Abstract
We have previously demonstrated that intranasal (i.n.) administration of the major encephalitogenic peptide, Ac1–9 of myelin basic protein (MBP), inhibited T-cell responsiveness in vitro and induced tolerance in the H-2u mouse model of experimental autoimmune encephalomyelitis (EAE). The peptide analogue Ac1–9[4Y] with high-affinity binding to the I-Au major histocompatibility complex (MHC) class II molecule was the most effective tolerogen. Here, we show that mice pretreated with 4Y i.n. and primed with myelin had strongly reduced levels of anti-MBP immunoglobulin G2a (IgG2a) and IgG1, demonstrating that both T helper 1 (Th1) and Th2 functions were inhibited in vivo. Since peptide administered i.n. was shown to be functionally relevant in the thymus, the time interval between 4Y i.n. and subsequent priming was varied in euthymic and adult thymectomized (ATx) mice, to examine the duration of in vitro cell unresponsiveness in the presence or absence of a thymus. For intervals of 1–6 weeks, inhibition of T-cell proliferation was virtually complete in both euthymic and ATx mice. From 8 weeks onwards, responsiveness slowly recovered in euthymic but not in ATx mice. With an interval of 16 weeks, substantial recovery of T-cell responsiveness in vitro in euthymic mice was reflected by a low degree of protection from EAE in vivo. By contrast, anti-MBP IgG2a and IgG1 antibody responses were still significantly reduced. These findings suggest that T-cell unresponsiveness by peptide i.n. represents a thymus-independent, peripheral phenomenon, the reversal of which is confined to new T cells being exported from the thymus. As observed for EAE and antibody responses, the kinetics of recovery may vary for different effector functions.
INTRODUCTION
Nasal administration of soluble peptide antigens has been demonstrated to be a highly effective strategy for tolerance induction in experimental autoimmune encephalomyelitis (EAE) and in other experimental models of autoimmune diseases.1–7 EAE is mediated by CD4+ T cells and represents an instructive model for inflammatory autoimmune disease with many similarities to multiple sclerosis in humans.8 Our previous studies in murine EAE have shown that intranasal (i.n.) peptide administration protected mice from EAE, inhibited T-cell responses in vitro, and induced bystander inhibition of responses to other myelin epitopes.1,2,7 Although such phenomena have been discussed in terms of peripheral mechanisms of tolerance, several studies have highlighted links between thymic and peripheral regulation of mature T cells, suggesting that the role of the thymus in peptide-induced T-cell unresponsiveness warrants closer scrutiny. Peptide given intraperitoneally (i.p.), intravenously (i.v.), or i.n. may induce apoptosis in thymocytes (refs. 9–11; B. Metzler, unpublished data). Conversely, intrathymic injection of antigen may contribute to acquired peripheral tolerance.12–15 Furthermore, tolerance induced by peptide administered via the i.p, i.v. and i.n. routes may be preceded by transient activation,10,11,16 and mature activated T cells have been shown to re-enter the thymus.17 There is also considerable evidence for a thymic contribution to either ‘natural’ or experimentally induced immunoregulation of mature peripheral T cells.18,19 Together, these findings raise the possibility that under some conditions, the thymus might support ‘peripheral’ tolerance. Other reports, however, demonstrated that peripheral administration of antigen could lead to thymus-independent T-cell unresponsiveness,20,21 the reversal of which in one study was exclusively accounted for by new thymic emigrant T cells.21 By contrast, in other systems, recovery of T-cell responsiveness was observed for previously tolerized T cells themselves.22–25 To address the impact of the thymus on the induction and duration of T-cell unresponsiveness in our system, we compared the effect of a strongly tolerogenic peptide, myelin basic protein (MBP) Ac1–9[4Y] (4Y) given i.n. on euthymic and adult thymectomized (Atx) mice leaving intervals of increasing length, 1–16 weeks, between i.n. peptide and subsequent priming. We have observed slow reversal of T-cell unresponsiveness with euthymic but not with Atx mice. As expected, partial recovery of T-cell responsiveness in vitro was reflected by inefficient protection from EAE 16 weeks after 4Y i.n.. By contrast, anti-MBP immunoglobulin G2a (IgG2a) and IgG1 responses, which were completely abolished or strongly reduced upon priming 1 week after 4Y i.n., were still significantly impaired after a 16-week interval. These findings rule out selective T helper type 1 (Th1) inhibition or Th2 deviation as a relevant mechanism of peptide i.n. in this model. They further suggest, that T-cell unresponsiveness induced by peptide i.n. is thymus-independent and irreversible over this long period of time, since recovery of responsiveness depended on new T cells being exported from the thymus. They further suggest that the kinetics of recovery in vivo can vary for different immune responses.
