Abstract
Infections of the dental pulp commonly result in infraosseus inflammation and bone destruction. However, the role of phagocytic leucocytes in the pathogenesis of pulpal infections has been uncertain. In this work we used P/E−/− selectin-deficient mice, which lack rolling adhesion of leucocytes to endothelium and mimic the human syndrome, leucocyte adhesion deficiency II (LAD-II), to test the hypothesis that phagocytic leucocytes protect against pulpal infection and subsequent periapical infraosseus bone resorption. P/E−/− mice and P/E+/+ wild-type controls were subjected to surgical pulp exposure, and both groups were infected with a mixture of pulpal pathogens including Prevotella intermedia, Fusobacterium nucleatum, Peptostreptococcus micros and Streptococcus intermedius. Animals were killed after 20 days, and the extent of infraosseus bone destruction was quantified by histomorphometry. In two separate experiments, P/E−/− mice had significantly greater bone resorption than P/E+/+ controls. The increased bone destruction correlated with a twofold decrease in polymorphonuclear (PMN) infiltration into periapical inflammatory tissues of P/E−/− mice. P/E−/− mice had higher tissue levels of the bone resorptive cytokine, interleukin (IL)-1α. Tissue levels of IL-2, IL-4, IL-10, tumour necrosis factor-α (TNF-α) and interferon-γ (IFN-γ) were all higher in P/E−/− mice, but the increases were not statistically significant. Only IL-12 was higher in P/E+/+ mice, possibly reflecting a greater number of infiltrating monocytes in wild-type mice. These findings demonstrate that phagocytic leucocytes are protective in this model, and suggest that elevated expression of inflammatory cytokines is responsible for the observed bone destruction.
INTRODUCTION
Infection of the dental pulp occurs subsequent to caries, trauma and operative dental procedures. These infections, which are predominantly caused by Gram-negative anaerobes, commonly result in pulpal necrosis and consequent infraosseus inflammation and bone destruction.1 The earliest pulpal responses to bacterial invasion and/or the diffusion of bacterial products through dentinal tubules include the influx of polymorphonuclear leucocytes (PMNs) and monocytes.2–4 With more persistent infection the response becomes mixed, with infiltration of T cells, B cells and plasma cells in addition to the earlier migrating phagocytes.5–7
It is unknown whether phagocytic cell responses are predominantly protective or destructive of bone following pulpal infection. In this regard, soluble poly-β1-6-glucotriosyl-b1-3-glucopyranose glucan (PGG-glucan), a biological response modifier that increases PMN and monocyte production and priming, decreases osteolysis in this model.8 It is also well established that individuals with PMN defects, including chronic granulomatous disease, cyclic neutropenia, Papillon–Lefevre Syndrome, Chediak–Higashi syndrome and leucocyte adhesion deficiencies, experience increased incidence and severity of oral bacterial infections, including periodontal diseases.9–11 Perhaps best studied are the leucocyte adhesion deficiencies (LADs), of which there are currently two recognized types:12–14 LAD-I is caused by mutations in β2 integrin, whereas LAD-II is secondary to defects in the fucosylation of the ligands for selectins.15 LAD-II leads to a deficiency in the initial rolling adhesion of leucocytes, whereas LAD-I prevents firm adhesion and transmigration. Both syndromes result in an inability of PMNs to emigrate from the vasculature into tissues in response to infection.
Genetically engineered knockout mice lacking both P- and E-selectins (P/E−/−) provide an animal model that, to a certain extent, mimics LAD-II.16,17 The endothelium of P/E−/− mice lacks constitutively expressed P-selectin, as well as E-selectin, which is induced by inflammatory stimuli such as lipopolysaccharide (LPS) and tumour necrosis factor-α (TNF-α).18 P/E−/− mice exhibit a 16-fold elevation in circulating neutrophils and a 10-fold increase in monocytes, impaired leucocyte rolling and adhesion, and decreased PMN and monocyte infiltration into local sites of inflammation or infection. P/E−/− mice are susceptible to ulcerative skin lesions and other opportunistic bacterial infections.
In this work, the effect of combined P- and E-selectin deficiency on the extent of infection-stimulated bone destruction was determined, using a well-characterized murine model of infraosseus inflammation.
MATERIALS AND METHODS
Animals
Genetically engineered 12–20-week-old mice, doubly deficient in P- and E-selectins,16 on a background of C57Bl/6 × 129Sv, were bred and maintained under specific pathogen-free conditions in the M. I. T. Animal Facility. Age-matched wild-type C57Bl/6 mice were used as controls. All animals were free of skin lesions and were in apparent good general health.
