits with a synthetic peptide corresponding to (GSKHLKNGPESIMNSTEYP HGG) of CasL. This peptide was predicted to have a low homology with p130Cas. NP-40 lysates of H9 T cells were immunoprecipitated either with U71 or Cas2 sera, and immunoblotted with clone 21 mAb. Although Cas2 and clone 21 were developed as antibodies recognizing p130Cas, both were shown to cross-react with CasL, as described previously.1 As shown in Fig. 1(a), while Cas2 precipitated proteins with apparent molecular weights of 115/125 (p130Cas) and 105 (CasL), U71 recognized only the 105 000 MW protein. In this particular experiment, CasL appeared to migrate as a single band; an additional band with slower mobility (110 000 MW) was occasionally detected in other experiments (see Fig. 1b and Fig. 3). Both forms were regarded as the product of the same transcript of the CasL gene, as described previously.1 This result indicates that U71 specifically recognizes CasL but not p130Cas. Using U71, we confirmed our previous findings9 that CasL undergoes tyrosine phosphorylation upon anti-CD3 cross-linking of H9 T cells (Fig. 1b), while the expression of CasL was not significantly different before and after anti-CD3 stimulation (lower panel).
Figure 1.
Anti-CD3 cross-linking induces tyrosine phosphorylation of CasL. (a) H9 T cells were immunoprecipitated with U71 (lane 1) and Cas2 (lane 2), and were immunoblotted with clone 21. (b) H9 T cells were immunoprecipitated with U71 before (lane 1) and after (lane 2) anti-CD3 cross-linking, followed by antiphosphotyrosine immunoblotting (upper panel). This membrane was reprobed with clone 21 to verify that the same amount of protein was loaded into each lane.
Figure 3.
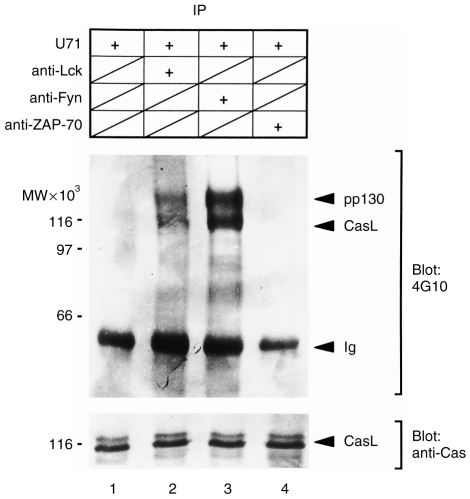
Fyn and Lck, but not ZAP-70, kinases phosphorylate CasL in vitro. CasL, Fyn, Lck and ZAP-70 were separately immunoprecipitated from cell extracts of unstimulated H9 T cells. The CasL immunoprecipitate was divided into four: CasL immunoprecipitate alone as a control (lane 1); or mixed with anti-Lck (lane 2), anti-Fyn (lane 3) or anti-ZAP-70 (lane 4) immunoprecipitates. Each mixture was subjected to an in vitro kinase assay in the presence of ATP. CasL was reprecipitated with U71 and subjected to antiphosphotyrosine immunoblotting (upper panel), as detailed in the Materials and methods. The same membrane was reprobed with clone 21 (lower panel). IP, immunoprecipitate.