MATERIALS AND METHODS
Mice
(PL/J×B10.PL) F1 mice were bred under specific pathogen-free (SPF) conditions in isolators and subsequently maintained in conventional facilities in the School of Medical Sciences, Bristol University. Mice were used at 6–12 weeks of age. Experimental groups were age- and sex-matched.
Thymectomy
Mice were thymectomized at 5–6 weeks of age under general anaesthesia with Metomidate (Hypnodil; Janssen, Beerse, Belgium) and fentamyl citrate (Sublimaze; Janssen). The chest was opened by a 1–2-cm longitudinal incision and the thymus was removed by suction with a modified Pasteur pipette inserted into the anterior mediastinum. The incision was sealed with surgical staples. Mice were rested for at least 2 weeks after thymectomy.
Antigens and antigen administration
The acetylated N-terminal peptide of murine myelin basic protein (MBP Ac1–9, Ac-ASQKRPSQR) and the 4Y peptide analogue (Ac1–9[4Y]) were synthesized using standard Fmoc chemistry on an AMS 422 multiple peptide synthesizer (Abimed, Langenfeld, Germany). Ac1–9[4Y] was solubilized in phosphate-buffered saline (PBS) at 4 mg/ml and 25 μl was administered i.n. under light ether anaesthesia. Mouse spinal cord homogenate (SCH) was prepared as previously described1 and used as a source of whole myelin. Mice were primed subcutaneously (s.c.) at the base of the tail with 0·1 ml of an emulsion consisting of equal volumes of complete Freund’s adjuvant (CFA; Difco, West Molesey, UK) and PBS containing heat-killed Mycobacterium tuberculosis, strain H37Ra (Difco) at 4 mg/ml, and either 50 μg Ac1–9 or 1 mg SCH. MBP was purified from mouse brains and spinal cords according to the method of Deibler et al.26 The purity of the protein was confirmed by sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS–PAGE), and its identity was confirmed by Western blot analysis using clone 12 rat anti-MBP IgG.27
Induction and assessment of EAE
Mice were primed with 1 mg SCH/CFA s.c. at the base of the tail. Pertussis toxin (Sigma, Poole, UK) was injected i.p. (200 ng in PBS per dose) at the time of priming and 2 days later. Mice were monitored daily for disease and EAE was graded as follows: (1) flaccid tail, (2) partial hind limb paralysis and impaired righting, (3) total hind limb paralysis, (4) fore and hind limb paralysis, and (5) moribund. For statistical analysis, the incidence of EAE between groups was compared by the Fisher exact test, the median day of disease onset by the median test in combination with the Fisher exact test, and differences between the mean maximum grades of EAE were assessed by Student’s t-test.
T-cell proliferation assay
Mice were primed with 50 μg Ac1–9/CFA s.c. at the base of the tail. Ten days later, single cell suspensions prepared from draining inguinal lymph nodes were restimulated with Ac1–9 in serum-free lymphocyte growth medium (Promocell, Heidelberg, Germany) at 5×105 cells/well in 96-well flat-bottomed microtitre plates. For standardization purposes, proliferative responses to 50 μg/ml bovine purified protein derivative (PPD; Central Veterinary Laboratory, Weybridge, UK) were measured in separate wells as positive controls. Cells were cultured at 37° for a total of 4 days, pulsed with 0·5 μCi/well [3H]thymidine (Amersham, Little Chalfont, UK) for the last 18 hr of culture, and incorporated radioactivity was measured by liquid scintillation counting.
