Abstract
The migration of immune cells through the extracellular matrix (ECM) towards inflammatory sites is co-ordinated by receptors recognizing ECM glycoproteins, chemokines and proinflammatory cytokines. In this context, galectins are secreted to the extracellular milieu, where they recognize poly-N-acetyllactosamine chains on major ECM glycoproteins, such as fibronectin and laminin. We investigated the possibility that galectin-1 could modulate the adhesion of human T cells to ECM and ECM components. T cells were purified from human blood, activated with interleukin-2 (IL-2), labelled, and incubated further with intact immobilized ECM and ECM glycoproteins in the presence of increasing concentrations of human recombinant galectin-1, or its more stable, related, C2-S molecule obtained by site-directed mutagenesis. The presence of galectin-1 was shown to inhibit T-cell adhesion to intact ECM, laminin and fibronectin, and to a lesser extent to collagen type IV, in a dose-dependent manner. This effect was specifically blocked by anti-galectin-1 antibody and was dependent on the lectin’s carbohydrate-binding properties. The inhibition of T-cell adhesion by galectin-1 correlates with the ability of this molecule to block the re-organization of the activated cell’s actin cytoskeleton. Furthermore, tumour necrosis factor-α (TNF-α) and interferon-γ (IFN-γ) production was markedly reduced when IL-2-activated T cells were incubated with galectin-1 or its mutant. This effect was prevented by β-galactoside-related sugars. The present study reveals an alternative inhibitory mechanism for explaining the suppressive properties of the galectin-1 subfamily on inflammatory and autoimmune processes.
INTRODUCTION
Galectin-1 is a member of a growing family of animal β-galactoside-binding proteins that have been highly conserved throughout animal evolution1–3 and share remarkable sequence similarities in the carbohydrate recognition domain.1,4 Although the precise functions of individual members of this family in vivo have been difficult to determine because of their widespread expression and overlapping specificities,3,5,6 galectin-1 has been proposed to play key roles in a wide variety of biological events involving cell–cell and cell–extracellular matrix (ECM) interactions, cell growth regulation,7,8 metastasis9 and immunomodulation.10,11
Adhesion and migration of immune cells, through the ECM, towards inflammatory sites is a multi-step process, co-ordinated by receptors recognizing a number of ECM glycoproteins, chemokines and proinflammatory cytokines.12,13 Under these circumstances, and despite the lack of a secretion signal sequence, galectin-1 is secreted into the extracellular environment,14 where it recognizes poly-N-acetyllactosamine chains on major ECM components. A variety of intracellular and extracellular candidate ligands has been reported to bind galectin-1, such as laminin (LN),15–17 fibronectin (FN),18 lysosome-associated membrane proteins 1 and 2,19,20 CD4521,22 and lactosamine-containing glycolipids.17
Galectin-1 has been thought to act as a modulator of cell–cell and cell–ECM interactions.23 However, whether galectin-1 exerts a positive or a negative effect on cell adhesion to ECM glycoproteins remains controversial, raising the possibility that this protein could promote cell attachment or detachment according to the cell type or according to the cell’s activation status or developmental stage. Galectin-1 has been reported to inhibit myoblast interaction with LN by blocking the α7β1 integrin, thus allowing myoblasts to fuse into myotubes.16 On the other hand, this β-galactoside-binding protein showed pro-adhesive properties on other cell types, such as melanocytes,24 teratocarcinoma cells,15 olfactory neurones,17 rhabdomyosarcoma cells18 and fibroblasts.15 In addition, galectin-1 is expressed in activated macrophages and T cells.25–27
The present study deals with the potential role of galectin-1 in modulating human T-cell adhesion to ECM, and the specificity and selectivity of this effect for different ECM components such as LN, FN and collagen type IV (CO-IV). We examined the effects of galectin-1 on the morphology of activated T cells, as well as the modulatory role of galectin-1 on interleukin-2 (IL-2)-induced T-cell adhesion to ECM proteins, because IL-2, in both its immobilized or soluble forms, has been shown to induce a β1 integrin-mediated T-cell adhesion.28 Finally, because proinflammatory cytokines have been shown to modulate T-cell adhesion and migration,29 we also investigated the potential role of galectin-1 in modulating tumour necrosis factor-α (TNF-α) and interferon-γ (IFN-γ) secretion from activated T cells.
MATERIALS AND METHODS
Reagents
The following reagents were obtained from the sources indicated: recombinant human IL-2 (specific activity 18×106 U/mg of protein) from Chiron B.V. (Amsterdam, the Netherlands); FN from Chemicon (Temecula, CA); fibroblast growth factor, bovine serum albumin (BSA), thiodigalactoside (TDG) and LN from Sigma Chemical Co. (St Louis, MO); CO-IV from ICN (Costa Mesa, CA); HEPES buffer, antibiotics, heat-inactivated fetal calf serum (FCS), sodium pyruvate, glutamine and RPMI-1640 medium from Biological Industries (Beit Haemek, Israel); and tissue culture dishes from Falcon, Becton Dickinson (Oxnard, CA).