Induction of infraosseus bone destruction
For induction of pulpal infection and infraosseus periapical inflammation and bone resorption, mice were anaesthetized with ketamine HCl (62·5 mg/kg) and xylazine (12·5 mg/kg), in sterile phosphate-buffered saline (PBS), by intraperitoneal (i.p.) injection. All four first molar pulps were exposed to the oral environment using a no. 1/4 round bur and a variable speed electric handpiece (Osada Electric, Los Angeles, CA) under a surgical microscope (MC-M92; Seiler, St. Louis. MO).19 The exposure size was equivalent to the diameter of the bur. Animals without exposures (three mice per group) were used as controls.
Infection regimen
To equalize the infectious challenge in the P/E−/− and wild-type groups, exposed pulps were infected with a mixture of four common endodontic pathogens, including Prevotella intermedia (ATCC 25611), Fusobacterium nucleatum (ATCC 25586), Peptostreptococcus micros (ATCC 33270) and Streptococcus intermedius (ATCC 27335), as described by Teles et al.20 This regimen results in reproducible colonization of the root canal with pathogens in this model. In brief, organisms were inoculated onto tryptic soy broth with yeast (TSBY) agar plates, and were grown for 7 days under anaerobic conditions (80% N2, 10% H2, 10% CO2). Colonies were harvested into prereduced, anaerobically sterilized, Ringer’s solution under nitrogen influx. The final concentration of each organism was determined spectrophotometrically, and the four species of micro-organism were mixed to yield 109 cells of each bacterial species/ml in methylcellulose (10 μg/ml). At the time of pulp exposure (day 0), both groups of animals were infected with 100 μl of the bacterial mixture directly into the exposed pulp and the oral cavity.
Sample preparation
Animals were killed on day 20 (Exp. 1) or day 14 (Exp. 2) after pulp exposure. After removal of soft tissue, both hemi-mandibles were isolated and were fixed in fresh 4% paraformaldehyde in PBS. For the maxillae, periapical tissues surrounding the mesial and distal root apices were carefully extracted, together with surrounding bone, in a block specimen under a surgical microscope. The gingiva, oral mucosa, tooth crown and bone marrow were dissected free and discarded. Periapical tissues were rinsed in PBS, freed of clots, weighed and immediately frozen in dry ice/ethanol. Spleens were also obtained, weighed and frozen at −70°. Spleens obtained from C57Bl/6 mice immunized i.p. (50 μg) with bovine serum albumin (fraction V; Sigma, St. Louis, MO) in Freund’s complete adjuvant (Sigma) were used as a positive control for cytokine assays.
Histomorphometry
After 24 hr of fixation, hemi-mandibles were decalcified in EDTA (10% wt/vol) in 0·1 m Tris-HCl, pH 6·96. Six-micrometre paraffin sections were cut and stained with haematoxylin and eosin. Slides were encoded and evaluated twice by an examiner blinded to the genotypes. Sections that included the crown and distal root of the mandibular first molar, and which exhibited a patent root canal apex representing the central portion of the tooth and periapical region, were selected for analysis. A minimum of three sections per tooth were evaluated histomorphometrically using an Optimas Bioscan image analysis system (Media Cybernetics, Bethell, WA). The largest values of the cross-sectional area of periapical resorption (mm2), were averaged from the two independent measurements (left and right first molars) to obtain a summary value of bone resorption for each animal.
Protein preparations and enzyme-linked immunoassay
For protein extract preparation, frozen periapical tissue samples were ground using a precooled sterile mortar and pestle, and the tissue fragments were dispersed in 650–800 μl of lysis buffer consisting of 100 μg/ml bovine serum albumin (fraction V, Sigma), 100 μg/ml Zwittergent-12 (Boehringer Mannheim, Indianapolis, IN), 50 μg/ml gentamycin (Life Technologies, Gaithersburg, MD), 10 mm HEPES buffer (Life Technologies), 1 μg/ml aprotinin (Sigma), 1 μg/ml leupeptin (Sigma) and 0·1 μm EDTA (Fisher Scientific, Pittsburgh, PA) in RPMI-1640 (Mediatech, Herndon, VA), as previously described.19 The incubation mixture was placed on ice and was subjected to a 20–30-second sonication. The supernatant was collected after centrifugation and stored frozen at −70° until assay.
Assays for cytokines in extracts employed commercially available enzyme-linked immunosorbent assay (ELISA) kits obtained from the following sources: interleukin (IL)-1α (Endogen, Cambridge, MA; sensitivity 6 pg/ml); IL-2 (13 pg/ml), IL-4 (5 pg/ml), IL-6 (8 pg/ml), IL-10 (13 pg/ml), IL-12 (2 pg/ml), interferon-γ (IFN-α; 1 pg/ml) and TNF-α (3 pg/ml) (all from BioSource International, Camarillo, CA). All assays were conducted in accordance with the manufacturer’s instructions. The concentration of each cytokine was calculated with reference to a standard curve that was constructed using recombinant cytokines provided with each kit. Results were expressed as pg cytokine/mg periapical tissue.