Anti-MBP antibody enzyme-linked immunosorbent assay (ELISA)
Sera were prepared from mice primed with 1 mg SCH/CFA s.c. at the base of the tail. Polyvinyl microtitre plates (3012, Falcon Labware, NJ) were coated with saturating concentrations of purified mouse MBP (10 μg/ml) at 4° overnight, and blocked with 1% bovine serum albumin (BSA)/PBS for 2 hr at room temperature. After washing with PBS/Tween-20, plates were incubated with serially diluted sera in PBS for 2 hr at room temperature. Plates were then washed and incubated (2 hr at room temperature) with horseradish peroxidase-linked goat anti-mouse, isotype-specific antibodies; IgM (Sigma), IgG (γ chain-specific, Sigma), IgG1 (SERA-LAB, Loughborough, UK), IgG2a (sera-lab). After washing, plates were incubated with the developing substrate o-phenylenediamine dihydrochloride in citrate/phosphate buffer in the presence of hydrogen peroxide. The reaction was stopped with 1 m sulphuric acid, and the absorbance was read at 492 nm. Standard curves were generated for each plate using titrations ranging from 1/50 to 1/5000 dilutions of positive anti-mouse MBP sera, and results were expressed as arbitrary units per ml (U/ml). One unit corresponds to the absorbance of the standard serum at a dilution of 1/500 or 1/250 and ≈50% maximum absorbance. Immunoglobulin levels between groups were compared by the median test and the statistical significance of differences evaluated by the Fisher exact test.
RESULTS
Establishment and duration of T-cell unresponsiveness in euthymic and Atx mice
In the H-2u mouse model of EAE, i.n. administration of a single peptide representing the immunodominant epitope of MBP, Ac1–928 protected mice from peptide- or myelin-induced disease and inhibited in vitro T-cell responsiveness. T-cell inhibition was improved with analogues of Ac1–9 (Ac1–9[4A] and Ac1–9[4Y]) that display higher binding affinity to the I-Au MHC class II molecule.29,30 Here we compare the effect of the highest affinity peptide analogue, 4Y, given i.n., on the induction and duration of T-cell unresponsiveness in Atx versus euthymic mice. Our previous studies had routinely included a 1-week interval between peptide i.n. and priming. During initial experiments with intervals increased up to 6 weeks, there had been no significant differences in the ability to inhibit T-cell proliferative responses with euthymic and Atx mice. After an 8-week interval, however, T-cell responsiveness appeared to have recovered to some extent in euthymic (untreated or sham-operated) but not in Atx mice (not shown). To confirm and extend these studies, another series of experiments included 1–16-week intervals. The results presented in Fig. 1 illustrate the trend of T-cell responsiveness with three intervals, 2 weeks (Fig. 1a,b), 8 weeks (Fig. 1c,d), and 16 weeks (Fig. 1e,f) between 4Y i.n. and subsequent priming. With short or intermediate time spans (1–5 weeks, here shown for 2 weeks, Fig. 1a,b), T-cell responsiveness remained poor or completely inhibited with or without previous thymectomy. After 8 weeks, however, all lymphocyte samples from euthymic but not Atx mice exhibited a low but significant degree of proliferation (Fig. 1c,d). The recovery of responsiveness in euthymic mice was more pronounced with a 12-week interval (not shown), and even more advanced 16 weeks after 4Y i.n. (Fig. 1e). By contrast, T-cell proliferative responses did not recover after thymectomy (Fig. 1f).
Figure 1.
Establishment and duration of T-cell unresponsiveness in euthymic and adult thymectomized (ATx) mice. Atx mice (b, d, f) or euthymic littermates (a, c, e) received a single 100 μg dose of Ac1–9[4Y] i.n. (•) or PBS (○). Mice were subsequently primed with 50 μg Ac1–9/CFA at one of the indicated time-points after 4Y i.n.; 2 weeks (a, b), 8 weeks (c, d), or 16 weeks (e, f). Ten days later, primed lymphocytes were tested for proliferative responses to Ac1–9 in vitro. Each symbol represents the arithmetic means of triplicate samples, standard deviations were less than 20%. Lymphocyte samples were either derived from individual mice, or pooled from two mice. Each plot shows proliferation data of a representative set of lymphocyte samples with comparable positive (PPD) and negative (medium only) control responses. All samples (three to six independent cell samples per group in two or three experiments per time-point) with comparable negative and positive controls showed similar differences in proliferation.