Recombinant galectin-1 expression, site-directed mutagenesis and antibody production
Human recombinant galectin-1 was obtained as described elsewhere.30 Briefly, the expression plasmid pH14GAL was constructed from the plasmid pUC540 (Kan®) and a cDNA for galectin-1 was derived from the human lung cDNA library. Escherichia coli strains of SCS1 and Y1090 were then transformed with pH14GAL, and galectin-1 expression was assessed by Western blot. The protein was purified by affinity chromatography on an asialofetuin–agarose column. The haemagglutinating activity was measured as described previously31 and the N-terminal amino acid sequence was determined with an Applied Biosystem 477A pulsed-liquid sequencer. The lipopolysaccharide content of the purified sample was determined with a colorimetric endotoxin determination reagent (Pyrodick; Seikagaku Kogyo Co. Ltd, Tokyo, Japan). The antiserum was raised against human galectin-1 as described by Hirabayashi et al.30 The recombinant, more stable, galectin, C2-S, substituting cysteine with serine, was generated by site-directed mutagenesis as described elsewhere,32 by using an Amersham in vitro site-directed mutagenesis kit.
Preparation of intact ECM and ECM glycoprotein-coated microtitre plates
Flat-bottomed microtitre wells (Falcon, Becton Dickinson Labware, NJ) were coated with ECM as described previously.33 Briefly, purified bovine corneal cells (5×104 cells/ml) were cultured in flat-bottomed 96-well plates in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 5% dextran T-40 (Sigma), 10% calf serum, fibroblast growth factor (100 ng/ml) and antibiotics. After 6–8 days in culture (37° in a 10% CO2 humidified atmosphere), the cell layers became confluent and were dissolved. This yielded an intact ECM attached to the entire surface area of the wells that was free of serum proteins, nuclei, cytoskeletal and cellular debris. FN, LN or CO-IV was added to the plates at a concentration of 1 μg/50 μl phosphate-buffered saline (PBS)/well for 2 hr at 37° in a 10% CO2 humidified atmosphere.28 After incubation, unbound FN, LN or CO-IV was removed by gentle washing and the remaining binding sites of control or coated wells were blocked with 1% BSA in PBS. The microtitre plates were then washed three times with PBS at 22° and stored sterile at 4° until used.
T-cell adhesion assay
Human T cells were purified from peripheral blood leucocytes of healthy donors. The mononuclear cells were isolated on a Ficoll gradient, washed, and incubated at 37° in a 10% CO2 humidified atmosphere. After 2 hr, the non-adherent cells were removed and incubated on nylon-wool columns (Uni-Sorb, Novamed, Jerusalem, Israel) for 45 min at 37° in a 10% CO2 humidified atmosphere. Non-adherent cells were eluted (>94% purity), washed and their adhesion to various immobilized protein substrates examined as described previously.29,33 Briefly, IL-2-activated (10 U/ml) or non-activated T cells were labelled with Na251[Cr]O4 (Amersham), washed and resuspended in adhesion medium (RPMI-1640 medium supplemented with 1% BSA). 51Cr-labelled T cells (105 cells/100 μl of adhesion medium/well) were added to ECM- or glycoprotein-coated wells. To test the effect on T-cell adhesiveness, recombinant galectin-1 or its mutant C2-S was added at concentrations ranging from 0·004 to 4 μg/ml. In order to determine the specificity of the effect, the anti-galectin-1 antibody at two different dilutions (1:50 or 1:100), or β-galactoside-related or non-related sugars (100 mm TDG or glucose, respectively), was added to some wells. The microtitre plates were incubated for 30 min at 37° in a humidified 10% CO2 incubator. The wells were then washed three times with pre-warmed PBS to remove non-adherent cells. The radiolabelled adherent cells were lysed with 1% Tween-20 in 1 N NaOH, and finally the resulting supernatants were removed and analysed in a γ-counter (Packard). For each experimental group, the results are expressed as the mean percentage (±SD) of bound T cells in quadruplicate wells.
Cytokine secretion assay
Human T cells were purified from peripheral blood leucocytes of healthy donors as described above and their TNF-α and IFN-γ secretion was examined. Briefly, T cells (3×106/well) were incubated (18 hr) in RPMI-1640 supplemented with 5% FCS, 10 mm HEPES, 1 mm sodium pyruvate, 2 mm glutamine and 1% antibiotics in the presence of IL-2 (100 U/ml) and in the presence or absence of galectin-1 (0·04–4 μg/ml). Some treatments included, in addition to IL-2 (100 U/ml) and galectin-1 (4 μg/ml), anti-galectin-1 antibody (1:100 or 1:50) or β-galactoside-related sugars (100 mm TDG). After incubation, the supernatants were collected and the TNF-α and IFN-γ contents determined using a standard enzyme-linked immunosorbent assay (ELISA) (Pharmingen, San Diego, CA).