Anti-CD3 cross-linking induces association of CasL with Fyn and Lck, but not with ZAP-70
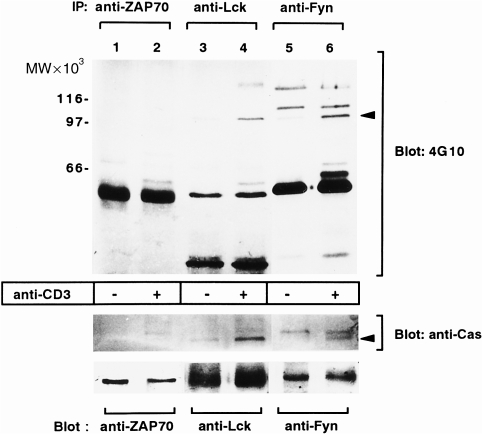
Three TCR-associated PTKs: Fyn, Lck, and ZAP-70, are known to act immediately downstream of the TCR in the activation signalling process.11,12 We therefore examined which PTKs are associated with CasL after TCR engagement. To achieve this, Fyn, Lck, or ZAP-70 were immunoprecipitated from NP-40 lysates of H9 cells and immunoblotted with antiphosphotyrosine antibody. As shown in Fig. 2(a) (upper panel), a 105 000 MW phosphoprotein was co-precipitated with Fyn and Lck, but not with ZAP-70, after anti-CD3 cross-linking. This band was confirmed as CasL by reprobing the upper half of the membrane with clone 21 (Fig. 2a, middle panel). In the anti-Fyn immunoprecipitates (lanes 5 and 6), an additional band with a slower gel mobility was detectable both in unstimulated and stimulated H9 cells. Although the molecular nature of this protein has not yet been clarified, it may represent p130Cas because it is reactive with clone 21 (Fig. 1a) and is constitutively associated with Fyn through the Fyn-SH3 domain (H. Kanda et al., unpublished observation) There was no band corresponding to CasL in anti-ZAP-70 precipitates. The lower portion of the membrane was reprobed with antibodies against Fyn (lanes 1 and 2), Lck (lanes 3 and 4) or ZAP-70 (lanes 5 and 6), to confirm that equal amounts of each protein were precipitated (Fig. 2a, bottom panel). Thus, our results suggest that ligation of the TCR induces an association of CasL with Fyn and Lck, but not with ZAP-70.
Figure 2.
Anti-CD3 cross-linking induces association of CasL with Fyn and Lck kinases but not with ZAP-70. Cell extracts from H9 T cells, either unstimulated (lanes 1, 3 and 5) or stimulated (lanes 2, 4 and 6) with anti-CD3 cross-linking, were immunoprecipitated with antibodies against ZAP-70 (lanes 1 and 2), Lck (lanes 3 and 4) and Fyn (lanes 5 and 6). The immunoprecipitates were subjected to immunoblotting with antiphosphotyrosine antibody. The arrow indicates the 105 000 MW protein whose tyrosine phosphorylation was enhanced in response to anti-CD3 cross-linking and is associated with Lck and Fyn but not with ZAP-70. The upper part of the membrane (>90 000 MW) was reprobed with clone 21 (middle panel), while the lower part (<90 000 MW) was reprobed with antibodies against ZAP-70 (lane 1 and 2), Lck (lanes 3 and 4) and Fyn (lanes 5 and 6). IP, immunoprecipitate.
Fyn and Lck, but not ZAP-70, are able to phosphorylate CasL in vitro
As Fyn and Lck associate with CasL in vivo, we next investigated whether or not these kinases are able to phosphorylate CasL in vitro. For this purpose, CasL was immunoprecipitated with U71 from unstimulated H9 cell lysates. Fyn, Lck and ZAP-70 kinases were also immunoprecipitated from the same lysates. U71 immunoprecipitates were left as controls or mixed with anti-Fyn, anti-Lck or anti-ZAP-70 immunoprecipitates. These samples were then subjected to in vitro kinase assay in the presence of ATP. After stopping the reaction, CasL was reprecipitated with U71 antibody from the reaction mixture, and analysed by immunoblotting with 4G10 to detect tyrosine phosphorylation of CasL (Fig. 3, upper panel). The same membrane was reprobed with anti-CasL (clone 21) to verify that equal amounts of CasL were precipitated in each lane (lower panel). It is apparent that two proteins with faster (≈115 000 MW) and slower (≈130 000 MW) gel mobilities were intensely phosphorylated when incubated with anti-Lck (lane 2) or anti-Fyn (lane 3) immunoprecipitates in the presence of ATP. Identity of the 115 000 MW protein as phosphotyrosyl CasL was confirmed because its mobility in the gel exactly matched that of CasL determined by the clone 21 immunoblot of the same membrane. However, the 130 000 MW protein (pp130) was not recognized by clone 21. Moreover, pp130 is not present in in vitro kinase assays using anti-Fyn and anti-Lck immunoprecipitates without adding CasL (data not shown). Thus, the pp130 protein is distinct from, but associated with, CasL, and is phosphorylated in an in vitro kinase assay by either Fyn or Lck. However, at present, the molecular identity of pp130 is not known. In the absence of either kinase (Fyn or Lck), tyrosine phosphorylation of CasL was negligible (lane 1). Likewise, CasL phosphorylation was undetectable in the absence of ATP, even when mixed with anti-Fyn and anti-Lck immunoprecipitates (data not shown). In contrast to Lck and Fyn, ZAP-70 failed to phosphorylate either CasL or pp130 (lane 4). Taken together, these results suggested that Fyn and Lck, but not ZAP-70, were able to phosphorylate CasL in vitro, which is consistent with the in vivo association of CasL with these PTKs.