Recovery of responsiveness in vivo
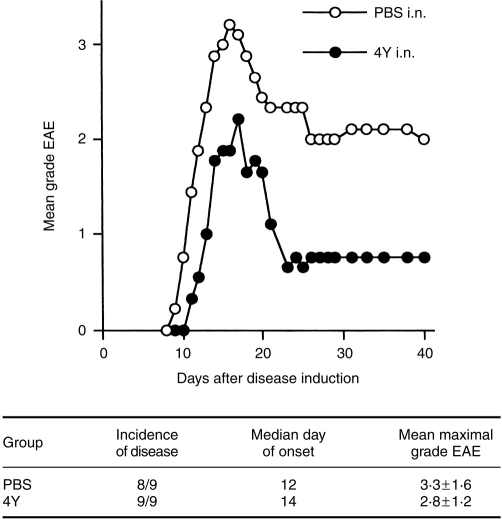
In previous experiments, 4Y i.n. 1 week prior to EAE induction with SCH significantly reduced the incidence of disease from 90–100% in controls to 20–60% and mean maximal grades from 2·4–4·3 to 0·4–1·2.1,2 The degree of protection by peptide i.n. or i.p, which varied with different peptides or peptide analogues. was reflected by the level of in vitro T-cell unresponsiveness (refs. 7, 31, and unpublished data). We therefore expected that protection from EAE would be partially diminished in euthymic mice 16 weeks after 4Y i.n.. As shown in Fig. 2, there was still a tendency for 4Y i.n.-treated mice to develop lower grade EAE, although this was not statistically significant, and there was no difference in disease incidence.
Figure 2.
Partial reversal of protection from EAE 16 weeks after 4Y i.n. Groups of nine mice received a single 100 μg dose of Ac1–9[4Y] i.n. (•) or PBS (○).Sixteen weeks later, EAE was induced with 1 mg SCH/CFA.
To further investigate the impact of 4Y i.n. on immune responses in vivo, we measured anti-MBP immunoglobulin responses: IgM as a readout for T-cell-independent B-cell activation, total IgG for T-cell-dependent immunoglobulin class switching, IgG2a and IgG1 to test the possibility of differential effects on Th1- and Th2-related functions, respectively.32 Mice were primed with myelin 1 week (Fig. 3a) or 16 weeks (Fig. 3b) after 4Y i.n., and sera were analysed for antibody concentrations 3 weeks later. The data in Fig. 3(a,b,i+ii) show that anti-MBP IgM was not inhibited by 4Y i.n. By contrast, IgG levels were strongly reduced by 4Y i.n. 1 week before priming (Fig. 3a, iii+iv, P = 0·00054), and, although recovered to some extent, were still substantially lower compared to controls when 16 weeks had passed after 4Y i.n. (Fig. 3b, iii+iv, P = 0·027). With a 1-week interval, IgG2a was not detectable in any of the sera (Fig. 3a, vi), whereas three of 10 mice produced significant levels of IgG1 (Fig. 3a, viii). This observation was reproducible in another independent experiment where 10 of 10 sera lacked IgG2a after 4Y i.n. while two of 10 contained IgG1 (not shown). An analysis of sera collected 5 and 11 days after priming excluded the possibility of an earlier Th2-dominated response (not shown). With 16 weeks between 4Y i.n. and priming, the majority of mice now produced significant levels of both isotypes IgG2a and IgG1. In contrast to the more advanced recovery for EAE, however, these anti-MBP IgG responses were still significantly lower compared with controls (P = 0·027 for both isotypes).
Figure 3.
Effect of 4Y i.n. on anti-MBP antibody responses. Groups of nine or 10 mice received a single intranasal dose of 100 μg Ac1–9[4Y] (ii, iv, vi, viii; filled bars) or PBS (i, iii, v, vii; open bars). One week (a) or 16 weeks (b) later, all were primed with 1 mg SCH/CFA. After 3 weeks, serum samples from individual mice were assayed by ELISA for anti-MBP antibody concentrations: IgM (i, ii), total IgG (iii, iv), IgG2a (v, vi), and IgG1(vii, vii). Results are expressed in arbitrary units per ml serum as determined from standard curves with positive sera. One unit corresponds to ≈50% maximum absorbance obtained with the standard titration at 1/250 or 1/500 dilution. Sera are numbered in order of increasing concentrations of total IgG. The same numbers apply to all other isotype data plots.