Cell staining
T cells were incubated (18 hr, 37°, 7·5% CO2, humidified atmosphere) in culture media. Recombinant human IL-2 (100 U/ml) was then added and the cell cultures were incubated for 24 hr. The T cells were washed and seeded onto FN-covered cover slips in the presence of either phorbol myristate acetate (PMA; 50 ng/ml), IL-2 (100 U/ml) or galectin-1. After 1 hr at 37°, the adherent cells were fixed (3 min) with paraformaldehyde (3%) and Triton-X-100 (0·5%), washed, and fixed (20 min) again with paraformaldehyde. The fixed cells were washed, treated with TRITC phalloidin, and washed again. Photographs (×1000) were taken.
Statistical analysis
The data were analysed and expressed as mean±SD. Significance was determined by using the Student’s t-test.
RESULTS
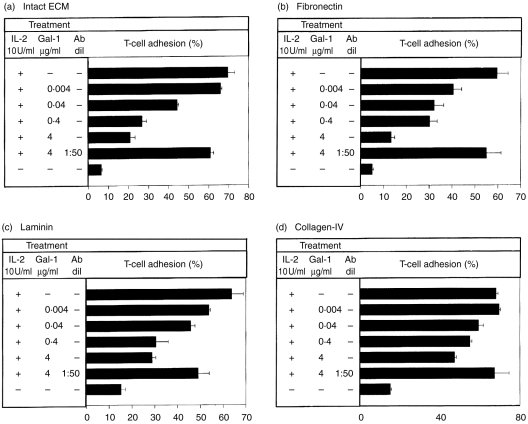
FN and LN, two major components of the ECM, have been shown to be specific extracellular ligands for galectin-1·15–18 To explore whether this β-galactoside-binding protein influences T-cell adhesiveness to intact ECM or to individual ECM components, we performed T-cell adhesion assays. As Fig. 1 shows, adding recombinant galectin-1 substantially decreased IL-2-activated T-cell attachment to whole ECM or intact FN or LN (Fig. 1a–c, respectively) in a dose-dependent manner when added at concentrations ranging from 0·004 to 4 μg/ml (P < 0·01, comparing the levels of T-cell adhesion to ECM and the ECM glycoproteins in the presence or absence of galectin-1, at the above mentioned amounts). This effect was reproduced when recombinant galectin-1 was added together with IL-2-activated T cells to FN- as well as to LN-coated wells (Fig. 1b,c, respectively). Finally, the effect was still apparent but much less strong when the cell-adhesive substrate was CO-IV (P < 0·05), which, accordingly, has not been assigned as a candidate ligand for galectin-1 (Fig. 1d). An antibody raised against galectin-1, when added at a dilution of 1:50, almost completely abrogated the anti-adhesive effect of this protein (at its highest concentration of 4 μg/ml) on the different ECM components. Moreover, TDG, a potent galectin sugar competitor, partially abolished a galectin-1-mediated decrease in T-cell attachment to intact ECM or individual components (data not shown). Hence, this effect was highly specific and mediated by the carbohydrate recognition domain of this protein. Moreover, the extent of inhibition depended on the particular ECM protein used as a substrate.
Figure 1.
IL-2-induced human T-cell adhesion to ECM components is specifically inhibited by galectin-1. 51[Cr]-labelled human T cells were incubated for 30 min in microtitre plates pre-coated with intact ECM (a), FN (b), LN (c) and CO-IV (d) in the presence of IL-2 (10 U/ml) and the indicated concentrations of recombinant galectin-1. In some wells, T cells were also incubated in the presence of IL-2 (10 U/ml), galectin-1 (4 μg/ml) and an antibody raised against galectin-1 (diluted 1:50). Non-stimulated T cells were used as negative controls. T cell-adhesion was then analysed. Results are expressed as mean percentage±SD of bound T cells from quadruplicate wells per experimental group. One experiment representative of four is shown.
C2-S is a more stable version of galectin-1, which has been generated by site-directed mutagenesis by substituting cysteine for serine, with no noticeable effect on the sugar-binding properties.32 When this mutant was assayed in our system, it behaved similarly to the wild-type galectin-1 (data not shown).