Statistical analysis
Differences in bone destruction, body weight and PMN accumulation were analysed by the Student’s t-test. ELISA data were analysed by the non-paired Student’s t-test with Bonferroni’s correction for multiple comparisons.
RESULTS
Effect of P- and E-selectin deficiency on infraosseus bone destruction
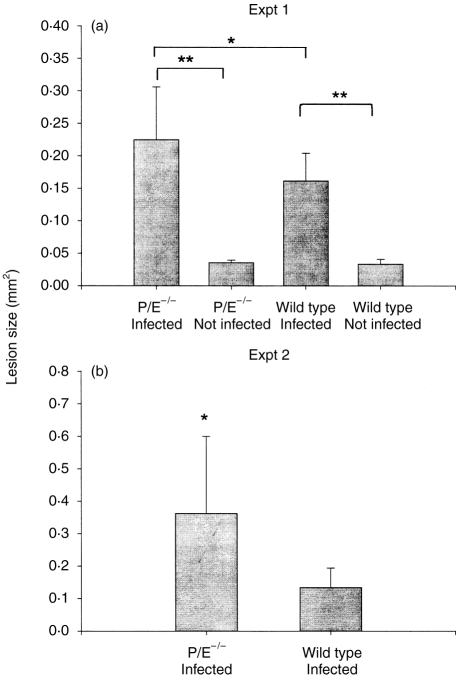
The mandibular first molars of P/E−/− and wild-type mice (P/E+/+) were subjected to surgical pulp exposure and infected with a mixture of four bacterial pathogens.20 The teeth of controls of both genotypes remained unexposed and uninfected (three mice per group). After 20 days, the extent of infraosseus periapical bone destruction was quantified by histomorphometry. As expected, both wild-type and P/E−/− mice with pulpal infections exhibited significant periapical resorption compared with uninfected control mice (P < 0·0001 for both comparisons) (Fig. 1a). Note that the data for the uninfected animals represents the cross-sectional area of the normal periodontal ligament. Of importance, P/E−/− mice had 39% more periapical bone resorption than wild-type mice (P = 0·034). These findings were confirmed in a second experiment (Fig. 1b), in which P/E−/− mice exhibited more than twice as much resorption than wild-type mice (P = 0·024). These data demonstrate that animals with an LAD-II-like PMN migration deficiency have increased susceptibility to infection-stimulated infraosseus bone destruction.
Figure 1.
Periapical bone destruction in P/E−/− and P/E+/+ mice. Vertical bars: standard deviation. (a) Exp. 1, 20 days postinfection: wild type (n = 9); P/E−/− (n = 7). (b) Exp. 2, 14 days postinfection: wild type (n = 5); P/E−/− (n = 7). Statistical significance was determined by the Student’s t-test. **P < 0·0001; *P < 0·05.
Morbidity of P/E−/− mice with pulpal infections
At killing on day 20, the presence of local infection appeared to result in some in morbidity of P/E−/− mice. This was manifested by a significant loss of weight in P/E−/− animals with pulpal infections, compared with uninfected controls (Table 1). In contrast, the weight of infected wild-type mice remained stable. Despite the weight loss, only one of 14 P/E−/− animals died following the infection procedure, compared to none 14 of the wild-type mice in the two experiments. Splenomegaly was also consistently observed in P/E−/− mice, as previously reported.16 This finding was independent of pulpal infection because no further increase in spleen size was seen in infected animals. No animals of either genotype developed orofacial abcesses, which have been observed in this model in RAG2 severe combined immunodeficiency (SCID) animals which lack specific T and B cells.20
Table 1.