DISCUSSION
Administration of aqueous antigen often leads to specific unresponsiveness in mature peripheral T cells. Loss of productive immune reactivity can manifest itself after several not mutually exclusive events such as physical or functional elimination of T cells by deletion and anergy, or the induction of regulatory T cells. The relevance of each mechanism may depend on the make-up of the antigen itself, and the dose, frequency, and route of administration.33–36 The nasal route is highly effective in delivering peptide systemically in an immunologically active form via both the blood circulation and lymphatics (B. Metzler et al. submitted for publication). This systemic distribution probably accounts for the very similar results observed after either i.p. or i.n. peptide administration.1,7,31 The fate of T cells affected by peptide i.n. has not been fully characterized and is the subject of further investigation. Although Ac1–9[4Y], given i.p or i.n., readily gains access to the thymus and induces apoptosis in transgenic thymocytes (ref. 10, B. Metzler et al. submitted for publication), we show in this report, that the thymus is not required for, and does not appear to contribute to the induction of T-cell unresponsiveness by 4Y i.n.. This is in agreement with previous studies on peripheral tolerance by soluble human gammaglobulin given i.p.,20 and for tolerance induction with a variety of antigens under the cover of non-depleting anti-CD4 and anti-CD8 antibodies.21 Furthermore, as also described for the latter case by Qin et al., T-cell unresponsiveness, once established after 4Y i.n., appeared to be irreversible, since slow recovery of responsiveness was observed with euthymic but not Atx mice. This differs from systems where reversal of peripheral tolerance was independent of new thymic emigrant T cells, and was observed when the specific tolerogen was no longer present.22–25 If continuous presence of peptide were essential for the maintenance of T-cell inhibition in our model, we would have expected fast recovery of T-cell responsiveness, since 4Y was no longer detectable in mouse tissues about 1 week after i.n. administration (B. Metzler et al. submitted for publication). Although we cannot rigorously exclude the existence of a cryptic source of 4Y, such a putative source was not capable of either inducing unresponsiveness in new thymic emigrant T cells or enabling previously tolerized T cells to induce ‘infectious tolerance’ in naive T cells, a mechanism also shown to require the presence of antigen.21,37 Tolerance by 4Y i.n. was confined to T cells, as illustrated by the lack of inhibition of anti-MBP IgM. This was not surprising since Ac1–9 of MBP constitutes a poor B-cell epitope (our unpublished data), and is consistent with previous observations that B cells are far less sensitive to tolerance induction than T cells.38 T-cell help required for class switching to IgG was profoundly inhibited by 4Y i.n.. This encompassed both Th1- and Th2-associated isotypes IgG2a and IgG1, respectively, although a minority of 4Y i.n.-treated mice still produced some IgG1 (Fig. 3a). This resembles observations made in one study on tolerance to human gammaglobulin,39 and suggests that any incomplete tolerance is more likely to affect Th2-dependent responses. Considering the low incidence of residual IgG1 together with concomitant inhibition of both Th1- and Th2-related in vitro cytokine production,7 it seems unlikely, however, that regulatory mechanisms induced by peptide i.n. rely on Th2 deviation. Inhibition of both Th1 and Th2 responses by peptide i.n. was also observed in the Der p I model.40 By contrast, in the non-obese diabetes model, nasal administration of a mixture of peptides3 or repeated administration of a single peptide4 to very young mice was reported to induce a Th2 response. In collagen-induced arthritis, repeated nasal peptide administration to rats resulted in elevated levels of IgG1.5 The different effects of peptide i.n. in different models might depend on several factors such as background genes, the age of animals when given peptide i.n., and the dose and frequency of peptide administration.
As expected from the partial recovery of in vitro T-cell responsiveness 16 weeks after 4Y i.n., euthymic mice treated with peptide were no longer efficiently protected from EAE. Anti-MBP IgG responses, however, were still substantially reduced, and IgG1 and IgG2a levels appeared to recover with similar slow kinetics. Assuming, as our data suggest, that the recovery of responsiveness relies entirely on new T cells being exported from the thymus, it is conceivable that fewer T cells are required to generate sufficient levels of pro-inflammatory cytokines for EAE compared to cytokines required to support IgG class switching. The kinetics of recovery can also be expected to vary among different systems. In the Der p I model, T-cell responsiveness did not recover for 6 months after peptide i.n..40 It is possible that a lower peptide-specific precursor frequency among thymic emigrant T cells and/or initial multiple doses of peptide i.n. might have delayed recovery of responsiveness beyond the time intervals observed in our study.