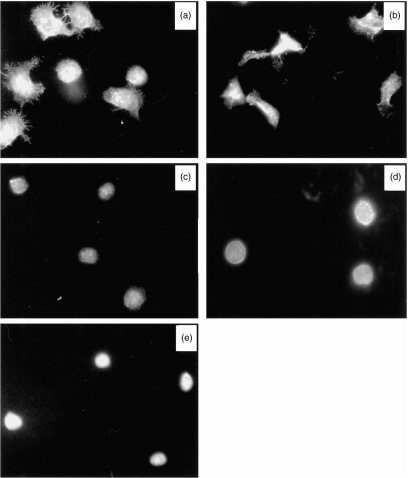
Immune cell adhesion to ECM and ECM proteins is accompanied with cell shape changes. In fact, morphological changes, such as the acquisition of spheric forms and the formation of pseudopods and of uropods, are the hallmarks of activated, matrix-adherent and mobile lymphocytes. Because galectin-1 inhibits T-cell adhesion to ECM ligands, we examined its effects on the acquisition of morphological changes by ECM-interacting activated T cells. Figure 2 shows that galectin-1 prevented the actin cytoskeleton-mediated spreading of IL-2- or PMA-activated T cells. Thus, the anti-adhesive effects of galectin-1 may involve the inhibition of the re-organization of the T cell’s actin cytoskeleton.
Figure 2.
The morphology and shape of FN-adherent T cells exposed to IL-2 (a), PMA (b), IL-2 and galectin-1 (c), PMA and galectin-1 (d) or medium alone (e). Photographs were taken 30–60 min after seeding of cells on FN-coated microtitre wells and exposure to the activating or inhibiting compounds.
As it has been postulated that cytokines may also be present and functionally active in the context of the ECM,12,13 the next issue we attempted to elucidate was related to the role of galectin-1 on proinflammatory cytokine T-cell secretion induced by IL-2. IL-2-activated T cells were cultured in the presence or absence of recombinant galectin-1 and their supernatants were subsequently tested for TNF-α and IFN-γ secretion. Galectin-1 was able to reduce the secretion of both cytokines in a dose-dependent manner (Fig. 3a, TNF-α; Fig. 3b, IFN-γ) when added at concentrations ranging from 0·04 to 4 μg/ml. Moreover, galectin-1 at 4 μg/ml exerted its maximum inhibitory effect, reducing TNF-α and IFN-γ secretion to background levels (P < 0·005). Finally, this latter inhibitory effect was not totally reversed by using the anti-galectin-1 antibody. However, it was partially prevented when TDG was added simultaneously to cell cultures. Hence, these results reveal an alternative inhibitory mechanism for explaining the suppressive properties of the galectin-1 subfamily on inflammatory and immunological processes.
Figure 3.
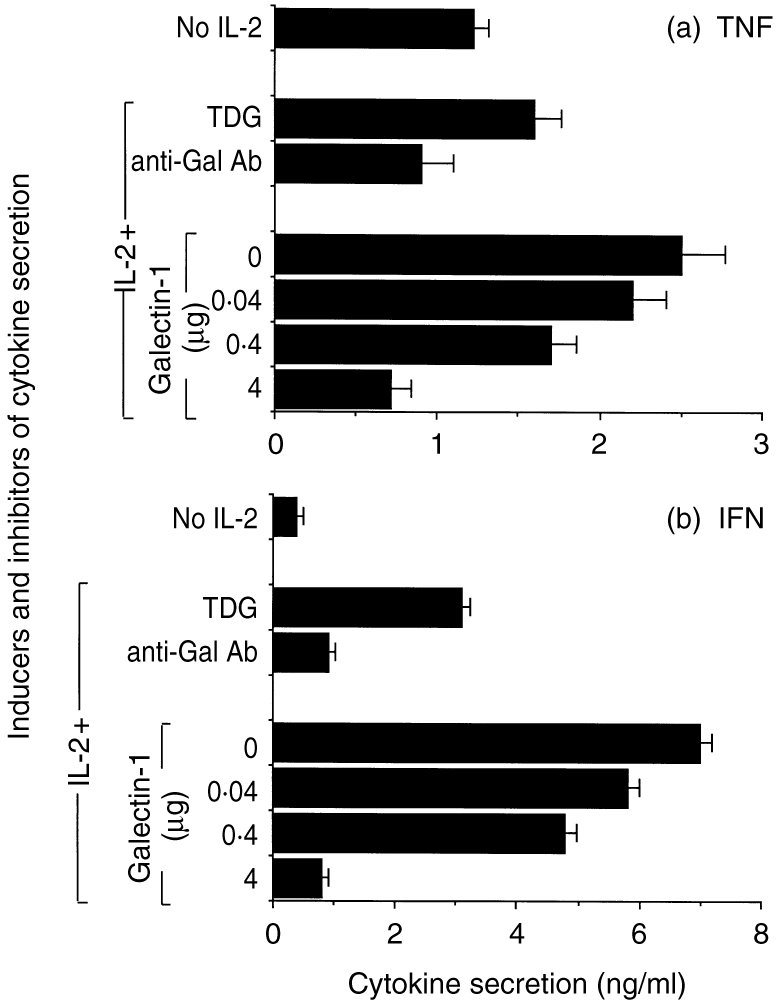
IL-2-induced proinflammatory cytokine secretion by T cells is inhibited by galectin-1. Human T cells were purified and cultured in the absence or presence of IL-2 (100 U/ml) and recombinant galectin-1 at concentrations ranging from 0·04 to 4 μg/ml for 18 hr at 37°. In some treatments, galectin-1 (4 μg/ml) was added together with a specific antibody (diluted 1:50) or a carbohydrate competitor (100mm TDG). Unstimulated T cells were used as negative controls Supernatants were collected and assayed for TNF-α (a) and IFN-γ (b) secretion by standard ELISA. Results are expressed as mean±SD from quadruplicate wells per experimental group. One experiment representative of four is shown.