CasL is constitutively hyperphosphorylated in splenocytes of MRL-MP-lpr/lpr mice
It has previously been reported that the atypical CD4−CD8− T cells found in lpr mice overexpress Fyn and Lck and that these PTKs may be involved in aberrant signal transduction in these cells.14 If Fyn and Lck are overexpressed in T cells from these animals, and these PTKs are responsible for CasL phosphorylation, enhanced CasL phosphorylation might be expected. Therefore, we examined expression and tyrosine phosphorylation of CasL in splenocytes obtained from 20-week-old lpr mice and compared them with those from congenic +/+ mice. As shown in Fig. 4(a), antipTyr immunoblotting of CasL immunoprecipitates revealed that although tyrosine phosphorylation of CasL was almost undetectable in +/+ splenocytes, it was extensive in lpr splenocytes. When the amounts of CasL protein were examined by reprobing the same membrane, it was revealed that lpr splenocytes did express slightly higher amounts of CasL (Fig. 4a). Densitometric analysis revealed that the expression of CasL was 1·2-fold higher in lpr than in +/+ splenocytes. On the other hand, CasL phosphorylation was 7·4 times stronger in lpr than in control splenocytes. This indicates that hyperphosphorylation of CasL in lpr splenocytes is not simply explained by overexpression. Lysates were also immunoprecipitated with antibodies against Fyn and Lck, and immunoblotted with clone 21. CasL was clearly detected in anti-Fyn and anti-Lck precipitates from lpr lymphocytes (Fig. 4b, 4c, respectively). In contrast, CasL was totally undetectable in either anti-Fyn or anti-Lck immunoprecipitates from +/+ splenocytes. Moreover, we failed to detect CasL in anti-ZAP-70 immunoprecipitates from either control or lpr lymphocytes (data not shown). Thus, CasL is specifically and constitutively associated with Fyn and Lck kinases in lpr lymphocytes. Given the constitutive activation of Fyn and Lck kinase activities in lpr lymphocytes, hyperphosphorylation of CasL in these abnormal T cells would be mediated by both kinases. These results again favour the view that Fyn and Lck are principal regulators for CasL phosphorylation in T cells.
Figure 4.
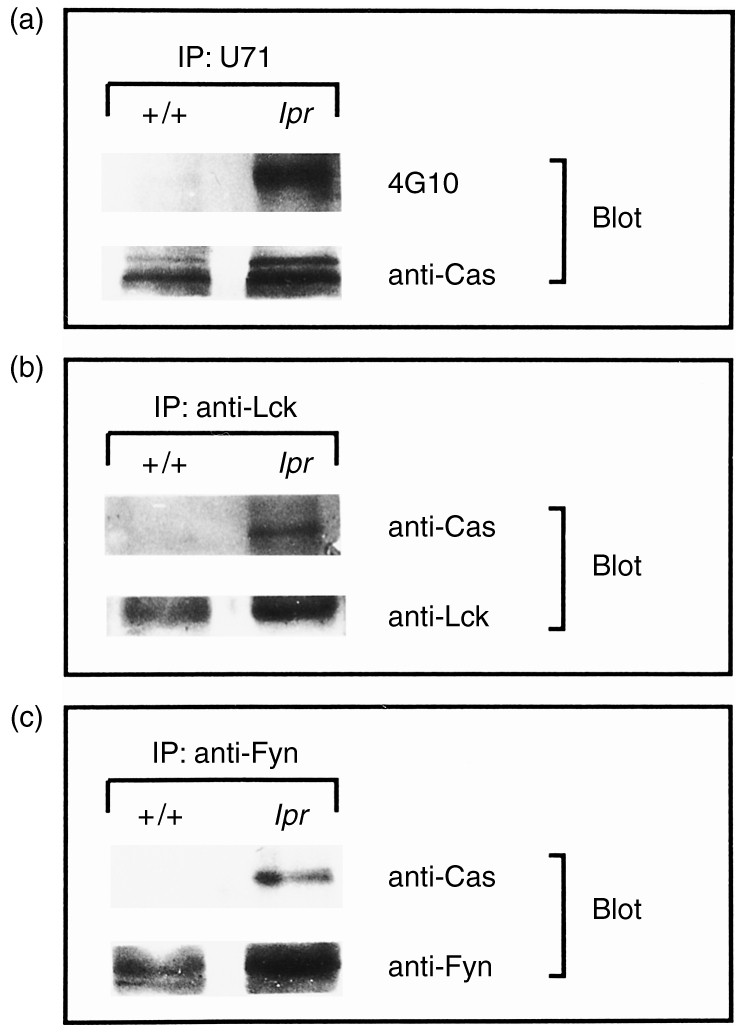
CasL expressed by lpr splenocytes was highly tyrosine phosphorylated and was associated with Lck and Fyn kinases. (a) Splenocytes obtained from 20-week-old lpr and +/+ mice were immunoprecipitated with U71, followed by antiphosphotyrosine immunoblotting. The same membrane was reprobed with clone 21 (anti-Cas). (b) Splenocyte extracts from 20-week-old lpr and +/+ mice were immunoprecipitated with anti-Lck, and probed with clone 21 immunoblotting. The same membrane was reprobed with anti-Lck. (c) Splenocyte extracts from 20-week-old lpr and +/+ mice were immunoprecipitated with anti-Fyn, and probed with clone 21 immunoblotting. The same membrane was reprobed with anti-Fyn. IP, immunoprecipitate.
DISCUSSION
In the present work, we showed that Fyn and Lck are the principal regulators of TCR-mediated tyrosine phosphorylation of CasL. This is based on the following evidence:
TCR engagement leads to the in vivo association of CasL with Fyn and Lck;
both kinases are capable of phosphorylating CasL in vitro; and
CasL is constitutively hyperphosphorylated in splenocytes from lpr mice expressing activated Fyn and Lck.
In contrast, ZAP-70, another TCR-linked PTK, neither associates with CasL in vivo nor phosphorylates CasL in vitro. CasL has the YDYVHL sequence in its C-terminal portion, which is conserved among all Cas-related proteins and provides a binding site for the SH2 domain of Src-family kinases, including Fyn and Lck.1–5 CasL was originally identified as a protein whose tyrosine phosphorylation is enhanced by integrin-mediated T-cell adhesion.6,7 A recent report by Tachibana et al. has shown that integrin-dependent tyrosine phosphorylation of CasL is primarily mediated by FAK.8 Indeed, FAK forms a stable complex with CasL in T cells through binding to the CasL-SH3 domain. Moreover, TCR stimulation was shown to result in tyrosine phosphorylation of FAK,15 suggesting that FAK also participates in the TCR signalling. However, Ohashi et al. have recently reported that a CasL mutant that lacks the SH3 domain could not be phosphorylated by integrin ligation, but still underwent tyrosine phosphorylation in response to TCR ligation.10 This suggests that FAK is not essential for TCR-mediated phosphorylation of CasL. Given the results presented here, CasL phosphorylation may be differentially regulated by integrin- and TCR-mediated signalling pathways. We and others have previously shown that the kinetics of CasL phosphorylation are different after TCR and integrin stimulation.9,10 While the response to TCR engagement is rapid and transient, integrin ligation induces CasL phosphorylation more slowly and in a prolonged fashion. Concordantly, TCR-mediated activation of Fyn and Lck is transient, whereas integrin-mediated activation of FAK is relatively sustained.6 Differential usage of distinct kinases may account for the observed difference in kinetics of CasL phosphorylation, although other mechanisms involving protein tyrosine phosphatases cannot be excluded.
An additional novel finding in this work is that, when compared with control mice, CasL expressed by lpr splenocytes was constitutively hyperphosphorylated on tyrosine residues. We also noted that CasL was constitutively associated in vivo with Fyn and Lck in lpr lymphocytes, but not in +/+ lymphocytes. As described, these findings further support a potential role of Fyn and Lck in CasL phosphorylation because markedly enhanced enzyme activity of these kinases is a characteristic feature of CD4−CD8− double-negative T (DNT) cells from this autoimmune mouse.14 In accordance with increased PTK activities, several other molecules have been described to be highly tyrosine phosphorylated in these lpr DNT cells.16,17 Interestingly, lpr DNT cells respond poorly to TCR stimulation or mitogens.18 The functional relationship between excessive phosphorylation and hyporesponsiveness of lpr DNT cells has not yet been elucidated. In this regard, it would be important to delineate the effect of hyperphosphorylation of CasL on its downstream signals.