The persistence of unresponsiveness in Atx mice could be due to two not mutually exclusive mechanisms. T cells could be deleted or irreversibly ‘locked’ in the unresponsive state. Tolerance by peptide i.p. or i.n. is preceded by transient activation,10,16 and leads to long-term reduction in the numbers of specific T cells from the periphery of transgenic mice (B. Metzler et al. submitted for publication). Although the relevance of deletion for the maintenance of T-cell unresponsiveness is not clear, the phenomenon of bystander inhibition indicates that apoptosis cannot be complete at early stages after peptide i.n. when active regulation clearly plays an important role.7 These regulatory mechanisms remain to be defined in detail and are the subject of further investigation. Recent evidence suggests, however, that interleukin-10 plays a role in immunoregulation induced by 4Y i.n. in transgenic mice (Burkart et al. submitted for publication). This does not exclude the possibility of more extensive T-cell death at later stages after 4Y i.n. It is also possible, as previously shown in the HY model,41 that T cells rendered tolerant by 4Y i.n. are more likely to disappear from the periphery in the presence than in the absence of new thymic emigrant T cells. Further studies using limiting transfer of peptide-specific transgenic T cells to euthymic and Atx recipients will help to clarify the underlying mechanism of long-term T-cell unresponsiveness.
We conclude that a single dose of intranasal peptide can induce long-lasting thymus-independent peripheral T-cell unresponsiveness, the recovery of which relies entirely on new T cells being exported from the thymus. Depending on the requirements for effector functions in vivo, the kinetics of recovery may vary among different T-cell-dependent immune responses.
Acknowledgments
We thank Heather Pope for technical advice and Pauline Lowrey for peptide synthesis. This work was supported by the Multiple Sclerosis Society of Great Britain and Northern Ireland.
Abbreviations
- Atx
adult thymectomized
- EAE
experimental autoimmune encephalomyelitis
- i.n
intranasal
- MBP
myelin basic protein
- SCH
spinal cord homogenate
REFERENCES
- 1.Metzler B, Wraith DC. Inhibition of experimental autoimmune encephalomyelitis by inhalation but not oral administration of the encephalitogenic peptide: influence of MHC binding affinity. Intern Immunol. 1993;5:1159. doi: 10.1093/intimm/5.9.1159. [DOI] [PubMed] [Google Scholar]
- 2.Metzler B, Wraith DC. Mucosal tolerance in a murine model of experimental autoimmune encephalomyelitis. Ann N Y Acad Sci. 1996;778:228. doi: 10.1111/j.1749-6632.1996.tb21131.x. [DOI] [PubMed] [Google Scholar]
- 3.Tian J, Atkinson MA, Clare-Salzler M, et al. Nasal administration of glutamate decrboxylase (GAD65) peptides induces Th2 responses and prevents murine insulin-dependent diabetes. J Exp Med. 1996;183:1561. doi: 10.1084/jem.183.4.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Daniel D, Wegmann DR. Protection of nonobese diabetic mice from diabetes by intranasal or subcutaneous administration of insulin peptide B-(9) Proc Natl Acad Sci USA. 1996;93:956. doi: 10.1073/pnas.93.2.956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Staines NA, Harper N, Ward FJ, Malmstrom V, Holmdahl R, Bansal S. Mucosal tolerance and suppression of collagen-induced arthritis (CIA) induced by nasal inhalation of synthetic peptide 184 of bovine type II collagen (CII) expressing a dominant T cell epitope. Clin Exp Immunol. 1996;103:368. doi: 10.1111/j.1365-2249.1996.tb08289.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Prakken BJ, Van Der Zee R, Anderton SM, Van Kooten PJS, Kuis W, Van Eden W. Peptide-induced nasal tolerance for a mycobacterial heat shock protein 60 T cell epitope in rats suppresses both adjuvant arthritis and nonmicrobially induced experimental arthritis. Proc Natl Acad Sci USA. 1997;94:3284. doi: 10.1073/pnas.94.7.3284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Anderton SM, Wraith DC. Hierarchy in the ability of T cell epitopes to induce tolerance to antigens from myelin. Eur J Immunol. 1998;28:1251. doi: 10.1002/(SICI)1521-4141(199804)28:04<1251::AID-IMMU1251>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 8.Martin R, McFarland HF. Immunological aspects of experimental allergic encephalomyelitis and multiple sclerosis. Crit Rev Clin Lab Sci. 1995;32:121. doi: 10.3109/10408369509084683. [DOI] [PubMed] [Google Scholar]