Effect of pulpal infection on body weight and splenomegaly

PMN infiltration into inflammatory tissues
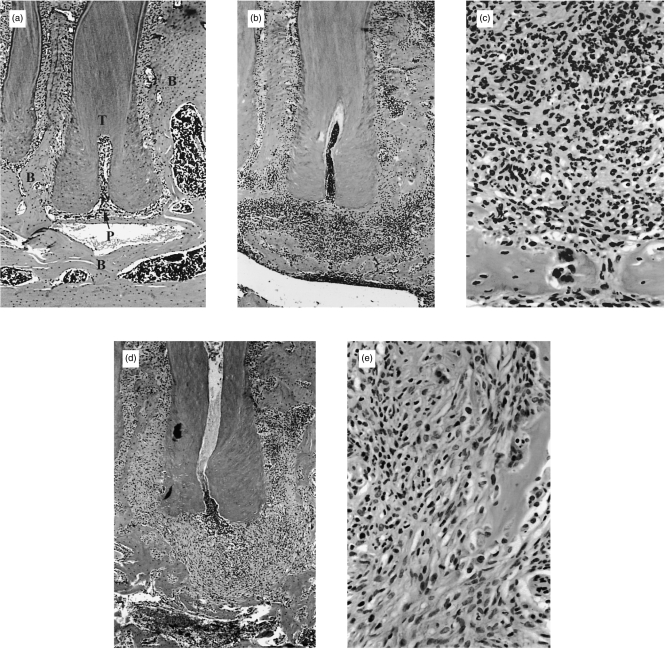
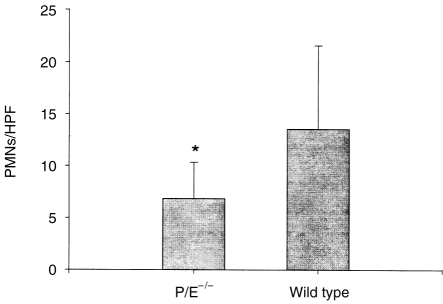
Cells with PMN morphology were present in the granulomatous lesions of both P/E−/− and wild-type mice on day 20 after pulp exposure and infection (Fig. 2). In addition, microabcesses were present in both groups and were localized to the interface between the infected necrotic pulp tissue and the periapical granuloma. However, a higher level (≈twofold) of infiltrating PMNs were observed in the surrounding granulomatous tissue of wild-type compared to P/E−/− mice (Fig. 3). Thus, although PMNs from P/E−/− animals have a deficiency in rolling adhesion, they are nonetheless eventually able to infiltrate into a site of chronic infection, albeit in numbers lower than normal.
Figure 2.
Periapical bone destruction and polymorphonuclear (PMN) infiltration surrounding the distal root of the first molar in P/E−/− and P/E+/+ mice after 20 days. (a) Periapical region, non-infected control tooth from P/E+/+ mice; (b) infected P/E+/+ mice; note infiltrated granulation tissue and area of bone resorption (50× magnification); (c) higher magnification (400×) of infiltrated tissue from (b); (d) infected P/E−/− mice; note decreased infiltrate and increased bone resorption; (e) higher magnification (400×) of infiltrated tissue from (d). B, bone; T, tooth root; P, periodontal ligament space.
Figure 3.
Polymorphonuclear (PMN) infiltration of periapical granulomatous tissue in P/E−/− and P/E+/+ mice 20 days after pulp exposure and infection. P/E−/−, n = 9; P/E+/+, n = 7. HPF: high-power fields; vertical bars: standard deviation. *P < 0·05, P/E−/− versus P/E+/+ mice.
Infraosseus cytokine responses
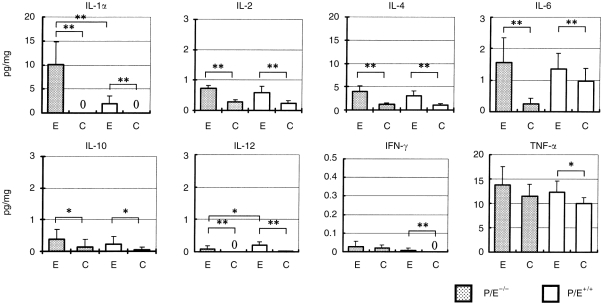
The local infraosseus cytokine responses generated in response to pulpal infection were assessed on day 20 and comprised IL-1α, IL-2, IL-4, IL-6, IL-10, IL-12, IFN-γ and TNF-α. As shown in Fig. 4, significant elevations of most mediators were seen following pulp exposure and infection (E) in both genotypes compared with uninfected controls (C). For tissues from infected teeth, the level of the bone resorptive mediator IL-1α was markedly increased in inflammatory tissues from P/E−/− versus wild-type mice (P < 0·001). Tissue levels of IL-2, IL-4, IL-6, IL-10, TNF-α and IFN-γ were all higher in P/E−/− than in wild-type mice, but the increases were not statistically significant. Only IL-12 was higher in wild-type mice (P < 0·05). Many of the cytokines evaluated, particularly TNF-α, were also present in periapical tissues from unexposed teeth, which represented normal periodontal ligament and some surrounding bone. The exceptions were IL-1α, IL-12 and IFN-γ, which were present in measurable quantities only in inflamed tissues.
Figure 4.
Periapical tissue cytokine concentrations in P/E−/− and P/E+/+ mice. Cytokine concentrations were measured by enzyme-linked immunosorbent assay (ELISA) on day 20 and values were normalized to the weight of periapical granuloma tissue. Shaded bar: P/E−/− (n = 9); open bars: P/E+/+ (n = 7). E, pulp exposure and infection; C, non-exposed, uninfected controls (n = 3 for both P/E−/− and P/E+/+). Vertical line: standard deviation. **P < 0·01; *P < 0·05.