Once phosphorylated, CasL binds to the Crk adaptor protein, which in turn recruits C3G, a guanine nucleotide exchange factor for a Rap-1 small GTPase of the Ras family.8,9 Although Rap-1 was initially discovered through an expression cloning strategy for suppressors of Ras transformation,19 a recent study using the phaeochromocytoma cell line, PC12, indicated that Rap-1 mediates sustained activation of mitogen-activated protein (MAP) kinases by acting through Raf-B.20,21 In other cell types, however, Rap-1-mediated signalling seems to impair the activation of MAP kinases.22,23 It is still not known how Rap-1 mediates contrasting signals in different cell types. Furthermore, it is not yet clear how CasL phosphorylation and interaction with C3G effects C3G function, Rap-1 activity or downstream pathways (such as activation or inactivation of MAP kinases) in normal or in lpr T cells. Nevertheless, it is now apparent that CasL acts as a docking protein that may link TCR-coupled PTKs to small GTPase pathways. Further studies will be necessary to define the signalling pathways lying downstream of CasL in both TCR and integrin-mediated signal transduction.
REFERENCES
- 1.Minegishi M, Tachibana K, Sato T, Iwata S, Nojima Y, Morimoto C. Structure and function of Cas-L, a 105-kD Crk-associated substrate-related protein that is involved in beta 1 integrin-mediated signaling in lymphocytes. J Exp Med. 1996;184:1365. doi: 10.1084/jem.184.4.1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Law SF, Estojak J, Wang B, Mysliwiec T, Kruh G, Golemis EA. Human enhancer of filamentation 1, a novel p130cas-like docking protein, associates with focal adhesion kinase and induces pseudohyphal growth in Saccharomyces cerevisiae. Mol Cell Biol. 1996;16:3327. doi: 10.1128/mcb.16.7.3327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ishino M, Ohba T, Sasaki H, Sasaki T. Molecular cloning of a cDNA encoding a phosphoprotein, Efs, which contains a Src homology 3 domain and associates with Fyn. Oncogene. 1995;11:2331. [PubMed] [Google Scholar]
- 4.Alexandropoulos K, Baltimore D. Coordinateactivation of c-Src by SH3- and SH2-binding sites on a novel p130Cas-related protein, Sin. Genes Dev. 1996;10:1341. doi: 10.1101/gad.10.11.1341. [DOI] [PubMed] [Google Scholar]
- 5.Sakai R, Iwamatsu A, Hirano N, et al. A novel signaling molecule, p130, forms stable complexes in vivo with v-Crk and v-Src in a tyrosine phosphorylation-dependent manner. EMBO J. 1994;13:3748. doi: 10.1002/j.1460-2075.1994.tb06684.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nojima Y, Rothstein DM, Sugita K, Schlossman SF, Morimoto C. Ligation of VLA-4 on T cells stimulates tyrosine phosphorylation of a 105-kD protein. J Exp Med. 1992;175:1045. doi: 10.1084/jem.175.4.1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nojima Y, Tachibana K, Sato T, Schlossman SF, Morimoto C. Focal adhesion kinase (pp125FAK) is tyrosine phosphorylated after engagement of alpha 4 beta 1 and alpha 5 beta 1 integrins on human T-lymphoblastic cells. Cell Immunol. 1995;161:8. doi: 10.1006/cimm.1995.1002. [DOI] [PubMed] [Google Scholar]
- 8.Tachibana K, Urano T, Fujita H, et al. Tyrosine phosphorylation of Crk-associated substrates by focal adhesion kinase. A putative mechanism for the integrin-mediated tyrosine phosphorylation of Crk-associated substrates. J Biol Chem. 1997;272:29083. doi: 10.1074/jbc.272.46.29083. [DOI] [PubMed] [Google Scholar]