- 9.Murphy KM, Heimberger AB, Loh DY. Induction by antigen of intrathymic apoptosis of CD4+CD8+ TCRlo thymocytes in vivo. Science. 1990;250:1720. doi: 10.1126/science.2125367. [DOI] [PubMed] [Google Scholar]
- 10.Liu GY, Fairchild PJ, Smith RM, Prowle JR, Kioussis D, Wraith DC. Low avidity recognition of self-antigen by T cells permits escape from central tolerance. Immunity. 1995;3:407. doi: 10.1016/1074-7613(95)90170-1. [DOI] [PubMed] [Google Scholar]
- 11.Liblau RS, Tisch R, Shokat K, et al. Intravenous injection of soluble antigen induces thymic and peripheral T-cell apoptosis. Proc Natl Acad Sci USA. 1996;93:3031. doi: 10.1073/pnas.93.7.3031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Khoury SJ, Sayegh MH, Hancock WW, Gallon L, Carpenter CB, Weiner HL. Acquired tolerance to experimental autoimmune encephalomyelitis by intrathymic injection of myelin basic protein or its major encephalitogenic peptide. J Exp Med. 1993;178:559. doi: 10.1084/jem.178.2.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goss JA, Nakafusa Y, Roland CR, Hickey WF, Flye MW. Immunological tolerance to a defined myelin basic protein antigen administered intrathymically. J Immunol. 1994;153:3890. [PubMed] [Google Scholar]
- 14.Sayegh MH, Perico N, Gallon L, et al. Mechanisms of acquired thymic unresponsiveness to renal allografts: thymic recognition of immunodominant allo-MHC peptides induces peripheral T cell anergy. Transplantation. 1994;58:125. [PubMed] [Google Scholar]
- 15.Chen W, Sayegh MH, Khoury SJ. Mechanisms of acquired thymic tolerance in vivo: intrathymic injection of antigen induces apoptosis and peripheral T cell anergy. J Immunol. 1998;160:1504. [PubMed] [Google Scholar]
- 16.Hoyne GF, Askonas BA, Hetzel C, Thomas WR, Lamb JR. Regulation of house dust mite responses by intranasally administered peptide: transient activation of CD4+ T cells precedes the development of tolerance in vivo. Intern Immunol. 1996;8:335. doi: 10.1093/intimm/8.3.335. [DOI] [PubMed] [Google Scholar]
- 17.Agus DB, Surh CD, Sprent J. Reentry of T cells to the adult thymus is restricted to activated T cells. J Exp Med. 1991;173:1039. doi: 10.1084/jem.173.5.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Saoudi A, Seddon B, Fowell D, Mason D. The thymus contains a high frequency of cells that prevent autoimmune diabetes on transfer into prediabetic recipients. J Exp Med. 1996;184:2393. doi: 10.1084/jem.184.6.2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang Y, Goldschneider I, Foss D, Wu DY, O’Rourke J, Cone RE. Direct thymic involvement in anterior chamber-associated immune deviation. J Immunol. 1997;158:2150. [PubMed] [Google Scholar]
- 20.Gahring LC, Weigle WO. The induction of peripheral T cell unresponsiveness in adult mice by monomeric human γ-globulin. J Immunol. 1989;143:2094. [PubMed] [Google Scholar]
- 21.Qin S, Wise M, Cobbold SP, et al. Induction of tolerance in peripheral T cells with monoclonal antibodies. Eur J Immunol. 1990;20:2737. doi: 10.1002/eji.1830201231. [DOI] [PubMed] [Google Scholar]
- 22.Ramsdell F, Fowlkes BJ. Maintenance of in vivo tolerance by persistence of antigen. Science. 1992;257:1130. doi: 10.1126/science.257.5073.1130. [DOI] [PubMed] [Google Scholar]
- 23.Migita K, Ochi A. The fate of anergic T cells in vivo. J Immunol. 1993;150:763. [PubMed] [Google Scholar]
- 24.Rocha B, Tanchot C, Von Boehmer H. Clonal anergy blocks in vivo growth of mature T cells and can be reversed in the absence of antigen. J Exp Med. 1993;177:1517. doi: 10.1084/jem.177.5.1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alferink J, Schittek G, Schonrich G, Hammerling GJ, Arnold B. Long life span of tolerant T cells and the role of antigen in maintenance of peripheral tolerance. Intern Immunol. 1995;7:331. doi: 10.1093/intimm/7.2.331. [DOI] [PubMed] [Google Scholar]