Systemic cytokine responses
The systemic immunostimulation in P/E−/− animals, as determined by the cytokine content of splenic tissue, was also assessed. As shown in Table 2, spleens from P/E−/− mice with pulpal infections had significantly increased levels of the proinflammatory cytokines IL-1α, IL-2, IL-6 and IL-12, compared with wild-type mice, whereas the levels of the anti-inflammatory mediators IL-4 and IL-10, and the bone resorption inhibitor IFN-γ, were similar. There were no significant changes in cytokine levels induced by pulpal infection per se, with the exception of a decrease in IL-6 in P/E−/− mice and a decrease in IL-12 in wild-type mice. IL-1α was undetectable in serum from either P/E−/− or wild-type animals (data not shown). These findings indicate that P/E−/− mice have widespread immunostimulation, perhaps reflective of the presence of subclinical infections at extraoral sites, which is not significantly impacted by pulpal infection.
Table 2.
Splenic cytokine expression in P/E−/− and wild-type mice

DISCUSSION
In the present work, we tested the hypothesis that non-specific responses mediated by phagocytic leucocytes are protective against infections of the dental pulp and subsequent infraosseus inflammation and bone resorption. Our data demonstrate that P/E−/− knockout mice have significantly increased infection-stimulated periapical bone destruction compared with wild-type controls. The bone destruction in P/E−/− animals correlated with decreased neutrophilic infiltration of periapical tissues, as well as with higher levels of locally produced IL-1α, previously shown to be the primary mediator of bone resorption in this model.19,21 These findings suggest that impairment in rolling adhesion, and subsequent decreased extravasation of phagocytic cells, results in compromised antibacterial immunity and consequent hard-tissue inflammation and bone destruction.
P-selectin, which is constitutively expressed on endothelium in many tissues, is important in immediate leucocyte responses, whereas E-selectin is induced, within 3–5 hr, by inflammation.16,18,22 The effects of single deficiencies of P- or E-selectin on leucocyte emigration are quite mild.17,23 In contrast, P/E−/− double knockouts exhibit a greater than fivefold reduction in intraperitoneal PMN extravasation in response to thioglycollate compared with wild-type mice, despite severe neutrophilia. P/E−/− mice also exhibit ulcerative dermatitis owing to infection with opportunistic micro-organisms.16 Such infections are not seen in mice lacking individual selectins.24–26 In the present study, local PMN infiltration into infrabony lesions was diminished by twofold, which resulted in 40% increased bone destruction. It is noteworthy that the decrease in PMN infiltration was less dramatic in this model of chronic inflammation compared to more acute models.16 The residual PMN migration in P/E−/− animals is likely to be mediated by l-selectin, which is probably responsible for the increasing leukocytic infiltrate seen with time after infection.27 In the pulp exposure model, the extent of periapical bone resorption is directly proportional to the degree of pulp necrosis, and is initiated when the infection destroys greater than ≈one-half of the radicular pulp.8 It might be speculated that the slowed PMN infiltration permits the infection to progress more rapidly, reaching the critical point sooner in P/E−/− than in wild-type animals. This hypothesis is supported by studies with the biological response modifier PGG-glucan, which increases PMN number and activity, and significantly reduces pulpal and periapical destruction in rats.8 The therapeutic effect of PGG-glucan was secondary to decreased soft tissue destruction, with only 3% of first molar pulps exhibiting complete pulpal necrosis in treated animals, compared to 41% of controls.