DISCUSSION
We have provided evidence that galectin-1 inhibits IL-2-induced T-cell adhesion to ECM glycoproteins. These anti-adhesive properties were found to be specific, as an antibody raised against galectin-1 and β-galactoside-related sugars was able to abrogate the inhibitory effect. It was also selective, because inhibitory properties were found to be more pronounced when T cells were exposed to FN and LN. Moreover, we have demonstrated that galectin-1 inhibits IL-2-induced proinflammatory cytokine secretion from T cells, below background levels.
Carbohydrate recognition is a phylogenetically ancient binding principle represented throughout the biological world.4,34 By virtue of this specific recognition, it has been proposed that galectins are involved in critical functions such as immunomodulation,10,11 cell growth regulation,7,8 apoptosis22,26,35 and metastasis.9 Galectin-1 has been shown to exert these functions via modulation of cell–cell and cell–ECM interactions in different biological systems.23 However, results have been controversial as to whether extracellular secreted galectin-1 promoted cell attachment or detachment in the context of the ECM. It seems that the pro- or anti-adhesive effect varies according to the cell type, cell activation status and, more importantly, relies on the relative cell-surface expression of particular LN and FN receptors.23
Galectin-1 has been shown to inhibit cell adhesion, apparently by sterically blocking the access of cell-surface receptors to LN. This is indeed true for skeletal muscle, where galectin-1 blocked myoblast interaction with LN by alosterically inhibiting the α7β1 integrin from recognizing LN.16 In contrast, this β-galactoside-binding protein has been able to promote cell adhesion, apparently by bridging oligosaccharides between specific cell-surface glycoconjugates and LN or FN on melanoma cells,24 F9 teratocarcinoma cells,15 olfatory neurones,17 rhabdomyosarcoma cells18 and CHO fibroblasts15.
To exert a physiological role, galectin-1 must be secreted. Despite the fact that this protein lacks a typical secretion signal sequence, it belongs to a category of proteins that are exported extracellularly by a non-classical secretory pathway.14 In this regard, it has been found recently, by using a mRNA differential display polymerase chain reaction, that galectin-1 gene is induced in activated T cells, and secreted to the extracellular milieu27 to exert its functions. This secreted protein has been thought to act as an autocrine negative growth factor.27 In good agreement with this, recent studies22,26,35 have shown that galectin-1 exerted its inhibitory properties by inducing apoptosis of activated T cells, especially those bearing the polylactosamine-enriched CD45RO splicing product.22 Consistent with this, we have recently reported the purification, biochemical characterization and pro-apoptotic activity of a galectin-1-like protein in activated rat macrophages.26 Particularly attractive, the protein’s total and surface expression was found to be increased fivefold in activated macrophages and twofold in inflammatory-elicited macrophages.25 The possibility that the anti-adhesive properties of galectin-1 could be attributed to its pro-apoptotic properties should not be ruled out. However, it should be emphasized that both the concentrations of recombinant lectin (from 0·004 to 4 μg/ml) and the incubation period (30 min) were far below the apoptotic threshold previously reported.26,35 Moreover, apoptotic assays such as DNA fragmentation and TUNEL were found to be negative at the galectin concentrations tested (data not shown). The fact that the anti-galectin-1 antibody used in our studies inhibited T-cell adhesion to ECM and its major glycoprotein ligands, but did not seem to reverse markedly the suppressive activities of the galectin-1 on T-cell cytokine secretion, needs further clarification. It can be postulated, however, that different domains of galectin-1 are involved in its regulation of these proinflammatory functions of human T cells. If this is true, it would be interesting to examine whether galectin-1 also binds different receptors on T cells, and thereby mediate different cellular functions.