- 26.Deibler GE, Martenson RE, Kies MW. Large scale preparation of myelin basic protein from central nervous tissue of several mammalian species. Preparative Biochemistry. 1972;2:139. doi: 10.1080/00327487208061467. [DOI] [PubMed] [Google Scholar]
- 27.Hruby S, Alvord EC.J, Groome NP, Dawkes A, Martenson RE. Monoclonal antibodies reactive with myelin basic protein. Mol Immunol. 1987;12:1359. doi: 10.1016/0161-5890(87)90132-5. [DOI] [PubMed] [Google Scholar]
- 28.Zamvil SS, Mitchell DJ, Moore AC, Kitamura K, Steinman L, Rothbard JB. T cell epitope of the autoantigen myelin basic protein that induces encephalomyelitis. Nature. 1986;324:258. doi: 10.1038/324258a0. [DOI] [PubMed] [Google Scholar]
- 29.Wraith DC, Smilek DE, Mitchell DJ, Steinman L, McDevitt HO. Antigen recognition in autoimmuneencephalomyelitis and the potential for peptide-mediated immunotherapy. Cell. 1989;59:247. doi: 10.1016/0092-8674(89)90287-0. [DOI] [PubMed] [Google Scholar]
- 30.Fairchild PJ, Wildgoose R, Atherton E, Webb S, Wraith DC. An autoantigenic T cell epitope forms unstable complexes with class II MHC: a novel route for escape from tolerance induction. Intern Immunol. 1993;5:1151. doi: 10.1093/intimm/5.9.1151. [DOI] [PubMed] [Google Scholar]
- 31.Liu GY, Wraith DC. Affinity for class II MHC determines the extent to which soluble peptides tolerize autoreactive T cells in naive and primed adult mice – implications for autoimmunity. Intern Immunol. 1995;7:1255. doi: 10.1093/intimm/7.8.1255. [DOI] [PubMed] [Google Scholar]
- 32.Snapper CM, Paul WE. Interferon-gamma and B cell stimulatory factor-1 reciprocally regulate Ig isotype production. Science. 1987;236:944. doi: 10.1126/science.3107127. [DOI] [PubMed] [Google Scholar]
- 33.Degermann S, Pria E, Adorini L. Soluble protein but not peptide administration diverts the immune response of a clonal CD4+ T cell population to the T helper 2 cell pathway. J Immunol. 1996;157:3260. [PubMed] [Google Scholar]
- 34.Critchfield JM, Racke MK, Zuniga-Pflucker JC, et al. T cell deletion in high antigen dose therapy of autoimmune encephalomyelitis. Science. 1994;263:1139. doi: 10.1126/science.7509084. [DOI] [PubMed] [Google Scholar]
- 35.Guery J-C, Galbiati F, Smiroldo S, Adorini L. Selective development of T helper (Th) 2 cells induced by continuous administration of low dose soluble proteins to normal and β2-microglobulin-deficient BALB/c mice. J Exp Med. 1996;183:485. doi: 10.1084/jem.183.2.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Miller A, Zhang ZJ, Sobel RA, Al-Sabbagh A, Weiner HL. Suppression of experimental autoimmune encephalomyelitis by oral administration of myelin basic protein. IV. Suppression of adoptively transferred disease and differential effects of oral vs. intravenous tolerization. J Neuroimmunol. 1993;46:73. doi: 10.1016/0165-5728(93)90235-q. [DOI] [PubMed] [Google Scholar]
- 37.Qin S, Cobbold SP, Pope H, et al. ‘Infectious’ transplantation tolerance. Science. 1993;259:974. doi: 10.1126/science.8094901. [DOI] [PubMed] [Google Scholar]
- 38.Adelstein S, Pritchard-Briscoe H, Anderson TA, et al. Induction of self-tolerance in T cells but not B cells of transgenic mice expressing little self antigen. Science. 1991;251:1223. doi: 10.1126/science.1900950. [DOI] [PubMed] [Google Scholar]
- 39.Romball CG, Weigle WO. In vivo induction of tolerance in murine CD4+ cell subsets. J Exp Med. 1993;178:1637. doi: 10.1084/jem.178.5.1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hoyne GF, Jarnicki AG, Thomas WR, Lamb J. Characterization of the specificity and duration of T cell tolerance to intranasally administered peptides in mice: a role for intramolecular epitope suppression. Intern Immunol. 1997;9:1165. doi: 10.1093/intimm/9.8.1165. [DOI] [PubMed] [Google Scholar]
- 41.Tanchot C, Rocha B. Peripheral selection of T cell repertoire: the role of continous thymic output. J Exp Med. 1997;186:1099. doi: 10.1084/jem.186.7.1099. [DOI] [PMC free article] [PubMed] [Google Scholar]