There are no reports concerning the frequency or severity of pulpal infections and periapical bone destruction in humans with LAD-II and other PMN deficiencies. However, conflicting data have been obtained using animal models. Kawashima et al.28 reported that administration of cyclophosphamide (CP), which causes severe neutropenia, resulted in increased periapical bone destruction and an absence of PMNs in the inflammatory infiltrate. Bacteria were observed both in the pulp and in the periapical region of CP-treated animals, indicating increased bacterial egress from the root canal system in the absence of PMNs. In contrast, Yamasaki et al.29 reported that methotrexate treatment, which also resulted in decreased PMN number, inhibited periapical lesion development. In periodontal disease models, CP-treated animals experienced greater alveolar bone loss, as well as septicaemia, compared with controls.30,31 Similar results were obtained in methotrexate-treated rats.32
P/E−/−-selectin mice have increased circulating levels of IL-3 and granulocyte–macrophage colony-stimulating factor (GM-CSF), which are probably a compensatory mechanism responsible for the observed neutrophilia and monocytaemia.16 However, local tissue and splenic cytokine responses in these animals have not previously been evaluated in models of infection. Our data demonstrate that the bone resorptive cytokine, IL-1α, is significantly elevated in periapical tissues in P/E−/− mice, suggesting a likely mechanism for the observed bone destruction.21,33 In contrast, the level of IL-12 was significantly decreased. IL-12 is predominantly a macrophage product, and it is possible that diminished monocyte infiltration into P/E−/− lesions accounts for this observation. The levels of most other cytokines examined, including IL-2, IL-4, IL-6, IL-10, IFN-γ and TNF-α, were not significantly different, although there was a general tendency for levels of all these mediators to be higher in the P/E−/− mice. These data therefore suggest that the deficiency in phagocytic leucocyte emigration leads to increased bacterial tissue invasion and to greater stimulation of host cytokine responses by both inflammatory and resident connective tissue cells.34,35
Although our data support a primary effect on the recruitment of PMNs, and possibly monocytes, to inflammatory sites, P- and E-selectins may also mediate the migration of T lymphocytes.36,37 Oxazolone-induced delayed type hypersensitivity reactions are impaired in P/E−/− mice,23 and T helper 1 (Th1) but not T helper 2 (Th2) cells bind P- and E-selectin to efficiently enter inflamed tissues.38 Our data (Fig. 4) show that IFN-γ, primarily a Th1 and natural killer (NK) cell product, was actually expressed at somewhat higher levels in P/E−/− mice, suggesting that these cells did migrate into lesions and were producing this mediator. As Th1 cells stimulate delayed-type hypersensitivity and proinflammatory pathways, including macrophage activation and IL-1 production, it is more likely that a decrease in Th1 migration would have the opposite effect, i.e. a decrease in bone destruction. Also arguing against a primary effect on T-cell migration are reports that T-cell-deficient athymic nu/nu rats have similar39 or less,40 rather than more, periapical bone destruction compared with immunologically intact animals. Although ≈one-third of RAG2 SCID mice (lacking both T and B cells) developed orofacial abcesses following pulpal infection, there was no increase in local bone destruction in non-abcessed RAG2 animals.20 Taken together, these data favour the interpretation that P/E−/− deficiency is manifested primarily on PMNs, and that these cells protect the pulp/periapical complex against infection and consequent bone destruction.
Acknowledgments
The authors thank Ms Justine Dobeck and Laura Mark for their assistance with histology, and Mr Joe Buchanan for photography. This work was supported by grants DE-09018 and DE-11664 (to P.S.), grant HL-41484 and support from the Howard Hughes Medical Institute (to R.H.) and grant 08771680 from the Ministry of Education, Science and Culture, Japan (to N.K.).
REFERENCES
- 1.Bergenholtz G. Pathogenic mechanisms in pulpal disease. J Endon. 1990;16:98. doi: 10.1016/S0099-2399(06)81571-2. [DOI] [PubMed] [Google Scholar]
- 2.Bergenholtz G, Lindhe J. Effect of soluble plaque factors on inflammatory reactions in the dental pulp. Scand J Dental Res. 1975;83:153. doi: 10.1111/j.1600-0722.1975.tb01193.x. [DOI] [PubMed] [Google Scholar]