- 9.Kanda H, Mimura T, Morino N, et al. Ligation of the T cell antigen receptor induces tyrosine phosphorylation of p105CasL, a member of the p130Cas-related docking protein family, and its subsequent binding to the Src homology 2 domain of c-Crk. Eur J Immunol. 1997;27:2113. doi: 10.1002/eji.1830270840. [DOI] [PubMed] [Google Scholar]
- 10.Ohashi Y, Tachibana K, Kamiguchi K, Fujita H, Morimoto C. T cell receptor-mediated tyrosine phosphorylation of Cas-L, a 105-kDa Crk-associated substrate-related protein, and its association of Crk and C3G. J Biol Chem. 1998;273:6446. doi: 10.1074/jbc.273.11.6446. [DOI] [PubMed] [Google Scholar]
- 11.Chan AC, Desai DM, Weiss A. The role of protein tyrosine kinases and protein tyrosine phosphatases in T cell antigen receptor signal transduction. Annu Rev Immunol. 1994;12:555. doi: 10.1146/annurev.iy.12.040194.003011. [DOI] [PubMed] [Google Scholar]
- 12.Wange RL, Samelson LE. Complex complexes:signaling at the TCR. Immunity. 1996;5:197. doi: 10.1016/s1074-7613(00)80315-5. [DOI] [PubMed] [Google Scholar]
- 13.Nojima Y, Morino N, Mimura T, et al. Integrin-mediated cell adhesion promotes tyrosine phosphorylation of p130Cas, a Src homology 3-containing molecule having multiple Src homology 2-binding motifs. J Biol Chem. 1995;270:15398. doi: 10.1074/jbc.270.25.15398. [DOI] [PubMed] [Google Scholar]
- 14.Katagiri T, Ting JP, Dy R, Prokop C, Cohen P, Earp HS. Tyrosine phosphorylation of a c-Src-like protein is increased in membranes of CD4− CD8− T lymphocytes from lpr/lpr mice. Mol Cell Biol. 1989;9:4914. doi: 10.1128/mcb.9.11.4914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maguire JE, Danahey KM, Burkly LC, van-Seventer GA. T cell receptor- and beta 1 integrin-mediated signals synergize to induce tyrosine phosphorylation of focal adhesion kinase (pp125FAK) in human T cells. J Exp Med. 1995;182:2079. doi: 10.1084/jem.182.6.2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mimura T, Minota S, Nojima Y, et al. Constitutive tyrosine phosphorylation of the vav proto-oncogene product in MRL/Mp-lpr/lpr mice. J Immunol. 1997;158:2977. [PubMed] [Google Scholar]
- 17.Samelson LE, Davidson WF, Morse HC, Klausner RD. Abnormal tyrosine phosphorylation on T-cell receptors in lymphoproliferative disorders. Nature. 1986;324:674. doi: 10.1038/324674a0. [DOI] [PubMed] [Google Scholar]
- 18.Sobel ES, Kakkanaiah VN, Rapoport RG, Eisenberg RA, Cohen PL. The abnormal lpr double-negative T cell fails to proliferate in vivo. Clin Immunol Immunopathol. 1995;74:177. doi: 10.1006/clin.1995.1026. [DOI] [PubMed] [Google Scholar]
- 19.Kitayama H, Sugimoto Y, Matsuzaki T, Ikawa Y, Noda M. A ras-related gene with transformation suppressor activity. Cell. 1989;56:77. doi: 10.1016/0092-8674(89)90985-9. [DOI] [PubMed] [Google Scholar]
- 20.York RD, Yao H, Dillon T, et al. Rap1 mediates sustained MAP kinase activation induced by nerve growth factor. Nature. 1998;392:622. doi: 10.1038/33451. [DOI] [PubMed] [Google Scholar]
- 21.Vossler MR, Yao H, York RD, Pan MG, Rim CS, Stork PJ. cAMP activates MAP kinase and Elk-1 through a B-Raf- and Rap1-dependent pathway. Cell. 1997;89:73. doi: 10.1016/s0092-8674(00)80184-1. [DOI] [PubMed] [Google Scholar]
- 22.Cook SJ, Rubinfeld B, Albert I, McCormick F. RapV12 antagonizes Ras-dependent activation of ERK1 and ERK2 by LPA and EGF in Rat-1 fibroblasts. EMBO J. 1993;12:3475. doi: 10.1002/j.1460-2075.1993.tb06022.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Boussiotis VA, Freeman GJ, Berezovskaya A, Barber DL, Nadler LM. Maintenance of human T cell anergy: blocking of IL-2 gene transcription by activated Rap1. Science. 1997;278:124. doi: 10.1126/science.278.5335.124. [DOI] [PubMed] [Google Scholar]