Accordingly, a hypothetical model could be proposed in which galectin-1 is secreted in low physiological concentrations from immunocompetent or bystander cells after the completion or exacerbation of an inflammatory or immunological response. The presence of active galectin-1 in the extracellular microenvironment would contribute to regulate T-cell adhesion to ECM negatively as a compensatory mechanism. Furthermore, if this first regulatory mechanism is not sufficient to achieve immunological homeostasis, enhanced secretion of galectin-1, together with prolonged stimulation and persistence in the extracellular milieu, would finally induce apoptosis of activated T cells. Moreover, the sensitivity of galectin-1 carbohydrate-binding activity to oxidative inactivation in that milieu36 might function to limit the duration of its activity. Thus galectin-1 may evolve as a tool that cells could use to modify local interactions with ECM proteins temporarily, where the risk of oxidative inactivation is high.36
Interestingly, it seems that a novel paradigm is providing a breakthrough in galectin research. Overall, opposite functions have been assigned to galectin-1 and galectin-3, a 29 000 MW member of this protein family with similar carbohydrate specificity. Although galectin-1 has been shown to induce T-cell apoptosis,22,26,35 galectin-3 has been shown to prevent cell death.37 Thus galectins 1 and 3 may represent an additional family of proteins similar to the bcl-2 family, where different family members exhibit sequence similarity yet opposite effects on cell survival.38 However, the interplay between galectin-1 and -3 seems to be more conflicting regarding their role in the modulation of cell–ECM interactions. In the context of an inflammatory response, galectin-3 promoted neutrophil adhesion to LN,39 whereas it showed an inhibitory effect on melanoma cell adhesion to ECM.40 Nevertheless, this intriguing paradigm has not been completely lost. Strikingly, in the context of inflammation and immunity, galectin-1 exhibited anti-adhesive and T-cell-spreading properties, as shown here, while galectin-3 promoted cell adhesion.39 However, in the context of abnormal tumorigenic cells, galectin-1 has been shown to induce cell adhesion,24 whereas galectin-3 inhibited attachment to ECM glycoconjugates.40 The molecular mechanism involved in these antagonistic properties remains to be elucidated in the future.
Finally, inhibition of T-cell adhesion and proinflammatory cytokine secretion in the context of the ECM by galectin-1 could represent an alternative mechanism for explaining the anti-inflammatory and immunosuppressive properties10,11 of this evolutionary conserved protein family. A growing body of experimental evidence is now emerging to support the possible use of galectin or galectin antagonists in the treatment of autoimmune disorders, inflammatory episodes and tumour spreading.41 Thus, the carbohydrate-binding domain of galectins represents an attractive target for clinical investigations.
Acknowledgments
Dr Ofer Lider is the incumbent of the Weizmann League Career Development Chair in Children's Diseases. This study was partially supported by The Robert Koch Minerva Center for Research in Autoimmune Diseases, and by The Israeli Center for Emerging Diseases. Dr Gabriel Rabinovich is a recipient of a CONICET fellowship from Argentina. We thank Dr Susana Pesoa for continuous encouragement and critical reading of the manuscript. We also thank Drs Clelia Riera and Claudia Sotomayor for their intellectual support.
Abbreviations
- CO-IV
collagen type IV
- ECM
extracellular matrix
- FN
fibronectin
- gal-1
galectin-1
- LN
laminin
- TDG
thiodigalactoside.
REFERENCES
- 1.Barondes SH, Cooper DNW, Gitt MA, Leffler H. Galectins: structure and function of a large family of animal lectins. J Biol Chem. 1994;269:20807. [PubMed] [Google Scholar]
- 2.Barondes SH, Castronovo V, Cooper DNW, et al. Galectin: a family of animal β-galactoside-binding lectins. Cell. 1994;76:597. doi: 10.1016/0092-8674(94)90498-7. [DOI] [PubMed] [Google Scholar]
- 3.Kasai K, Hirabayashi J. Galectins: a family of animal lectins that decipher glycocodes. J Biochem. 1996;119:1. doi: 10.1093/oxfordjournals.jbchem.a021192. [DOI] [PubMed] [Google Scholar]