- 3.Okiji T, Kawashima N, Kosaka T, et al. Distribution of Ia antigen-expressing nonlymphoid cells in various stages of induced periapical lesions in rat molars. J Endod. 1994;20:27. doi: 10.1016/s0099-2399(06)80023-3. [DOI] [PubMed] [Google Scholar]
- 4.Kawashima N, Okiji T, Kosaka T, Suda H. Kinetics of macrophages and lymphoid cells during the development of experimentally induced periapical lesions in rat molars: a quantitative immunohistochemical study. J Endod. 1996;22:311. doi: 10.1016/S0099-2399(96)80266-4. [DOI] [PubMed] [Google Scholar]
- 5.Stern MH, Dreizen S, Mackler BF, et al. Quantitative analysis of cellular composition of human periapical granulomas. J Endod. 1981;7:117. doi: 10.1016/S0099-2399(81)80125-2. [DOI] [PubMed] [Google Scholar]
- 6.Nilsen R, Johannessen AC, Skaug N, Matre R. In situ characterization of mononuclear cells in human dental periapical inflammatory lesions using monoclonal antibodies. Oral Surg Oral Med Oral Pathol. 1984;58:160. doi: 10.1016/0030-4220(84)90131-2. [DOI] [PubMed] [Google Scholar]
- 7.Stashenko P, Yu SM. T helper and T suppressor cell reversal during the development of induced rat periapical lesions. J Dental Res. 1989;68:830. doi: 10.1177/00220345890680051601. [DOI] [PubMed] [Google Scholar]
- 8.Stashenko P, Wang CY, Riley E, et al. Reduction of infection-stimulated periapical bone resorption by the biological response modifier PGG glucan. J Dental Res. 1995;74:323. doi: 10.1177/00220345950740010701. [DOI] [PubMed] [Google Scholar]
- 9.Van Dyke TE, Taubman MA, Ebersole JL, et al. The Papillon–LeFevre syndrome: neutrophil dysfunction with severe periodontal disease. Clin Immunol Immunopathol. 1984;31P:419. doi: 10.1016/0090-1229(84)90094-1. [DOI] [PubMed] [Google Scholar]
- 10.Cohen MS, Leong PA, Simpson DM. Phagocytic cells in periodontal defense. Periodontal status of patients with chronic granulomatous disease of childhood. J Periodontol. 1985;56:611. doi: 10.1902/jop.1985.56.10.611. [DOI] [PubMed] [Google Scholar]
- 11.Waldrop TC, Anderson DC, Hallmon WW, et al. Periodontal manifestations of the heritable Mac-1, LFA-1, deficiency syndrome. J Periodontol. 1987;58:400. doi: 10.1902/jop.1987.58.6.400. [DOI] [PubMed] [Google Scholar]
- 12.Springer TA, Thompson WS, Miller LJ, et al. Inherited deficiency of the Mac-1, LFA-1, p150,95 glycoprotein family and its molecular basis. J Exp Med. 1984;160:1901. doi: 10.1084/jem.160.6.1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Anderson DC, Springer TA. Leukocyte adhesion deficiency: an inherited defect in the Mac-1, LFA-1 and p150,95 glycoproteins. Annu Rev Med. 1987;38:175. doi: 10.1146/annurev.me.38.020187.001135. [DOI] [PubMed] [Google Scholar]
- 14.Von Andrian UH, Chambers JD, Berg EL, et al. l-selectin mediates neutrophil rolling in inflamed venules through sialyl Lewis x-dependent and independent recognition pathways. Blood. 1993;82:182. [PubMed] [Google Scholar]
- 15.Etzioni A, Frydman M, Pollack S, et al. Severe recurrent infections due to a novel adhesion molecule defect. N Engl J Med. 1992;327:1789. doi: 10.1056/NEJM199212173272505. [DOI] [PubMed] [Google Scholar]
- 16.Frenette PS, Mayadas TN, Rayburn H, et al. Susceptibility to infection and altered hematopoiesis in mice deficient in both P- and E-selectins. Cell. 1996;84:563. doi: 10.1016/s0092-8674(00)81032-6. [DOI] [PubMed] [Google Scholar]
- 17.Bullard DC, Kunkel EJ, Kubo H, et al. Infectious susceptibility and severe deficiency of leukocyte rolling and recruitment in E-selectin and P-selectin double mutant mice. J Exp Med. 1996;183:2329. doi: 10.1084/jem.183.5.2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eppihimer MJ, Wolitzky B, Anderson DC, et al. Heterogeneity of E- and P-selectins in vivo. Circ Res. 1996;79:560. doi: 10.1161/01.res.79.3.560. [DOI] [PubMed] [Google Scholar]
- 19.Wang CY, Stashenko P. The role of interleukin-1α in the pathogenesis of periapical bone destruction in a rat model system. Oral Microbiol Immunol. 1993;8:50. doi: 10.1111/j.1399-302x.1993.tb00543.x. [DOI] [PubMed] [Google Scholar]
- 20.Teles R, Wang CY, Stashenko P. Increased susceptibility of RAG-2 SCID mice to dissemination of endodontic infections. Infect Immun. 1997;65:3781. doi: 10.1128/iai.65.9.3781-3787.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stashenko P, Wang CY, Tani-Ishii N, Yu SM. Pathogenesis of induced rat periapical lesions. Oral Surg Oral Med Oral Path. 1994;78:494. doi: 10.1016/0030-4220(94)90044-2. [DOI] [PubMed] [Google Scholar]
- 22.Walter UM, Issekutz AC. The role of E- and P-selectin in neutrophil and monocyte migration in adjuvant-induced arthritis in the rat. Eur J Immunol. 1997;27:1498. doi: 10.1002/eji.1830270628. [DOI] [PubMed] [Google Scholar]
- 23.Staite ND, Justen JM, Sly LM, et al. Inhibition of delayed-type contact hypersensitivity in mice deficient in both E-selectin and P-selectin. Blood. 1996;88:2973. [PubMed] [Google Scholar]
- 24.Mayadas TN, Johnson RC, Rayburn H, et al. Leukocyte rolling and extravasation are severely compromised in P selectin-deficient mice. Cell. 1993;74:541. doi: 10.1016/0092-8674(93)80055-j. [DOI] [PubMed] [Google Scholar]
- 25.Arbones ML, Ord DC, Ley K, et al. Lymphocyte homing and leukocyte rolling and migration are impaired in l-selectin-deficient mice. Immunity. 1994;1:247. doi: 10.1016/1074-7613(94)90076-0. [DOI] [PubMed] [Google Scholar]
- 26.Labow MA, Norton CR, Rumberger JM, et al. Characterization of E-selectin-deficient mice: demonstration of overlapping function of the endothelial selectins. Immunity. 1994;1:709. doi: 10.1016/1074-7613(94)90041-8. [DOI] [PubMed] [Google Scholar]
- 27.Jung U, Ramos CL, Bullard DC, Ley K. Gene-targeted mice reveal importance of l-selectin-dependent rolling for neutrophil adhesion. Am J Physiol. 1998;274:H1785. doi: 10.1152/ajpheart.1998.274.5.H1785. [DOI] [PubMed] [Google Scholar]
- 28.Kawashima N, Okiji T, Kosaka T, Suda H. Effects of cyclophosphamide on the development of experimentally-induced periapical lesions in the rat. Japan J Conserv Dentistry. 1993;36:1388. [Google Scholar]
- 29.Yamasaki M, Kumazawa M, Kohsaka T, Nakamura H. Effect of methotrexate-induced neutropenia on rat periapical lesions. Oral Surg Oral Med Oral Path. 1994;77:655. doi: 10.1016/0030-4220(94)90330-1. [DOI] [PubMed] [Google Scholar]
- 30.Sallay K, Sanavi F, Ring I, et al. Alveolar bone destruction in the immunosuppressed rat. J Perio Res. 1982;17:263. doi: 10.1111/j.1600-0765.1982.tb01153.x. [DOI] [PubMed] [Google Scholar]
- 31.Samejima Y, Ebisu S, Okada H. Effect of infection with Eikenella corrodens on the progression of ligature-induced periodontitis in rats. J Perio Res. 1990;25:308. doi: 10.1111/j.1600-0765.1990.tb00920.x. [DOI] [PubMed] [Google Scholar]
- 32.Yoshinari N, Kameyama Y, Aoyama Y, et al. Effect of long-term methotrexate-induced neutropenia on experimental periodontal disease in rats. J Perio Res. 1994;29:393. doi: 10.1111/j.1600-0765.1994.tb01240.x. [DOI] [PubMed] [Google Scholar]
- 33.Kawashima N, Stashenko P. Expression of bone resorption and regulatory cytokines in urine periapical inflammation. Arch Oral Biol. 1999;44:55. doi: 10.1016/s0003-9969(98)00094-6. [DOI] [PubMed] [Google Scholar]
- 34.Tani-Ishii N, Wang CY, Stashenko P. Immunolocalization of bone resorptive cytokines in rat pulp and periapical lesions following surgical pulp exposure. Oral Microbiol Immunol. 1996;10:213. doi: 10.1111/j.1399-302x.1995.tb00145.x. [DOI] [PubMed] [Google Scholar]
- 35.Wang CY, Tani-Ishii N, Stashenko P. Bone resorptive cytokine gene expression in developing rat periapical lesions. Oral Microbiol Immunol. 1997;12:65. doi: 10.1111/j.1399-302x.1997.tb00619.x. [DOI] [PubMed] [Google Scholar]
- 36.Diacovo TG, Roth SJ, Morita CT, et al. Interactions of human alpha/beta and gamma/delta T lymphocyte subsets in shear flow with E-selectin and P-selectin. J Exp Med. 1996;183:1193. doi: 10.1084/jem.183.3.1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lichtman AH, Ding H, Henault L, et al. CD45RA− RO+ (memory) but not CD45RA+RO− (naive) T cells roll efficiently on E- and P-selectin and vascular adhesion molecule-1 under flow. J Immunol. 1997;158:3640. [PubMed] [Google Scholar]
- 38.Austrup F, Vestweber D, Borges E, et al. P- and E-selectin mediate recruitment of T-helper-1 but not T-helper-2 cells into inflamed tissues. Nature. 1997;385:81. doi: 10.1038/385081a0. [DOI] [PubMed] [Google Scholar]
- 39.Wallstrom JB, Torabinejad M, Kettering J, McMillan P. Role of T cells in the pathogenesis of periapical lesions. A preliminary report. Oral Surg Oral Med Oral Pathol. 1993;76:213. doi: 10.1016/0030-4220(93)90207-k. [DOI] [PubMed] [Google Scholar]
- 40.Tani N, Kuchiba K, Osada T, et al. Effect of T cell deficiency on the formation of periapical lesions in mice: histological comparison between periapical lesion formation in BALB/c and BALB/c nu/nu mice. J Endod. 1995;21:195. doi: 10.1016/s0099-2399(06)80565-0. [DOI] [PubMed] [Google Scholar]