- 4.Hirabayashi J, Kasai K. The family of metazoan metal-independent β-galactoside-binding lectins: structure, function and molecular evolution. Glycobiology. 1993;3:297. doi: 10.1093/glycob/3.4.297. [DOI] [PubMed] [Google Scholar]
- 5.Poirrier F, Robertson EJ. Normal development of mice carrying a null mutation in the gene encoding the L-14 S-type lectin. Development. 1993;119:1229. doi: 10.1242/dev.119.4.1229. [DOI] [PubMed] [Google Scholar]
- 6.Leffler H. Introduction to galectins. Trends Glycosci Glycotech. 1997;45:9. [Google Scholar]
- 7.Wells V, Mallucci L. Identification of an autocrine negative growth factor: mouse β-galactoside-binding protein is a cytostatic factor and cell growth regulator. Cell. 1991;64:91. doi: 10.1016/0092-8674(91)90211-g. [DOI] [PubMed] [Google Scholar]
- 8.Adams L, Kenneth Scott G, Weinberg CS. Biphasic modulation of cell growth by recombinant human galectin-1. Biochem Biophys Acta. 1996;1312:137. doi: 10.1016/0167-4889(96)00031-6. [DOI] [PubMed] [Google Scholar]
- 9.Raz A, Lotan R. Endogenous galactoside-binding lectins: a new class of functional tumor cell surface molecules related to metastasis. Cancer Metast Rev. 1987;6:433. doi: 10.1007/BF00144274. [DOI] [PubMed] [Google Scholar]
- 10.Levy G, Tarrab-Hazdai R, Teichberg VI. Prevention and therapy with electrolectin of experimental autoimmune myasthenia gravis in rabbits. Eur J Immunol. 1983;13:500. doi: 10.1002/eji.1830130613. [DOI] [PubMed] [Google Scholar]
- 11.Offner H, Celnik B, Bringman TS, et al. Recombinant human β-galactoside-binding lectin suppresses clinical and histological signs of experimental autoimmune encephalomyelitis. J Neuroimmunol. 1990;28:177. doi: 10.1016/0165-5728(90)90032-i. [DOI] [PubMed] [Google Scholar]
- 12.Gilat D, Cahalon L, Hershkoviz R, Lider O. Interplay of T cells and cytokines in the context of enzymatically modified extracellular matrix. Immunol Today. 1996;17:16. doi: 10.1016/0167-5699(96)80563-9. [DOI] [PubMed] [Google Scholar]
- 13.Cahalon L, Hershkoviz R, Gilat D, et al. Functional interactions of fibronectin and TNFα: a paradigm of physiological linkage between cytokines and extracellular matrix moieties. Cell Adh Commun. 1994;2:269. [PubMed] [Google Scholar]
- 14.Cooper DNW, Barondes SH. Evidence for export of a muscle lectin from cytosol to extracellular matrix and for a novel secretory mechanism. J Cell Biol. 1990;110:1681. doi: 10.1083/jcb.110.5.1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhou Q, Cummings RD. L-14 lectin recognition of laminin and its promotion of in vitro cell adhesion. Arch Biochem Biophys. 1993;300:6. doi: 10.1006/abbi.1993.1002. [DOI] [PubMed] [Google Scholar]
- 16.Cooper DNW, Massa SM, Barondes SH. Endogenous muscle lectin inhibits myoblast adhesion to laminin. J Cell Biol. 1991;115:1437. doi: 10.1083/jcb.115.5.1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mahanthappa NK, Cooper DNW, Barondes SH, Schwarting GA. Rat olfactory neurons can utilize the endogenous lectin L-14, in a novel adhesion mechanism. Development. 1994;120:1373. doi: 10.1242/dev.120.6.1373. [DOI] [PubMed] [Google Scholar]
- 18.Ozeki Y, Matsui T, Yamamoto Y, Funahashi M, Hamako J, Titani K. Tissue fibronectin is an endogenous ligand for galectin-1. Glycobiology. 1995;5:255. doi: 10.1093/glycob/5.2.255. [DOI] [PubMed] [Google Scholar]
- 19.DOK -Y, Smith DF, Cummings RD. LAMP-1 in CHO cells is a primary carrier of poly-N-acetyllactosamine chains and is bound preferentially by a mammalian S-type lectin. Biochem Biophys Res Commun. 1990;173:1123. doi: 10.1016/s0006-291x(05)80902-7. [DOI] [PubMed] [Google Scholar]
- 20.Srincosky DM, Allen HJ, Bernacki RJ. Galaptin-mediated adhesion of human ovarian carcinoma A121 cells and detection of cellular galaptin-binding glycoproteins. Cancer Res. 1993;53:2667. [PubMed] [Google Scholar]
- 21.Perillo NL, Oittenbogaart CH, Nguyen JT, Baum LJ. Galectin-1, an endogenous lectin produced by thymic epithelial cells, induces apoptosis of human thymocytes. J Exp Med. 1997;97:1851. doi: 10.1084/jem.185.10.1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Perillo NL, Pace KE, Seilhamer JJ, Baum LG. Apoptosis of T-cells mediated by galectin-1. Nature. 1995;378:736. doi: 10.1038/378736a0. [DOI] [PubMed] [Google Scholar]
- 23.Cooper DNW. Galectin-1: secretion and modulation of cell interactions with laminin. Trends Glycosci Glycotech. 1997;45:57. [Google Scholar]
- 24.Van Den Brule FA, Buicu C, Baldet M, et al. Galectin-1 modulates human melanoma cell adhesion to laminin. Biochem Biophys Res Comm. 1995;209:760. doi: 10.1006/bbrc.1995.1564. [DOI] [PubMed] [Google Scholar]
- 25.Rabinovich GA, Castagna LF, Landa CA, Riera CM, Sotomayor CE. Regulated expression of a 16-kD galectin-like protein in activated rat macrophages. J Leuk Biol. 1996;59:363. doi: 10.1002/jlb.59.3.363. [DOI] [PubMed] [Google Scholar]
- 26.Rabinovich GA, Iglesias MM, Modesti NM, et al. Activated rat macrophages produce a galectin-1-like protein that induces apoptosis of T cells. Biochemical and functional characterization. J Immunol. 1998;160:4831. [PubMed] [Google Scholar]
- 27.Blaser C, Kaufmann M, Muller C, et al. Beta-galactoside-binding protein secreted by activated T cells inhibits antigen-induced proliferation of T cells. Eur J Immunol. 1998;28:2311. doi: 10.1002/(SICI)1521-4141(199808)28:08<2311::AID-IMMU2311>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 28.Ariel A, Yavin EJ, Hershkoviz R, et al. IL-2 induces T cell adherence to extracellular matrix: inhibition of adherence and migration by IL-2 peptides generated by leukocyte elastase. J Immunol. 1998;161:2465. [PubMed] [Google Scholar]
- 29.Alon R, Cahalon L, Hershkoviz R, et al. TNFα binds to the N-terminal domain of fibronectin and augments the β1-integrin-mediated adhesion of CD4+ T cells to this glycoprotein. J Immunol. 1994;152:1304. [PubMed] [Google Scholar]
- 30.Hirabayashi J, Ayaki H, Soma GI, Kasai K. Production and purification of a recombinant human 14 kDa β-galactoside-binding lectin. FEBS Lett. 1989;250:161. doi: 10.1016/0014-5793(89)80711-2. [DOI] [PubMed] [Google Scholar]
- 31.Nowak TP, Haywood TL, Barondes SH. Developmentally regulated in embryonic chick muscle and a myogenic cell line. Biochem Biophys Res Commun. 1976;68:650. doi: 10.1016/0006-291x(76)91195-5. [DOI] [PubMed] [Google Scholar]
- 32.Hirabayashi J, Kasai K. Effect of amino acid substitution by site-directed mutagenesis on the carbohydrate recognition and stability of human 14-kDa β-galactoside-binding lectin. J Biol Chem. 1991;266:23648. [PubMed] [Google Scholar]
- 33.Gilat D, Hershkoviz R, Mekori YA, Vlodavsky I, Lider O. Regulation of adhesion of CD4+ T lymphocytes to intact or heparinase-treated subendothelial extracellular matrix by diffusible or anchored RANTES and MIP-1β. J Immunol. 1994;153:4899. [PubMed] [Google Scholar]
- 34.Hughes RC. Mac-2, a versatile galactoside-binding protein of mammalian tissues. Glycobiology. 1994;4:5. doi: 10.1093/glycob/4.1.5. [DOI] [PubMed] [Google Scholar]
- 35.Rabinovich GA, Modesti NM, Castagna LF, Landa CA, Riera CM, Sotomayor CE. Specific inhibition of lymphocyte proliferation and induction of apoptosis by CLL-I, a β-galactoside-binding lectin. J Biochem. 1997;122:365. doi: 10.1093/oxfordjournals.jbchem.a021762. [DOI] [PubMed] [Google Scholar]
- 36.Tracey BM, Feizi T, Abbott WN, Carruthers RA, Green BN, Lawson AM. Subunit molecular mass assignment of 14,654 Da to the soluble beta-galactoside-binding lectin from bovine heart muscle and demonstration of intramolecular disulfide bonding associated with oxidative inactivation. J Biol Chem. 1992;267:10342. [PubMed] [Google Scholar]
- 37.Yang RY, Hsu DK, Liu FT. Expression of galectin-3 modulates T-cell growth and apoptosis. Proc Natl Acad Sci USA. 1996;93:6737. doi: 10.1073/pnas.93.13.6737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Oltavi ZN, Milliman CL, Korsmeyer SJ. Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that accelerates programmed cell death. Cell. 1993;74:609. doi: 10.1016/0092-8674(93)90509-o. [DOI] [PubMed] [Google Scholar]
- 39.Kuwabara I, Liu F-T. Galectin-3 promotes adhesion of human neutrophils to laminin. J Immunol. 1996;156:3939. [PubMed] [Google Scholar]
- 40.Ochieng J, Leite-Browning ML, Warfield P. Regulation of cellular adhesion to extracellular matrix proteins by galectin-3. Biochem Biophys Res Com. 1998;246:788. doi: 10.1006/bbrc.1998.8708. [DOI] [PubMed] [Google Scholar]
- 41.Akahani S, Inohara H, Nangia-Makker P, Raz A. Galectin-3 in tumor metastasis. Trends Glycosci Glycotechnol. 1997;9:69. [Google Scholar]